Abstract
Clinical research is a branch of healthcare science that assesses the safety and efficacy of treatments, drugs, and medical devices aimed at improving human health. The primary goals include enhancing our understanding of human biology, proving the efficacy of treatments, and determining the relationship between genetics and diseases. Clinical trials, a subset of clinical research, progress through phases: Phase 0 to explore initial drug kinetics, Phase I for safety, Phase II for efficacy and side effects, Phase III to compare with existing treatments, and Phase IV for post-market surveillance. Research designs play a key role, with quantitative designs focusing on measurable data and cause-effect relationships, while qualitative designs explore lived experiences and cultural contexts. Experimental research involves manipulating variables to assess causal effects, while non-experimental research relies on natural observations without intervention. Types of experimental research (includes pre-experimental, true experimental and quasi-experimental designs) and Non-experimental studies (like cohort, case-control, and cross-sectional designs) are vital in observational studies. Qualitative research methods provide deep insights into human experiences and societal behaviours. Each method contributes to advancing scientific knowledge, guiding evidence-based healthcare practices, and improving patient outcomes.
Keywords
Clinical research, Clinical trials, Research design, Quantitative Research design, Qualitative research design.
Introduction
A clinical research needs an organised method with deliberate preparation, execution, and sampling to acquire dependable and validated results. The researchers must have an extensive knowledge of each study methodology. Furthermore, choosing an inappropriate form of study is a prominent mistake that cannot be fixed after a study has started which leads to ineffective execution. Clinical research study findings expand our understanding of a disease's pathogenicity, a medicine that has been developed or is now in use, a surgical or diagnostic treatment, or a medical device. A number of variables affect the way the clinical research technique is implemented is consists of the study's design, methodology, population, sampling, statistical processes, methodology, and objectives. Clinical research design is primarily classified as Quantitative and Qualitative research design. Furthermore the Quantitative research methods are classified into experimental and non-experimental research. The experimental study designs have been regarded as the most essential in providing precise and trustworthy results irrespective of all the clinical research methodologies that are currently available. In the past, clinical research has frequently and successfully employed experimental designs (pre-experimental, true, and quasi-experimental designs) to precisely identify the internal and external factors influencing the disease and the drug's effect, thereby minimising these confounding variables (1). Since a number of factors cannot be modified ethically or technically, experimental designs are unable to resolve all of the research issues. Non-experimental research designs are used for these kinds of studies. Non-experimental study designs describe current incidents without changing an independent variable or subject conditions that may affect responses (2). To understand how people perceive their environment, qualitative research is conducted. While qualitative research can be approached in numerous of methods, all of them have a tendency to be flexible and emphasise maintaining profound significance in data interpretation. Common approaches include grounded theory, ethnography, action research, case studies, phenomenological research, and narrative research (3). In this review we comprehensively describe about the clinical research design, phases of clinical trials, and types of clinical research. (3)
1. Clinical Research
1.1 Definition:
Clinical research is a branch of healthcare science that determines the safety and efficacy of medicinal substances, a surgical or medical procedure, or a device intended for human use. This is conducted with the aim of patient care and treatment. Furthermore, it can refer to any study that assesses the pathophysiology, symptoms, risk factors and other aspects of a disease. It includes conducting studies of human health and illness. (4)
1.2 Goals:
- To acquire knowledge that improves human health or enhances understanding of human biology.
- To evaluate the safety and efficacy of a medicinal drug, a medical/surgical procedure, or a device as a part of treatment and patient care.
- To utilize scientific methods to demonstrate the beneficial aspects and limitations of the experimental treatment.
- To assess how genetics are linked to diseases.
1.3 Importance:
- It is crucial for enhancing medical advancements and improving global health.
- It assists researchers in finding effective methods for preventing, diagnosing, treating and understanding the disease.
- It systematically collects data and evidence which contributes to the development of evidence - based guidelines and best practices that establishes the standard of care for various medical conditions.
- Clinical Trials
2.1 Definition:
- Clinical trial is a research study that evaluates a new medical treatment or a new application of an existing treatment to determine if it offers a more effective method for preventing, diagnosing or treating a disease.
- Before entering clinical trials any new drug must undergo preclinical studies (which include in-vitro experiment) and clinical trials conducted on animal populations.(5)
- Clinical trials are carried out to evaluate the therapeutic effectiveness of a new drug in comparison to an existing drug or a placebo.
2.2 Phases of Clinical Trials: (6)
- Number of subjects-10-15
- Dose-Unrestricted
- Type of study-Exploratory
- Conducted by -Clinical pharmacologists
- Objectives-Explore pharmacokinetics and pharmacodynamics
Phase 0 is also called micro dosing. It is conducted in a small number of subjects 10-15 for a short duration less than 7 days. A very small dose is used to evaluate the pharmacodynamics and pharmacokinetics in human beings and is exposed to the drug for a short period. Analysis is done by highly sensitive methods like accelerated mass spectrometry and positron emission tomography (PET).
- Number of subjects-20-50 volunteers
- Dose-Very small sub therapeutic
- Type of study-non-therapeutic trial
- Conducted by-Clinical pharmacologists
- Objectives-To establish safety, to know biological effects, pharmacokinetics profile and to design a safe dose
Phase I trials are the first stage of testing in human subjects. Normally, a small (20 -80) group of healthy volunteers will be selected. This phase includes trials designed to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of a drug. These trials are often performed in clinical centers, where the subjects are kept under observations by full–time staff. The Phase I trials are also called as dose magnifying studies as they consist of normal ranging of doses so that the safest and appropriate dose can be determined. Phase 1 trials most often include healthy volunteers.
- Number of subjects-100-300 patients
- Dose-Often sub therapeutic but with ascending dose
- Type of study-Exploratory trial
- Conducted by - Clinical pharmacologists and Clinical investigators
- Objectives-To establish efficacy, detect adverse effects & pharmacokinetics.
Once the initial safety of the study drug has been confirmed in phase 1 trials, phase 2 trials are performed on larger groups (100-300) and are designed to assess how well the drug works, as well as to continue phase I safety assessments in a larger group of volunteers and patients. It is conducted in order to establish efficacy, to detect any adverse effects, appropriate dose and detailed pharmacology of the drug in patients suffering from disease for which the drug under trial has therapeutic prospects. Phase II studies divided into 2 phases: - Phase II A - Designed to assess dosing requirements
-Phase II B- Designed to study efficacy.
- Number of subjects - 250 to >10,000 patients
- Dose - Therapeutic dose
- Type of study-Therapeutic confirmatory trial
- Conducted by-Clinical investigators
- Objectives-To establish efficacy, safety, to identify latent side effects, tolerance, design ideal dose-range and to compare with existing drugs
The drug is given to a large member of selected patients to establish the benefits of the drug in the target disease, to identify the latent side effects, susceptibility to tolerance and to design ideal dosage regimen for different groups of patients.
- Number of subjects - Several 1000 patients
- Dose - Therapeutic dose
- Type of study- Post approval study
- Conducted by - Medical practitioners
- Object- Long-term safety and efficacy, to identify other possible therapeutic uses
Phase IV trial is also known as post marketing surveillance trial. If phase III studies are satisfactory new drug is marketed. Since the earlier phases involve a relatively smaller number of patients (3000) for short period (less than 1 year), they cannot be expected to provide full safety information thus post marketing surveillance is done for systematic detection and evaluation of long-term safety of the drug. Phase IV trials are conducted by medical practitioners.
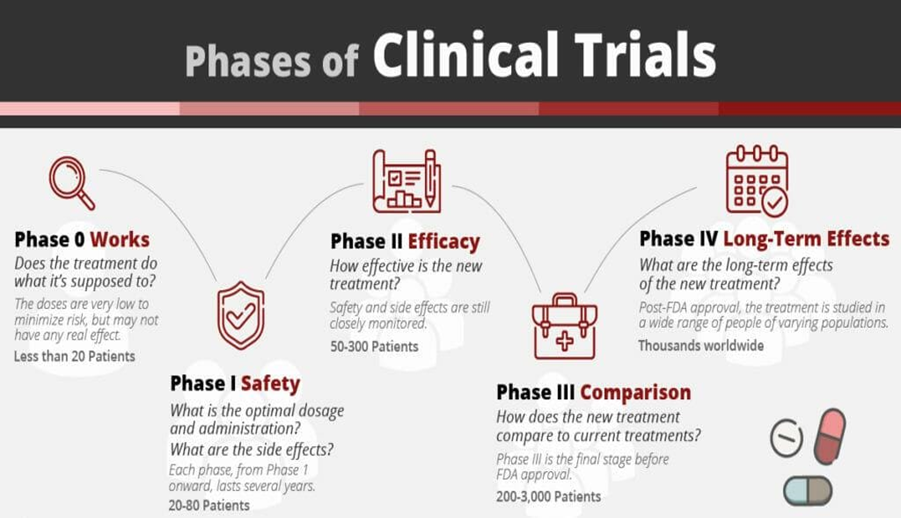
Figure 1: Phases of Clinical Trial
CLINICAL RESEARCH DESIGN
3.1 Definition: (7)
- The term Clinical Research design is a systematic approach for conducting studies in healthcare, concentrating on the methodology used to evaluate the effectiveness, safety, and outcomes of interventions.
- This kind of study concentrates on methods, drugs, goods and treatments with practical uses.
3.2 Objectives:
- To design a study that can yields valid and meaningful scientific conclusions using suitable statistical methods.
- To enhance the patient healthcare
- To identify the factors related to the disease
- To evaluate the safety and effectiveness of an investigational drug, procedure or device.
- To address key questions that enhances scientific understanding of clinical practices.
3.3 Scope:
- The scope of the research design in clinical studies involves developing the methodology and structure for studying medical treatments, interventions or health outcomes in human subjects.
3.4 Steps Involved in Clinical Research Design:
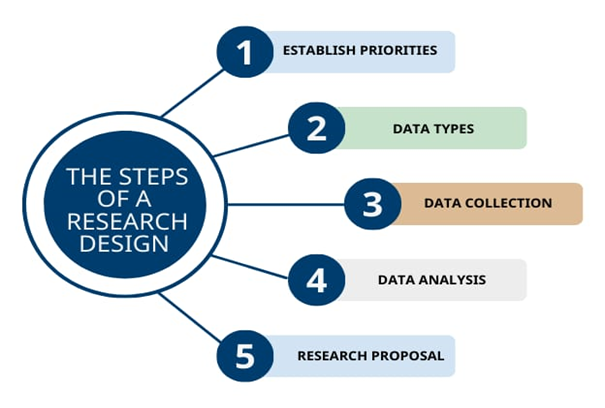
Figure 2: Steps involved in Clinical Research Design
Types Of Clinical Research Design:
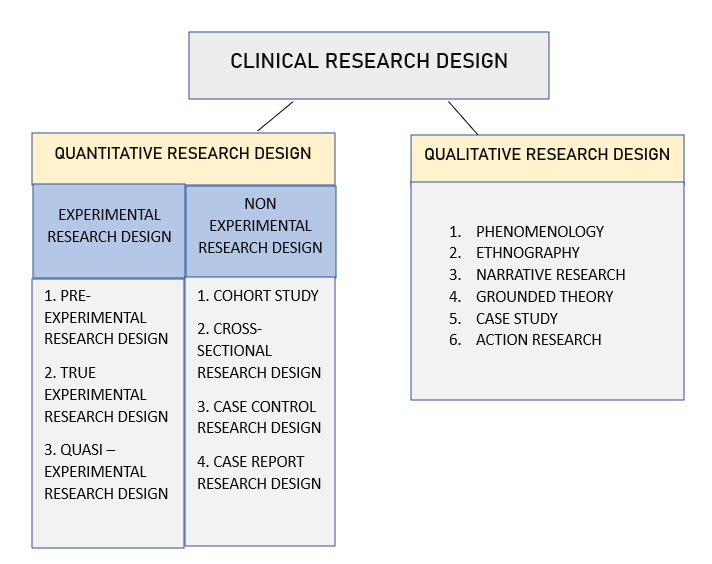
Figure 3: Types of Clinical Research Design
Quantitative Research Design
- Definition:
Quantitative research is defined as a systematic investigation of phenomena through the collection of numerical data and the application of statistical or mathematical techniques. It is grounded in positive paradigm, which supports methodologies that include statistical analysis, hypothesis testing, and various experimental designs, such as randomization, blinding, and structured questionnaires that offer a limited set of predetermined responses. (8)
4.2 Characteristics of Quantitative Research:
- It focuses on gathering and analysing numerical data to characterize, clarify, forecast, or manage variables and phenomena of interest.
- The findings are derived from larger sample sizes that accurately reflect the population.
- The study typically allows for replication or repetition due to its high reliability.
- It aims to illustrate existing situations and determine relationships between variables.
- Data is commonly collected through structured research tools.
- Advantages:
- Quantitative research enables the researcher to quantify and evaluate data effectively.
- It provides an in-depth examination of the relationship between independent and dependent variables.
- This objectivity is beneficial as it helps the researcher maintain impartiality regarding the research outcomes.
- Due to its statistical capabilities, quantitative research is suitable for testing hypotheses in experimental settings.
4.4 Disadvantages:
- One of the primary drawbacks of quantitative research is that it overlooks the context of the study or experiment.
- This type of research does not examine phenomena in their natural environments.
- It can be time-consuming.
- Additionally, a significant sample of the population must be analysed, which can be challenging.
5. Experimental Research Design
5.1 Definition:
Experimental research involves the formulation of a hypothesis and the identification of measurable variables within a controlled setting. Its main objective is to investigate the correlation and relationship between dependent and independent variables. The data collected in experimental studies must be quantifiable or measurable. This type of research is often referred to as intervention research or group comparison research. It is a quantitative research method used to evaluate the impact of specific activities or materials on participant outcomes. The researcher assesses this effect by applying a particular intervention to one group while withholding it from another group. (9)
5.2 Characteristics of Experimental Research Design:
- The researcher actively manipulates the independent variable.
- They determine both the type and magnitude of the treatment.
- Following the administration of the treatment, researchers observe or assess the groups receiving the treatments to identify any differences.
- Experimental research allows researchers to extend beyond mere description and prediction, seeking to identify the causes of observed effects.
Types of Experimental Research Design:

Figure 4: Types of Experimental Research Design
5.3.1 Pre-experimental research design:
Pre-experimental research design is the simplest form of experimental research design in statistics. This method involves identifying specific elements as causes and effects, followed by monitoring one or more groups. It is often employed to assess the need for further research within a target population, making it an efficient approach. In this design, one or more dependent groups are analysed to observe the effects of an independent variable that is presumed to induce change. Being the most fundamental type of experimental study design, it lacks a control group. (10)
Types of pre-experimental research design
- One shot case study research design
- One Group Pre-test – Post-test research design
- Static group comparison
5.3.1.1 One shot case study research design
A single group is examined after undergoing an intervention or treatment that is expected to produce change. This design does not include a pre-test or a control group for comparison. Consequently, it is challenging for the researcher to assess the impact of the intervention due to the absence of a control group and pre-testing of the variables.

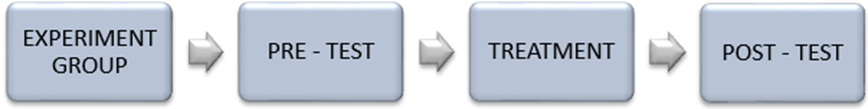
5.3.1.2 One group pre-test-post-test research design
This is the most basic form of pre-experimental research. Participants are non-randomly selected and first take a pre-test, followed by the administration of a treatment or intervention, after which a post-test is conducted. In this design, there is no control group for comparison, and it is typically conducted with an intact group.
5.3.1.3 Static group comparison
In this design, one group that has undergone a treatment or intervention is compared to another group that has not received the intervention. Participants are selected through a non-randomized method, typically involving pre-existing groups. A pre-observation or pre-test is conducted prior to the intervention.


Figure 7: Static group comparison
True experimental research design
True experimental research designs are inherently prospective, allowing for a strong argument in favour of a cause-and-effect relationship. These designs are the most effective way to demonstrate the efficacy of a new intervention or treatment. For instance, the Food and Drug Administration (FDA) requires robust evidence of efficacy from a true experimental study, such as a prospective, randomized, controlled, blinded clinical trial, before approving a new pharmaceutical product. However, these studies often demand significant investments of time, cost, and other resources. (11)
Types of True experimental research design:
- Post-test only design
- Pre-test - post-test design
- Solomon four group design
5.3.2.1 Post-test only design
This design involves two groups that are randomly assigned: an experimental group and a control group. Neither group undergoes pre-testing prior to the treatment. The treatment is administered to the experimental group, and a post-test is conducted for both groups to evaluate the effects of the treatment or manipulation. This design is often used when pre-testing the subjects is not feasible.
5.3.2.2 Pre-test-post-test design
In this design, subjects are randomly assigned to either the experimental group or the control group. Both groups undergo pre-testing for the independent variable. The experimental group then receives the treatment, and both groups are post-tested to evaluate the impact of manipulating the independent variable on the dependent variable.
5.3.2.3 Solomon four group design
The Solomon four-group design involves randomly assigning subjects into four distinct groups, comprising two experimental groups and two control groups. In this setup, only two of the groups undergo a pre-test. One of the pretested groups, along with the unpretested group, receives the treatment. All four groups participate in a post-test. The differences in the dependent variable observed initially are then analysed to assess the impact of the independent variable on the dependent variable based on the post-test outcomes. This approach effectively integrates elements from earlier designs and helps mitigate potential sources of error.
???????Quasi-experimental design
The term "quasi" means "resembling”, so quasi-experimental research refers to studies that have characteristics similar to experimental research but do not meet the criteria for true experiments. In this type of research, the independent variable is manipulated; however, participants are not randomly assigned to different conditions or the order in which they experience those conditions. This manipulation occurs before measuring the dependent variable, which helps resolve the directionality issue. Nevertheless, because there is no random assignment, other differences between the groups may persist, leaving the potential for confounding variables. As a result, quasi-experimental designs generally have an internal validity that is intermediate between correlational studies and true experiments. (12)
Types of Quasi-experimental design
There are two types:
- Non-Randomized Control Group Design.
- Time Series Design.
-
-
- Non-Randomized Control Group Design
- This design, often referred to as the "non-equivalent control group design," closely resembles the pre-test-post-test group design, with the key difference being the absence of random assignment of participants into experimental and control groups.
In this approach, dependent variables are measured in both the experimental and control groups prior to the intervention. Afterward, the experimental group receives the treatment, and post-test observations of the dependent variable are conducted for both groups to evaluate the impact of the intervention on the experimental group.


Figure 8: Non randomized control group design
Time Series Design
Time series design is particularly beneficial when a researcher aims to assess the effects of a treatment over an extended period. The researcher continues to provide the treatment and measures its effects multiple times throughout the experiment. In single-subject research, where the focus is on one individual or a small group, the researcher alternates between administering and withdrawing the treatment to evaluate the intervention's effectiveness.

Figure 9: Time series design
6. Non Experimental Research Design
6.1 Definition:
- Non-experimental research design is a broad category of research designs where the researcher observes phenomena in their natural occurrence without introducing external variables. In this approach, variables are not intentionally manipulated, and the environment is not controlled. (2)
- It is a research design where both the setting and the variables are specifically modified.
6.2 Characteristics of Non-Experimental Research Design:
- Non experimental research design does not involve the manipulation of variables.
- This method is used when experimentation is not possible because of ethical or practical reasons.
6.3 Steps involved in Non-Experimental Research Design: (2)
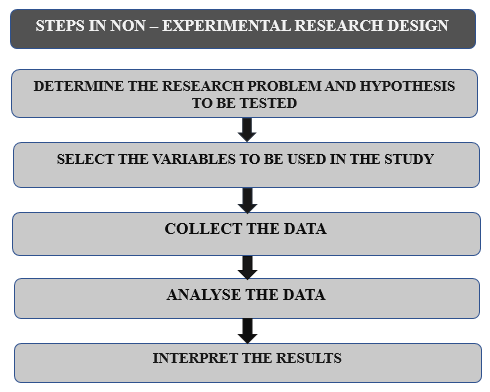
Figure 10: Steps involved in Non-Experimental Research Design
6.4 Advantages:
- It is easily adaptable while conducting research.
- The phenomenon's cause is established, and its impact is being studied.
- The study group's attributes can be specified by the researcher.
6.5 Disadvantages:
- The population as a whole is not represented by the groups.
- Biases in the research can emerge from practice errors.
6.6 Types of Non-Experimental Research Design:
- Cohort study
- Cross sectional research design
- Case control research design
- Case report research design
6.6.1. Cohort Study:
- "Cohort" is derived from the Latin word "cohors”, which denotes a group of soldiers within a legion.
- Cohort studies are observational analytical research studies which observe subject groups (cohorts) as they progress from an exposure to an outcome. (13)
- Types Of Cohort Study: (14)
There are two types of cohort study,
- Prospective cohort study - also called “Follow up” or “Longitudinal study”
- Retrospective cohort study – also called “Historic cohort study”
6.6.2. Cross-Sectional Research Design: (15)
- A cross-sectional study is an observational study design that examines data from a population at a single point in time.
- There is no prospective or retrospective follow-up. After selecting the subjects, the investigators will gather the data and evaluate the associations between exposures and outcomes.
- Cross sectional studies can be used for both analytical and descriptive purposes:
- An analytical research looks for explanations for the reasons why a particular result could exist.
- A Descriptive study uses descriptive statistics only for summarize the said outcomes.
Purpose:
- To understand the prevalence of a disease within a population.
- The aim of the research is to determine the occurrence of the expected outcome for the entire population or particular subgroups at a particular instant in time.
- Sometimes, cross-sectional studies are conducted to look at relationships between risk factors and the desired outcome.
- The study's objective is descriptive, and it often has the outline of a survey.
Strengths:
- Quick and cost-effective to carry out
- Free from ethical concerns
- Data on all variables gathered at a single time point
- Enables study of multiple outcomes and exposures
- Useful for generating hypotheses
- Findings often serve as a basis for more detailed research
Weakness:
- Incidence cannot be measured.
- Making causal inferences is challenging.
- Identified associations may be hard to interpret.
- Temporal relationships between outcomes and risk factors cannot be investigated.
- Not suitable for studying rare diseases.
- Vulnerable to biases, including nonresponse bias and recall bias.
6.6.3 Case Control Research Design: (16, 17)
- Case control study is the simplest analytical study design, involving a comparison between a group of diseased patients (cases) and a similar group of individuals who are not diseased (controls).
- Case-control studies are especially suitable for investigating disease outbreaks, rare diseases, or specific outcomes of interest.
Criteria:
Data on exposure (for e.g., to chemicals or pesticides) is expensive or hard for others to come across.
The disease (like AIDS in the 1980s) is uncommon, has a prolonged incubation period, or is not extensively studied.
Seeking out to the population to ask follow-up questions is challenging.
Strengths:
- Require less time and less expensive
- Require smaller sample sizes
- Can evaluate multiple exposures
- Useful for studying rare diseases or outcomes
Weakness:
- Cannot determine incidence or prevalence
- Cannot establish causality
- Not useful for studying rare exposures
- Prone to recall bias and selection bias
6.6.4. Case Reports Research Design:
- A case study, sometimes referred to as a case report, is a thorough examination of one person or one particular group.
- Because of their educational usefulness and historical origins, case reports continue to be widely used in medical literature.
- Case reports come in three primary forms:
1. Highly unique cases: These involve illnesses or syndromes that have never been reported before.
2. Unexpected associations: These cases demonstrate an unexpected connection between illnesses or their symptoms, which may point to a novel cause-and-effect relationship.
3. Unexpected outcomes: These draw attention to unexpected new positive or negative treatment effects.
Strengths:
- It gives extremely comprehensive details.
- It enables thorough analysis of conditions that would be impossible or unethical to carry out using a different study design.
Weakness:
- Bias among the researchers can be present.
- It is challenging to replicate.
7. QUALITATIVE RESEARCH DESIGN
-
- Definition:
- Qualitative Research design is defined as the form of research methodology that focuses on investigating and understanding complicated events and the interpretations that people or groups make of them. This type of study is particularly used when studying social and cultural phenomena. (3)
- Key Components:
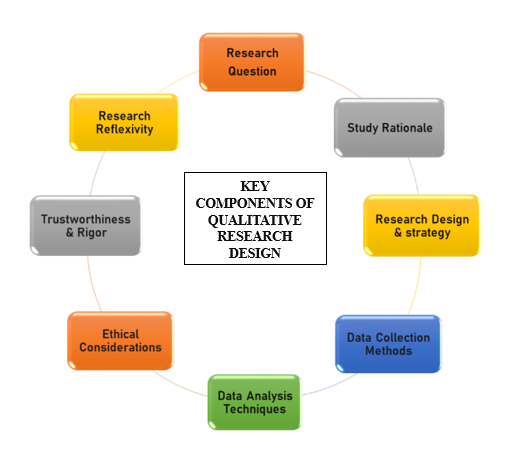
Figure 11: Components of QRD
Steps to Conduct a Qualitative Study:
- Identifying a research problem or stating the problem.
- Reviewing the literature.
- Specifying a purpose and research questions.
- Collecting the data.
- Analysing the data
- Determining the quality of data
- Reporting the research
7.4 Data Collection Methods: (18)
- Observation: Recording your observations, conversations and experiences in detailed field notes.
- Interviews: Directly questioning individuals in one-on-one discussions.
- Focus groups: Raising questions and encouraging discussion among group members.
- Surveys: Distributing questionnaires with open ended questions.
- Secondary research: Collecting existing data in the form of texts, images, audio or video recordings.
7.5 Types of Qualitative Research Design:

Figure 12: Types of QRD
Phenomenology:
- The phenomenological method is a qualitative research technique that seeks to understand the individuals’ perceptions, perspectives, and understandings of specific phenomena.
- It is related to specific procedures that are then used to individual situations or the precisely selected samples. Such particular investigations make it simpler to recognize problems that exhibit inconsistencies, mistakes, positive conclusions, and attention to specific circumstances.
- Additionally, this research methodology is important for contributing out the existence of factors and their consequences in various case contexts, but it only applies when the experimental approach has been used to estimate the size of the population that the participants or examples were taken from. (19)
7.5.2 Ethnography (20)
- Ethnographic research is a qualitative research method also referred to as cultural anthropology or naturalistic inquiry.
- It focuses on discovering and describing the culture of a particular group or community.
- Ethnography in qualitative research is also called thick description as it involves an up-close observation of the participants and a detailed description of their cultures, behaviours, mutual differences and practices.
7.5.3 Narrative Research (21)
- In this method researcher seeks to uncover about experiences of an individuals’ lives, expressed in their own words and contexts.
- It usually focuses on studying an individual person.
- It serves as both a method for data collection and an interpretive or analytical framework.
7.5.4 Grounded Theory (22)
- Grounded theory is a qualitative research methodology that aims to discover or develop theories from data that is systematically gathered and analysed through comparative analysis.
- Although the grounded theory is inherently inflexible, it is also a complex methodology.
- Researchers collect and analyse data to identify patterns, concepts and relationships, which then form basis for developing new theories.
7.5.5 Case Study (23)
- A case study is an extensive, in-depth examination of how one particular event, person, or condition evolved over time in real-life circumstances.
- Case studies are frequently used to examine and expose nuanced social and medical concerns.
- One of the earliest forms of research to be used globally among qualitative approaches was case studies.
- It primarily focuses on the experience of a single person, a family, a group, a community or an organisation.
-
- Action Research:
- Action research is a process which includes a strong emphasis on working together to find complications create solutions, and carry out advancements with participants.
- In contrast to traditional techniques, which prioritise information development, action research places an additional preference on practical solutions and practice improvement.
- It is also called as “cycle of action” or a “cycle of inquiry” as each step involves collaboration between the study participants and the researcher.
CONCLUSION
The Choice of Clinical research design is crucial for addressing specific research questions and achieving reliable results. Experimental research design offers high levels of evidence by minimising bias and allowing for causal inferences. Non Experimental research design provides valuable insights into associations and real world outcomes but may be limited by potential confounding variables. Qualitative research design helps to demonstrate the flexibility, depth in exploring and interpreting human experiences and social processes. Each design type is essential to select the appropriate approach based on the research objectives, available resources and the nature of the clinical question. Careful design and methodological rigor are the keys to producing robust and generalizable findings that can advance medical knowledge and improve patient care.
REFERENCES
-
-
-
- Venkataramana Kandi, Sabitha Vadakedath, “Clinical Research: An Overview of Study Types, Designs, and Their Implications in the Public Health Perspective”. Volume 9, Issue 2, American Journal of Clinical Medicine Research, 2021
- Dr. G. Radhakrishnan, Non-Experimental Research Designs: Amenable to Nursing Contexts, vol. 3 issue 1, Karnataka, Asian Journal of Nursing Education and Research, 2013.
- Ugwu, Chinyere N, EzeVal, Qualitative Research, Volume 8, issue 1, IDOSR (International Digital Organisation for Scientific Research) Journal of Computer and Applied Sciences, 2023.
- Venkataramana Kandi, Sabitha Vadakedath, Clinical Trials and Clinical research; A Comprehensive Review, Volume 15, issue 2, PubMed, 2023.
- Clinical trial Wikipedia, the free encyclopedia, Available from: URL http://en.wikipedia.org/wiki/clinical_trial , 2008.
- Kulkarni S. K., Handbook of Experimental Pharmacology, 3rd edition, New Delhi, Vallabh Prakashan, 2004.
- Hulley, S.B., Cummings, S.R.,Browner W.S., Grady D.G., & Newman, T.B, Designing Clinical Research,4th ed., Lippincott Williams & Wilkins, 2016.
- Neuman W. L., Social Research Methods: Qualitative and Quantitative Approaches, Pearson New International Edition. Pearson Education Limited, 2014.
- Anahita ghanad, An Overview of Quantitative Research Methods, volume 6, issue 8, International Journal of Multidisciplinary Research and Analysis, August 2023.
- Ahsanul Mahhub Zubair, Experimental Research Design – Types & process, Research gate, 2023.
- Cheryl Bagley Thompson, RN, Edward A. Panacek, Research Study Designs: Experimental and Quasi-Experimental, volume 25, issue 6, Air Medical Journal, 2006.
- Cook, T.D, Campbell, D.T, Quasi-experimentation: Design & analysis issues in field settings, 1979.
- Brenda Morrow, An Overview of Cohort Study Designs and their Advantages and Disadvantages, volume 17, issue 10, International Journal of Therapy and Rehabilitation, October 2010.
- Patrida Healy, Declan Devane, Methodological considerations in cohort study designs, volume 8, issue 3, RCN Publishing/Nurse Researcher, April 2011.
- Xiaofeng Wang, Zhenshun Cheng, Cross sectional Studies – Strengths, Weaknesses, and Recommendations, volume 158, Chest journal, 2020.
- Aamir omair, selecting the appropriate study design: Case-Control and Cohort study designs, volume 4, issue 1, The Journal of Health Specialities, 2016.
- Tanujit Dey, Anish Mukherjee, Sounak Chakraborty, A practical Overview of Case Control Studies in Clinical Practice, volume 158, 2020.
- Elise paradis, Bridget O’Brien, Laura Nimmon, Design: Selection of Data Collection Methods, volume 8, issue 2, Journal of Graduate Medical Education, May 2016.
- Neville Greening, Phenomenological Research Methodology, Scientific Research Journal, volume 7, issue 5, May 2019.
- Hemanth Lata sharma, Chiranjit sarkar, Ethnographic research: an overview, Research gate, volume 6, issue 2, June 2019.
- Kayi Ntinda, Narrative Research, Researchgate, July 2020.
- Ylona Chun Tie, Melanie Birks, Karen Francis, Grounded Theory Research: A Design framework for novice researchers, volume 7, SAGE Open Med, January 2002.
- Josephine Oranga, Audrey Matere, Qualitative Research: Essence, Types, and Advantages, volume 10, Open Access Library Journal, 2023


 Jesima Begum A*
Jesima Begum A*













 10.5281/zenodo.14294932
10.5281/zenodo.14294932