Abstract
Microspheres are multiparticulate drug delivery systems which are prepared to obtain prolonged the controlled drug delivery to improve bioavailability, stability and to target the drug to specific site at a predetermined rate. They are made from polymeric waxy or other protective materials such as natural, semi synthetic and synthetic polymers. Microspheres are characteristically free flowing powders having particle size ranging from 1-1000 ?m consisting of proteins or synthetic polymers. The range of techniques for the preparation of microspheres provides multiple options to control as drug administration aspects and to enhance the therapeutic efficacy of a given the drug. These delivery systems offer numerous advantages compared to conventional dosage forms, which include improved efficacy, reduced toxicity, improved patient compliance and convenience. Such systems often use macromolecules as carriers for the drugs. The present review highlights various types of microspheres, different methods of preparation, its applications and also various parameters to evaluate their efficiency. Microspheres are various types like Bioadhesive microspheres, Magnetic microspheres, Floating microspheres, Radioactive microspheres, Polymeric microspheres, Biodegradable polymeric microspheres, Synthetic polymeric microspheres and are prepared by methods like Spray Drying, Solvent Evaporation, Single emulsion technique, Double emulsion technique, Phase separation coacervation technique, Spray drying and spray congealing, Solvent extraction, Quassi emulsion solvent diffusion. Microspheres have wide range of applications because of controlled and sustained release.
Keywords
Microspheres, Types of Microspheres, Materials used in Microspheres, Mechanism of Microspheres, Methods of preparation and Applications.
Introduction
A medicinal substance is delivered to the target site in a well-controlled and sustained model by a novel drug delivery system. Micro spheres, also known as micro particles, are free, spherical particles made of a drug and polymer matrix. They are made up of synthetic polymers or proteins that are biodegradable and have a particle size of less than 200 µm. Micro spheres may be referred to as tiny, spherical particles that have dimensions between 1 and 1000 micrometers. Another name for micro spheres is micro particles. The application of micro spheres in drug transport has been thoroughly investigated, and different polymers have been used to create the micro spheres, which have then been evaluated for unique objectives. Because a constant plasma concentration is maintained, the entire dosage and a small number of side effects can eventually be reduced. There are two kinds of micro spheres:
Micro capsules
Micro matrices
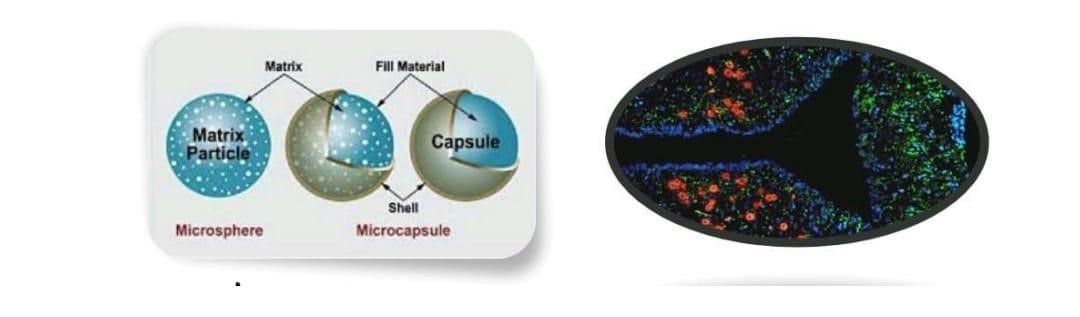
Figure 1; Micro capsules Micro matrices
Microcapsules have a distinct capsule wall enclosing the entrapped material, while micromatrices have the entrapped material scattered. (1)
The Ideal Properties of Microspheres:
1) The drug can be administered at higher concentrations to act as a depot;
2)The release rate can be controlled predetermined amount of time through out the
microspheres matrix.
3)Not harmful.
4) A fair amount of stability.
5)The capacity to bio reabsorb.
6) Boost the effectiveness of treatment.
7)Command over the release of content.(2)
8) The preparation's stability following synthesis, with a shelf life that is clinically
acceptable.
9)Controllable biodegradability and biocompatibility.
10)Managed medication dispersion and particle size in aqueous solvent for parenteral administration.
11)Dispersibility or water solubility.
12) Preserve the medication. (3)
Advantages of Microspheres:
1) The potency of the poorly soluble substance is boosted when the microsphere's size decreases because it increases its surface area.
2)Dose frequency and adverse effects can be reduced.
3)Increased patient compliance.
4)Drug package with polymer prevent drug from enzymatic cleavage therefore the drug can be protected from various enzymes.
5)Enhances bioavailability.
6)Gastric irritation can be reduced.
7)Biological half-life can be enhanced.
8)First pass metabolism can be reduced.
9)Unpleasant odour and taste of the drug can be masked. (4)
Disadvantages of Microspheres:
1)Reproducibility is less.
2)The cost of materials and processing is high compared to conventional preparations. 3)Change in process variables such as change in temperature, pH, solvent addition an 4)Evaporation/agitation may influence the stability of core particles. 5)The fate of polymer matrix and add additives (5)
Microsphere types include:
1.Bioadhesive microspheres
2.Magnetic microspheres
3.Floating microspheres
4.Radioactive microspheres
5.Polymeric microspheres
Bioadhesive microspheres
Adhesion is the process by which a drug sticks to a membrane by using the adhesive qualities of water-soluble polymers. The attachment of a drug delivery system to a mucosal membrane, such as the nasal, rectal, ocular, or buccal mucosa, is known as bioadhesion. These microspheres have a greater therapeutic impact and tighter interaction with the absorption site because they stay at the application site for a longer period of time. (6)
Figure 2: Bioadhesive Microspheres
Magnetic microspheres:
The medicine is localized to the target spot using this kind of delivery method. This kind of delivery system involves injecting a medication or therapeutic radioisotope that is attached to a magnetic component into the bloodstream, which is subsequently stopped by a strong magnetic field at the target site or ailment. Molecular particles known as magnetic microspheres are small enough to pass through capillaries without obstructing the esophagus (less than 4 ?m), but they are ferromagnetic, meaning they are very susceptible to being caught in micro vessels and pulled through nearby tissues by a magnetic field. Here, a smaller quantity of a magnetically targeted medicine can take the place of a greater quantity of a drug that is currently in circulation. Magnetic microspheres come in several varieties They're Anticancer drugs are directed to liver tumors using therapeutic microspheres. This aids in eradiating tumor cells without endangering neighboring cell. diagnostic microspheres by creating supra magnetic iron oxide nanoparticles, they can be utilized to image liver metastases and differentiate bowel loops from other abdominal structures. (7)

Figure 3: Magnetic Microspheres
Floating microspheres:
These types stay buoyant in the stomach without influencing the rate of gastric emptying because their bulk density is lower than that of the gastric fluid. If the system is floating on stomach content, increasing gastric residency and increasing variability in plasma concentration, the medicine is released gradually at the desired rate. Additionally, it lessens the likelihood of dosage dumping and striking. It also lowers dosing frequencies by producing a longer-lasting therapeutic impact. This form is used to administer the drug ketoprofen. (8)
Radioactive microspheres:
10–30 nm tiny, capillary-sized microspheres used in radio emobilization therapy are tapped in the first capillary bed they encounter. The arteries leading to the tumor of interest receive an injection of them. Therefore, under all of these circumstances, radioactive microspheres provide large doses of radiation to the targeted regions without causing harm to the surrounding normal tissues (9). It is not the same as a medicine delivery system because radioactivity is not emitted from microspheres but rather acts from within a normal distance of radioisotopes. There are three types of radioactive microspheres: a, ß, and y emitters. (10)

Figure 4: Radioactive Microspheres
Polymeric microspheres:
There are two varieties of polymeric microspheres: synthetic and
biodegradable.
a)Biodegradable polymeric microspheres: Due to their biodegradability, biocompatibility, and biostickiness, natural polymers such as starch are utilized. When biodegradable polymers come into touch with mucous membranes, their high degree of swelling property with an aqueous medium prolongs the residence period, which leads to gel formation. rate and amount of medication release are controlled by the polymer concentration and the release pattern through out time,The primary drawback is that biodegradable microspheres' drug loading efficiency in clinical settings is complicated, making it challenging to regulate drug release. Nonetheless, they have a wide range of uses in treatment based on microspheres. (11)
b) Synthetic polymeric microspheres: These are of relevance since they are frequently employed in clinical settings. Additionally, it has been shown to be safe and biocompatible when utilized as bulking agents, fillers, embolic particles, drug delivery vehicles, etc. However, these microspheres have the drawback of having a propensity to move away from the injection site, which increases the chance of an embolism and additional organ damage. (12)
Materials used in the microspheres
In the formulation of microsphere mainly used polymers, classifoed as
Follows
Synthetic polymers
Natural polymers
Synthetic polymer;
Synthetic polymers are divided into two types:
a) Non-biodegradable polymer: Example- Poly methyl methacrylate(PMMA), Acrolein,Glycidyl methacrylate, Epoxy polymers. b) Biodegradable polymers-
Example- Lactides, Glycolides and their co polymers, Poly alkyl cyano acrylates, Poly anhydrides.
2) Natural polymers:
They are obtained from different sources like proteins, carbohydrates chemically modified carbohydrates. They are also used a protein like Albumin, Gelatin, and Collagen, Carbohydrates like Agarose, Carrageenan, Chitosan, Starch and also Chemically changed carbohydrates used like Poly dextran, poly starch. (13)
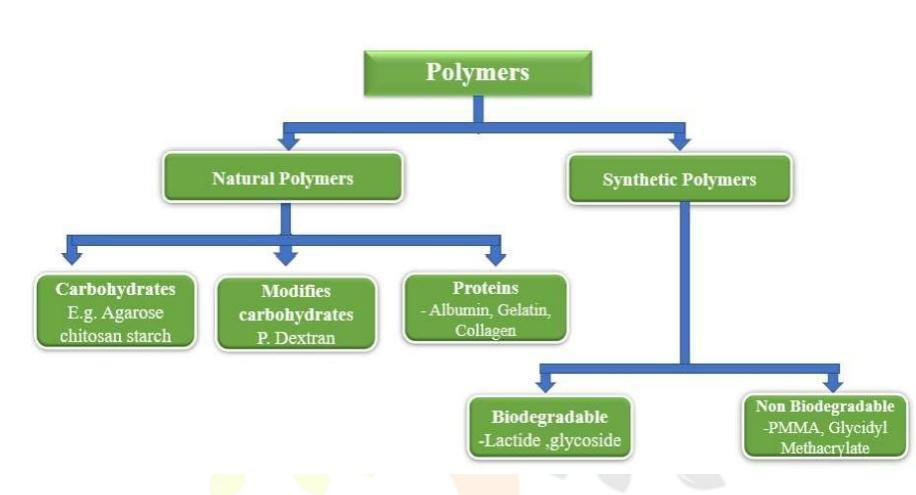
Figure 5: Flow chart of polymers used in Microspheres
Mechanism of microspheres:
The majority of drug delivery via microparticles inhibits the formation of a matrix like internal solid dispersion morphology structure. The drug can be insoluble in the matrix of polymer, and it is released through erosion. First, water diffuses into the matrix, dissolving the resulting near the device’s surface. The resulting osmotic pressure gets alleviated by forming a channel to the surface and releasing a predetermined amount of drug in the initial drug burst.Drug release from the microspheres occurs by a general mechanism including
1.Dissolution,
2.Diffusion,
3.Polymer degradation,
4.Hydrolysis/erosion. (14)

Figure 6: Mechanism of Microspheres
Techniques For Microsphere Preparation
1.Solvent evaporation
2.Single emulsion technique
3.Double emulsion technique
4.Phase separation technique
5.Spray drying and spray coacervation congealing
6.Solvent extraction
7.Freeze drying
8.polymerization
9.Quassi emulsion solvent diffusion
1.Solvent evaporation:
The process of solvent evaporation takes place during the vehicle
production phase. First, a volatile solvent that is incompatible with the liquid production vehicle phase is used to distribute the microcapsule coating. A core material to be microencapsulated is dissolved or distributed in a coated polymer solution. Agitation is used to distribute the core material combination throughout the liquid manufacturing vehicle phase in order to produce microcapsules of an acceptable size. The mixture is heated if required in order to evaporate the solvent. Matrix-type microcapsules are created when the core material dissolves in the covering polymer. Either water-soluble or water-insoluble materials could make up the core. The creation of an emulsion between a polymer solution and an immiscible continuous phase, whether aqueous (o/w) or non-aqueous, is known as solvent evaporation. (15)
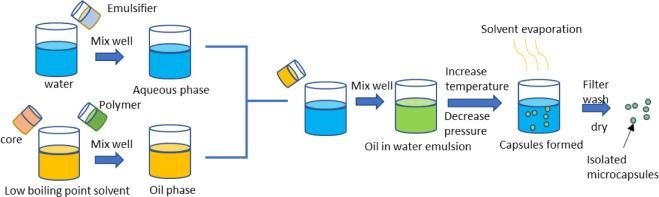
2.Single emulsion technique:
The single emulsion approach can be used to create the microparticulate carriers of natural polymers, such as proteins and carbohydrates. After being dissolved or dispersed in an aqueous media, the natural polymers are then disseminated in a non-aqueous medium, such oil. Either heat or chemical cross linkers can be used to accomplish cross linking in the following phase. Acid chloride, formaldehyde, glutaraldehyde, and other chemicals are utilized as cross-linking agents.
When it comes to thermolabile compounds, heat denaturation is not appropriate. Chemical cross linking suffers the disadvantage of excessive exposure of active ingredients to chemicals if added at the time of preparation.It is then subjected to centrifugation, washing and separation. The nature of the surfactants used to stabilize the emulsion phases can greatly influence the size, size distribution, surface morphology, drug loading, drug release and bio performance of the final multiparticulate product.(16)
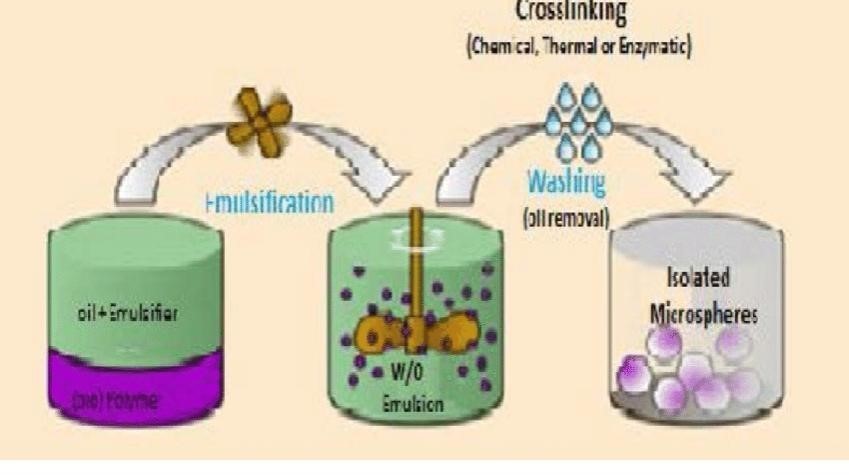

Figure 7: Single Emulsion Method
3.Double emulsion technique
Double emulsion method of microsphere involves the formation of the multiple emulsion or double emulsion of type w/o/w and is best suited for water soluble drugs, peptides, proteins and the vaccines. This method can be used with both the natural as well as synthetic polymers. The aqueous protein solution is dispersed in a lipophilic organic continuous phase. This protein solution may contain the active constituents. The continuous phase generally consists of the polymer solution that eventually encapsulates protein contained in dispersed aqueous phase. The primary emulsion is then subjected to the sonication before addition to the aqueous solution of the polyvinyl alcohol (PVA).The emulsion is then subjected to a solvent removal either by solvent evaporation or by solvent extraction. A number of hydrophilic drug like luteinizing hormone (LH-RH) agonist, vaccines, proteins/peptides and conventional molecules are successfully incorporated into the microspheres using method of double emulsion solvent evaporation/extraction. (17)
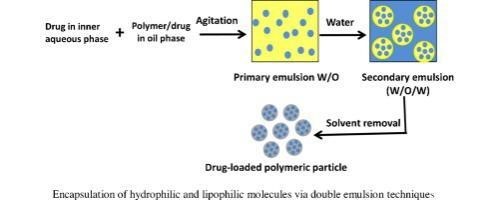
Figure 8: Double emulsion technique
4.Coacervation Phase Separation:
Microencapsulation by coacervation phase Separation process is divided in to steps under continuous agitation.
1.Formation of thee immiscible phases; a liquid manufacturing phase, a core material phase and a coating material phase. Core material is dispersed in solution of coating 3 solution (liquid manufacturing vehicle phase) for the formation of three phases. The coating material phase, an immiscible polymer in liquid state is formed by utilizing one of the methods of phase separation coacervation i.e. by changing temperature of polymer; or by adding salt, nonsolvent, or incompatible polymer to the polymer solution or by inducing polymer-polymer interaction.
2.Deposition of the liquid polymer coating on the core material by controlled, physical mixing of coating material and core material in manufacturing vehicle. Deposition of the liquid polymer coating around the core material occurs if polymer is adsorbed (which is prerequisite to effective coating) at the interface formed between the core material and the liquid vehicle phase. The continue deposition cause reduction in total free interfacial energy of the system brought about by decrease of coating material surface area during coalescence of the liquid polymer droplets.
3.Rigidizing the coating usually by thermal, cross linking or dissolution techniques to form a microcapsule.Equipment required for microencapsulation this method is relatively simple; it consists mainly of jacketed tanks with variable speed agitators. (18)
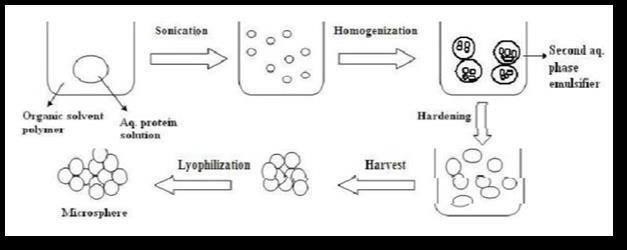
Figure 9: Coacervation phase seperation
5) Spray Drying and Congealing
Because of certain similarities of these two processes, they are discussed together. Both involve dispersing the core material in a liquefied coating substance and spraying or introducing the core-coating mixture into some environmental condition, whereby rapid formation and solidification of coating is effected. The principal difference between the this two methods are,Coating solidification in case of spray drying is effected by rapid evaporation of a solvent in which the coating material dissolved. Coating solidification in spray congealing methods however, is accomplished by thermally congealing a molten coating material or by solidifying a dissolved coating by introducing the coating core material mixture into non- solvent. Microencapsulation by spray drying is conducted by dispersing a core material in coating solution, in which the coating substance is dissolved and in which the core material is insoluble and then atomizing the mixture into an air stream. The latent heat of vaporization of heated air required to remove the solvent from the coating material, thus forming the microencapsulated product. The equipment used for spray drying include air heater, atomizer, main spray chamber, blower or fan, cyclone and product collector. The process variables include feed material properties such as viscosity, uniformity, and concentration of core and coating material, feed rate, method of atomization, and drying rate. The process produces microencapsules approaching a spherical structure in the range of 5 to 600 microns. Spray drying yields products of low density, owing to the porous nature of coated particles. Low active contents are required to provide the necessary protection except for volatile and liquid core materials. Microencapsulation by spray congealing can be accomplished with spray drying equipment, when the protective coating is applied as a melt. The process variables are same as that of spray drying, except core material is dispersed in a coating material melt rather than coating solution.(19)
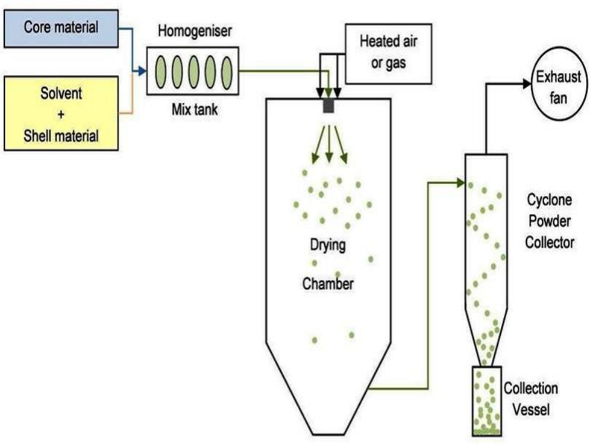
Figure 9: Spray drying and congealing
6)Solvent extraction:
A lot of PLA and PLGA microspheres containing different medications have been made using solvent evaporation. Numerous factors, including drug solubility, internal morphology, solvent type, diffusion rate, temperature, polymer composition, viscosity, and drug loading, have been found to have a substantial impact on microspheres properties. This process is especially useful with medications that are either insoluble or partially soluble in the liquid media because its effectiveness depends on the active ingredient being successfully trapped inside the particles. (20)
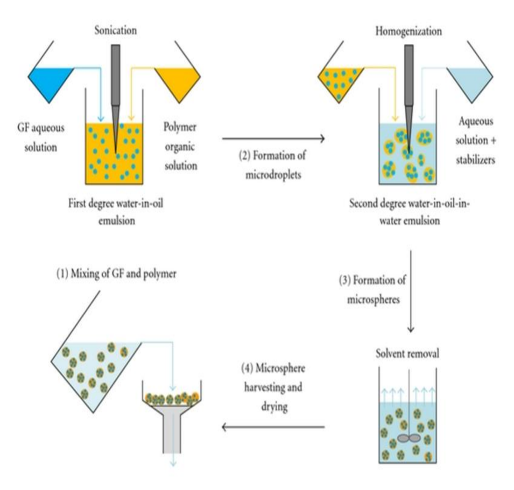
Figure 10: Solvent evaporation
7)Freeze-drying:
It is effectively used in protein API microspheres preparation. The method is freezing, sublimation, main drying, and secondary drying. At the freezing step, account is taken of the eutectic point of the components. During the process, lyoprotectants or cryoprotectants will stabilise API molecules by removing water, creating a glass matrix, lowering intermolecular interaction by forming hydrogen bonds between the molecules or dipole - dipole interactions. It's a beneficial cycle for heat tolerant molecules, given its high expense. Freeze-drying produces solidification and then enables the reconstitution of particles in an aqueous media. (21)
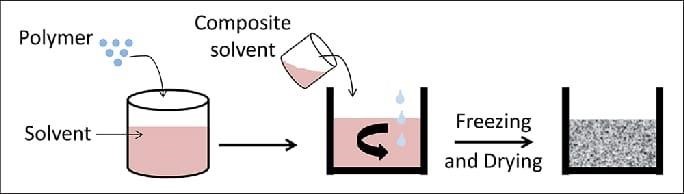
Figure 11: Freeze drying
8) Polymerization: The polymerization techniques conventionally used for the preparation of the microspheres are normally Classified as:
1.Normal polymerization
2.Interfacialpolymerization
Normal polymerization:
The two processes are carried out in a liquid phase.Normal polymerization proceeds and carried out Using different techniques as bulk, suspension, Precipitation, emulsion and miceller polymerization Process. The bulk polymerization, a monomer or a Mixture of monomer along with the initiator is usually heated to initiate polymerization and carry out the process. The catalyst or the initiator is added to the reaction mixture to facilitate or accelerate the rate of the reaction. The polymer so obtained may be moulded or fragmented as microspheres. For loading of drug, absorptive drug loading or adding drug during the process of polymerization may be opted.
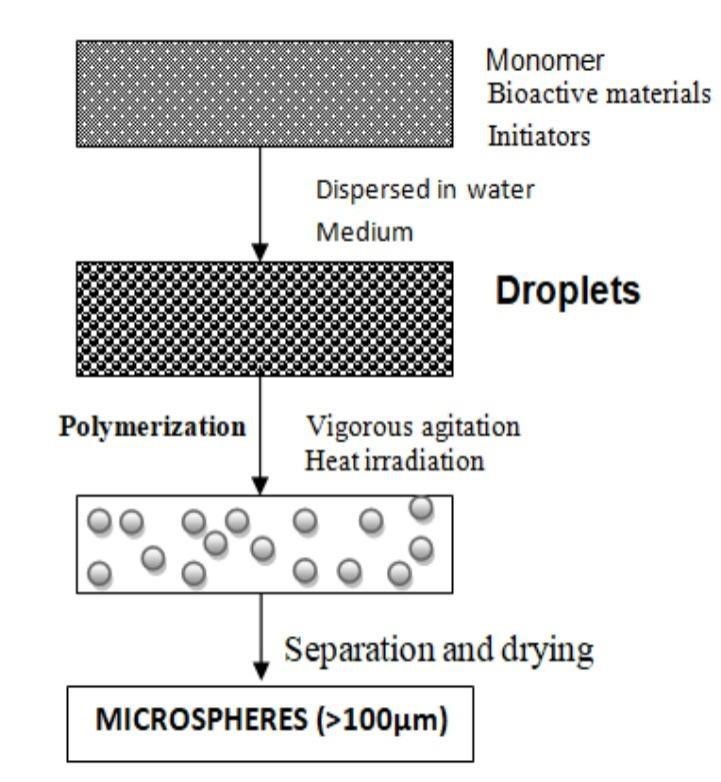
Figure 12: suspension polymerization
suspension polymerization which is also referred as the bead or pearl polymerization is carried out by heating the monomer or mixture of monomer with active principle as droplets dispersion in a continuous aqueous phase. The droplets may also contain an initiator and other additives. The emulsion Polymerization however differs from the suspension polymerization as due to presence of initiator in the aqeous phase, which later on diffuses to the surface of the micelles or the emulsion globules. The bulk polymerization has an advantage of formation of the pure polymer, but it also suffers a disadvantage, as it is very difficult to dissipate the heat of reaction, which can adversely affect the thermolabile active ingredients. On the other hand suspension and emulsion polymerization can be carried out at lower temperature, since continuous external phase is normally water through which heat can easily dissipate. The two processes also lead to the formation of the higher molecular weight polymer at relatively faster rate.(22) The major disadvantage of suspension and emulsion polymerization is, association of polymer with the unreacted monomer and other additives.
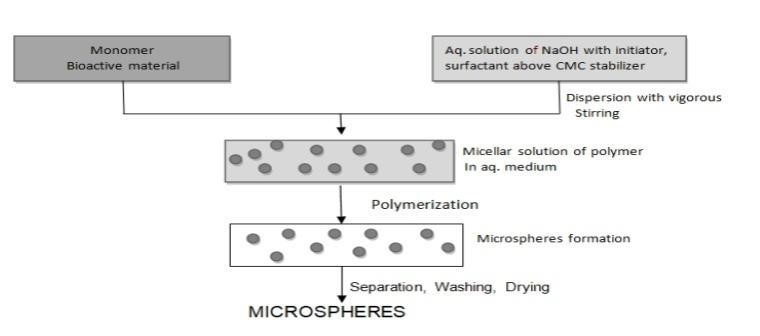
Figure 13: Schematic Representation of Emulsion Polymerization
Interfacial polymerization:
Interfacial polymerization essentially proceeds, involving reaction of various monomers at the interface between the two immiscible liquid phases to form a film of polymer that essentially envelops the dispersed phase. In this technique two reacting monomers are employed; one of which is dissolved in the continuous phase while the other being Dispersed in continuous phase. The monomers Present in either phases diffuse rapidly and polymerize rapidly at interface. Two conditions arise depending upon the solubility of formed polymer in The emulsion droplet. If the polymer is soluble in droplet it will lead to the formation of the monolithic type of carrier on the other hand if the polymer is insoluble in the monomer droplet, the formed carrier is of capsular (reservoir) type. The interfacial Polymerization is not widely used in the preparation of the microparticles because of certain drawbacks, which are associated with the process such as:
1.Toxicity associated with the unreacted monomer.
2.permeability of the film.
High degradation of the drug during
3.Polymerization
4.ragility of microcapsules.
Non-biodegradability of the microparticles. (23)
5.Quassi emulsion solvent diffusion:
Quasi-emulsion solvent diffusion method is used for the manufacturing of the controlled release microspheres of drugs with acrylic polymers. Microsponges can be manufactured by this method by using external phase which contains distilled water and polyvinyl alcohol. Internal phase consists of the drug, ethanol and polymers. Firstly, the internal phase is heated at 60ºC and added to the external phase in the room temperature. The mixture is stirred continuously for 2 hours. Then the mixture can be filtered for separation of the microsponges. (24)
Formulation of Microspheres :
|
S.no
|
Method
|
Drug
|
Polymer
|
Category
|
|
1
|
Cross linking
|
Insulin
|
Chitosan
|
Anti diabetic
|
|
2
|
Cross linking
|
Furosemide
|
Chitosan
|
Diuretic
|
|
3
|
Dry in oil
|
Fluorouracil
|
Glutarldehyde
|
Anti cancer
|
|
4
|
Double Emulsion
|
Gentamycin
|
PLGA
|
Antibiotic
|
Characterization Of Microsphere
- Particle size and shape
The microparticles are visualized by microscopic method using calibrated optical micrometer. The most widely used procedure for microparticular visualisation are standard light microscopy (LM) and Scanning electron microscopy (SEM) (25)
LM provides a control over coating parameters in double SEM Scanning electron microscopy (SEM) study The Samples were analyzed through SEM and it was well qualified from a back scattered electron sensor for image analysis and conducting the x Ray diffraction analysis (EDXA) for elemental structure determination where particular elements have been identified. In this method the sample was scanned in parallel lines using a centered electron beam. Microspheres were then placed on a sample holder for SEM characterization preceded by coating with a conductive metal like platinum or zirconium using a sputter coater. The sample was then scanned with a guided, fine electron beam. The surface properties of the sample were derived from the secondary electrons leaked from the sample surface. (26)
Floating behavior:
The polymer dissolves into solution when floating microspheres are
dispersed in stimulated stomach juice devoid of enzymes, leading to matrix erosion and the formation of pores on the microspheres. This causes the microspheres to float. The percentage of the floating was determined using the following formula. Size and form of molecules: microsphere Using scanning electron microscopy or light microscopy, one can ascertain the dimensions, form, and external structure
Determination of density:
The thickness of the microspheres is measured using a multi-volume pycnometer. The example states that a multi-volume pycnometer in a cup has something set in it. To enable an extension, helium is pumped into the chamber at a steady weight. Because of this progression, the results' weight is lessened within the gathering. When the ratio of two successive weight readings falls, the introduction weight is established. The thickness of the microsphere transporter may be determined by the volume based on two weight readings. (27)
Angle of contact:
The wetting property of a microparticle channel is ascertained using the angle of contact. Microspheres' tendency to be described by the term hydrophobicity is also referred to as hydrophilicity. It is necessary to identify the point of contact between the water, air, and strong contacts. Adding a bead to a roundabout cell that is positioned over the aim of an enhanced magnifying device allows one to discern the progressing and receding point of touch. The contact sites in a microsphere affidavit moment are roughly around 20°C.
Encapsulation Efficiency:
Lysate can determine the microspheres' catch ability or percent capture by allowing them to be washed. The lysate is then subjected to dynamic component assurance, as indicated by the monograph. Encapsulation efficiency is calculated using the following equation:
Actual content
% Entrapment=Theoretical content x 100
Electron spectroscopy for chemical analysis:
Electron spectroscopy for substance investigation (ESCA) is required for the surface science of the microsphere. The nuclear organization of these stocks' surfaces (ESCA) is made possible by the use of electron spectroscopy for compound assessment. The biodegradable microsphere's surface cleanliness is verified using the spectra. ECSA was used to create these spectra.
Attenuated Total Reflectance Fourier Transform-Infrared
Spectroscopy
FTIR is used to determine the degradation of the polymeric matrix of the
carrier system. The surface of the microspheres is investigated measuring alternated total reflectance (ATR). The IR beam passing through the ATR cell reflected many times through the sample to provide IR spectra mainly of surface material. The ATRFTIR provides information about the surface composition of the microspheres depending upon manufacturing procedure and condition. (28)
Invitro release Studies:
Dissolution studies:
Release studies for microspheres in phosphate saline buffer of pH 7.4, are carried out using rotating paddle apparatus or by using dialysis method. In case of the paddle apparatus the sample is agitated at 100 rpm. The samples are taken at specific time intervals and are replaced by same amount of saline. The active ingredient in the sample withdrawn is analysed as per the monograph requirement and release profile is determined using the plot of amount released as a function of time. The release profile of the drugs or proteins generally depends on the method of formulation, formulation conditions (process variable), and more importantly on the nature of the polymer used for the preparation. Degradation profile of the polymer is another important parameter, which determines, whether the release is sustained, prolonged or burst type. Dialysis is another method used to study the release of the drugs/proteins from the microspheres. The method involves the use of the assembly . The micro- spheres are kept in a dialysing bag or tube with membrane, while the dialysing media is continuously stirred and samples of dialysate are taken.The with drawn samples are estimated for drug content .Each time the volume is replacaed using fresh buffer solution.
Beaker method:
The dosage form in this method is made to adhere at the bottom of the beaker containing the medium and stirred uniformly using over head stirrer. Volume of the medium used in the literature for the studies varies from 50-500 ml and the stirrer speed form 60-300 rpm.
Interface diffusion system:
This method is developed by Dearden& Tomlinson. It consists of four compartments. the compartment A represents the oral cavity, and initially contained an appropriate concentration of drug in a buffer. The compartment B representing the buccal membrane, contained 1-octanol, and compartment C representing body fluids, contained 0.2 M HCl. The compartment D representing protein binding also contained 1-octanol. Before use, the aqueous phase and 1-octanol were saturated with each other. Samples were with drawn and returned to compartment A with a syringe.(29)
Modified Keshary Chien Cell:
A specialized apparatus was designed in the laboratory. It comprised of a Keshary
Chien cell containing distilled water (50ml) at 370 C as dissolution medium. TMDDS (Trans Membrane Drug Delivery System) was placed in a glass tube fitted with a 10# sieve at the bottom which reciprocated in the medium at 30 strokes per min.
In vivo release:
In addition to release behaviour, the in vivo examination considers biocompatibility, excessive toxicity, and inflammatory response. Tissue reactions in vivo are ;
1.local inflammation
2.When microspheres degenerate to a particular size, a thin fibre wall forms at the between them and the organization, and phagocytosis occurs.
Animal Models:
Animal models are used mainly for the screening of the series of compounds, investigating the mechanisms and usefulness of permeation enhancers or evaluating a set of formulations In general, the procedure involves anesthetizing the animal followed by administration of the dosage form. In case of rats, the esophagus is ligated to prevent absorption pathways other than oral mucosa. At different time Intervals, the blood is withdrawn and analyzed. (30)
Buccal absorption test:
The buccal absorption test was developed by Beckett &Triggs in 1967. It is a simple and reliable method for measuring the extent of drug loss of the human oral cavity for single and multi component mixtures of drugs. The test has been successfully used to Investigate the relative importance of drug structure, contact time, initial drug concentration and Ph of the solution while the drug is held in the oral cavity.
Angle of repose:
The powder mass was allowed to flow through the funnel orifice kept vertically to a plane paper kept on the horizontal surface, giving a heap angle of powder on paper. The angle of repose was calculated by the following equation.
Tan? = h/ r
Bulk density:-
Bulk density was obtained by dividing the 3mass of powder by the bulk volume in cm.
It was calculated by using equation:
Bulk density=mass of microsphere/bulk volume. (31)
Tapped density:
It is the ratio of total mass of the powder to the tapped volume of the powder.it is expressed in g/ml and is given by:
Tapped density = mass of microsphere/Tapped volume.
Swelling index:
It is conducted in a phosphate buffer of pH 6.8. Their diameter is measured periodically by using laser particle size distribution analyzer until they were decreased by erosion and dissolution.
Swelling index = (mass of swollen microsphere–mass of dry microspheres/ mass of dried microspheres)100.
Adhesion property:
Freshly cut piece of pig intestine is used (5 cm long),clean and wash it
with isotonic saline solution. Accurate weight of microspheres was placed on mucosal surface, phosphate buffer of pH 6.8 is warmed at 37 °c was peristaltically pumped at a rate of 5 ml/min over the tissue. The duration of complete washing microspheres from pig intestine was recorded. (32)
Isoelectric point:
Electrophoretic mobility of microspheres can be measured using
micro Electrophoretic from which the isoelectric point is determined. The electrophoretic mobility is related to the surface contained charge, ionisable behaviour or ion absorption nature of the microspheres. (33)
Application of Microspheres
A number of pharmaceutical microencapsulated products are currently on the market.
Microspheres in vaccine :
The precondition of a vaccine is safety toward the microbes and its
harmful component. An ideal vaccine should satisfy this same necessity of effectiveness, protection, affordability in application and charge. The aspect of protection and avoidance of severe effects is a complicated. The aspect of safeness and the extent of the manufacturing of antibody responses are intently linked to mode of application. Biodegradable delivery technology for vaccines which are provided by intravenous path may resolve the shortcoming of this same conventional vaccines. The involvement in parenteral (subcutaneous, intramuscular, intradermal) carrier exists even though those who offer significant benefits. (34)
Microspheres in Gene delivery:
Genotype drug delivery involves viral vectors, nonionic liposomes, polycation complexes, and microcapsules technologies. Viral vectors are beneficial for genotype delivery even though those who are extremely efficient and also have a broad variety of cell goals. Even so, if used in vivo they trigger immune responses and pathogenic effects. To resolve the restrictions of viral vectors, nonviral delivery systems have been regarded for gene therapy. Non viral delivery system does have benefits these as simplicity of preparation, cell / tissue targeting, reduced immune system, unrestricted plasmid size, as well as large-scale replacable production. Polymer will be used as a transporter of DNA for gene delivery application. (35)
Monoclonal Antibodies:
Monoclonal antibodies targeting microspheres are physiologically immuno microspheres. Monoclonal antibodies are highly precise compounds and can be attached to microspheres by one of the following methods
- Non-specific adsorption
- Specific adsorption
- Direct coupling
- Coupling via reagent.
- Oral drug delivery :
The potential of polymer matrix usually contains diazepam like an oral drug delivery has been evaluated through rabbits. Its findings showed that even a film consisting of a 1:0.5 drug-polymer combination may have been an effectual dosage form which is comparable to commercial tablet formulations. The capacity of polymer to establish films could allow use in the formulation of film dosage forms, as an option with drug tablets. The pH sensitivity, combined with both the reactions of the main amine groups, start making polymer a distinctive polymer for oral drug delivery applications. The capacity of polymer to establish films could allow use in the formulation of film dosage forms, as an option with drug tablets. The pH sensitivity, combined with both the reactions of the main amine groups, start making polymer a distinctive polymer for oral drug delivery applications.
Transdermal drug delivery:
Polymer has good film-forming characteristics. The release profile from of the devices is impacted by the membrane thickness as well as crosslinking of a film. Chitosan-alginate polyelectrolyte structure has also been prepared in-situ in beads and microspheres for potential uses in packaging, controlled release systems and surgical instruments. Polymer gel beads are an impressive highly biocompatible vehicle for chemotherapy of inflammatory cytokines for medications like prednisolone that also showed extended release action enhancing treatment effectiveness. The amount of drug discharge was found to also be depend on the characteristics of cell wall used. A mixture of chitosan membrane and chitosan hydrogel known to contain lidocaine hydrochloride, a local anaesthetic is a great comprehensive process for controlled drug release and release kinetics. (36)
Targeting by Using Micro Particulate Carriers:
The principle of trying to target is a well established dogma, that is trying to gain huge interest present a days. The response manufactured by drug depends itself on availability and ability to interact to binding site generally pellets technique is confirmed that can be formulated by utilising extrusion / Spheronization innovation e.g. microcrystalline cellulose (MCC) and chitosan.
Ocular drug delivery:
Microspheres are a good carrier for the ocular drug delivery. Using microspheres drug delivery bioavailability of the drug has been improved as compared to the aqueous ocular preparations. Due to their sustained or controlled release mechanism microspheres are used for the long lasting release of drug which leads to reduce the dosing frequency.
Intranasal drug delivery:
This route is mainly preferred for the delivery of proteins and the peptides. Conventional formulations are easily get drained off from nasal mucosa. Bioadhesive microspheres provide better bioavailability by exerting its sustained/controlled mechanism (37)
Buccal drug delivery:
Mucoadhesive microspheres serve as a reservoir for the drug; it releases the drug from the applied site for a longer period of time. Mucoadhesive polymers reside on the mucosa of the buccal cavity and act as a reservoir; it also improves the bioavailability of the drug by avoiding first-pass metabolism in the body.
Gastrointestinal drug delivery:
Microspheres are used for delivery of the potent drug to the specific site (Gastrointestinal tract [GIT]). Eudragit, ethylcellulose, carbopol, and alginate microspheres are used for delivery of the drug at a specific site in the GIT. It prevents the first pass hepatic metabolism of the drug and increases the bioavailability of the drug. (38)
Intra-tumoral and local drug delivery:
Anticancer drugs should be delivered at the tumor site inappropriate concentration, for example, paclitaxel loaded microspheres. Film forming polymers are used to sustain the release at local site, that is, oral cavity.
Colonic drug delivery:
Microspheres are used for delivering the drug at a specific site in intestine, that is, colon. Insulin loaded into chitosan microspheres targeted to release its drug at colon .(39)
Vaginal drug delivery:
Microspheres drug delivery used for treating vaginal infections such as mycotic infection of the genital tract. Chitosan, Gelatin, and PLGA polymers are used for fabricating the microspheres to treat vaginal infections .
Radioactive application:
Radioactive isotopes of elements have been utilized for medical use from decades radioactive isotopes loaded microspheres are used to treat several diseases such as liver and spleen tumor, for example, Yttrium 90 loaded microspheres are used to treat carcinoma .
Application in dentistry:
Microspheres are used in the dental preparations to treat various infections related to oral cavity such as Gingivitis and bleeding gums.
Microspheres are also used in craniofacial tissue regeneration
Application in bone tissue engineering:
Microspheres can be used as a stable carrier system for delivering MSC (Bone marrow-derived mesenchymal stem cell) which helps in earlier bone regeneration as well as rapid bone formation in tissue Engineering.(40)
CONCLUSION:
Microsphere is a short term but it is having wide applications in drug delivery systems. Most important are the targeted drug delivery (Bio adhesive microspheres-nasal, ocular, buccal, rectal etc., Magnetic microspheres and radioactive microspheres – For tumours), Controlled and sustained drug delivery (Polymeric microspheres, Floating microspheres). By combining various strategies, microspheres will find central place in novel drug delivery mainly particularly in cell sorting, diagnostics and Genetic engineering. From the study it is proved that Microspheres act as effective carriers for the novel drug delivery system
REFERENCES
- Chaudhari A, Jadhav K. R, Kadam VJ. An Overview: Microspheres as a Nasal Drug Delivery System. Int. J. of Pharmaceutical Sciences Review and Res. 2010; 5
- Li, S.P., Kowalski C.R., Feld K.M., Grim W.M., Recent Advances in Microencapsulation Technology and Equipment, Drug DevInd Pharm. 14, 1988, 353-376
- Alagusundaram. M, MadhuSudana Chetty. C, Umashankari. K, Attuluri Venkata Badarinath, Lavanya. C and Ramkanth. S. Microspheres as a novel drug delivery system – A Review. International Journal of Chem Tech Research. 1(3), 2009, 526-534.
- N. R. and Suvarna V, Microspheres: A Brief Review, Asian Journal of Biomedical and Pharmaceutical Sciences, 2015;3(4):13-15.
- Virmani Tarun and GuptaJyoti, Pharmaceutical Application of Microspheres: An Approach for the treatment of Various Diseases; International Journal of Pharmaceutical Sciences and Research, 2017; 8(1): 3257- 3259.
- Li S.P, Kowalski C.R, Feld K.M, Grim W.M. 1988. Recent Advances in Microencapsulation Technology and Equipment, Drug Dev, Ind Pharm. 14: 353-376.
- Shanthi N.C, Gupta R, Mahato K.A. 2010 Traditional and Emerging Applications of Microspheres: A Review, International Journal of Pharm Tech Research; 2(1):675-681.
- Najmuddin M, Ahmed A, Shelar S, Patel V, Khan T. 2010. Floating Microspheres Of Ketoprofen: Formulation and Evaluation, International Journal Of Pharmacy and Pharmaceutical sciences. 2(2):83-87.
- Hafeli U, 2002, Physics and Chemistry Basic of Biotechnology. Focus on biotechnology. Review. Radioactive Microspheres for Medical Application, 7:213-248.
- Yadav AV, Mote HH. 2008. Development of Biodegradable Starch Microspheres for Intranasal Delivery, Indian Journal of pharmaceutical Sciences. 70 (2):170-174.
- Saralidze K, Leo H, Koole, Menno L, Knetsch W. 2010. Polymeric Microspheres for Medical Applicatio, Materials; 3:3357-3564.
- Trivedi P, Verma L, Garud N. 2008.Preparation and Characterization of Acclofenac Microspheres, Asian Journal of pharmaceutics. 2(2): 110-115.
- M. Okubo, et. Al., Production of submicron-size monodisperse polymer particles having aldehyde groups by seeded aldol condensation polymerization, Colloid and Polymer Science, 1993, 271, Pages109–113.
- Ganesan, P., Johnson, A.J., Sabapathy, L. and Duraikannu, A., 2014. Review on microsphere. American Journal of Drug Discovery and Development, 4(3), pp.153-179.
- Veena Rani .E et. Al., Preparation and Evaluation of Aspirin Loaded Microspheres by Solvent Evaporation Technique, Journal of Medicine and Biology, 2019;1 (1):27- 32.
- Shweta Saini, et. Al., Microspheres as Controlled Drug Delivery System: An Updated Review, international journal of pharmaceutical science and research,4, 2018, 1760-1768.
- Prasanth V.V., Moy A. C., Mathew S. T., Mathapan R., Microspheres An overview, Int. J. Res. Pharm. Biomed. Sci., 2011; 2:332 8. DOI
- Saravana Kumar K, et. Al., A Review on Microsphere for Novel drug delivery System, Journal of Pharmacy Research, 2012,5(1), Page 420-424.
- Mathew Sam T, Devi Gayathri S, Prasanth VV, Vinod B. NSAIDs as microspheres, The Internet Journal of Pharmacology. 2008;6: 11-15.
- Yadav R, Bhowmick M, Rathi V, Rathi J, Design and Characterization of floating microspheres for Rheumatoid arthritis, Journal of Drug Delivery and Therapeutics 2019; 9(2-s):76-81.
- Xinghang Ma, et. Al., Stability Study of Drugloaded Proteinoid Microsphere Formulations during Freeze-drying, Iournal of Drug Targeting, 1994, 2, Page 9-21.
- Jain N K, Controlled and Novel drug delivery, (2004), 236-237, 21.
- Jayaprakash S, Halith S M, Mohamed Firthouse P U, Kulaturanpillai K, Abhijith, Nagarajan M. Preparation and Evaluation of biodegradable microspheres of Methotrexate. Asian J Pharm,3;2009,26-9.
- Abbaraju Krishna shailaja, et. Al., Biomedical Applications of microspheres, Journal of Modern Drug Discovery and Drug Delivery Research, 2015;4(2):1-5.
- Kannan. K., Karar. K.P., Manavalan. R., Formulation and Evaluation of Sustained Release Microspheres of Acetazolamide by Solvent Evaporation Technique, J. Pharm. Sci& Res. 2009; 1 (1):36-39.
- Chowdary K.P.R., Suri B.J., Permeability of Ethylene Vinyl Accetate Copolymer Microcapsules: Effect of Solvents, Indian Journal of pharmaceutical Sciences. 2003; 65(1):62 66.
- Abbaraju Krishna shailaja, et. Al., Biomedical Applications of microspheres, Journal of Modern Drug Discovery and Drug Delivery Research, 2015;4(2):1-5.
- Ghulam M., Mahmood A., Naveed A., Fatima R.A., Comparative study of various microencapsulation techniques. Effect of polymer viscosity on microcapsule charecterestics, Pak.J.Sci.2009
- Sandeep, Microspheres: A Recent Update, International Journal of Recent Scientific Research, 2015: 5859-5860.
- Farah Hamad Farah, Magnetic Microspheres: A Novel Drug Delivery System, World Journal of Pharmacy and Pharmaceutical Sciences, 2017, 2(2): 93.
- Saini Shweta, Kumar Sandeep, Choudhary Manjusha,Nitesh and Budhwar Vikaas, Budhwar Microspheres As Controlled Drug Delivery System: An Updated Review, International Journal of Pharmaceutical Sciences and Research, 2018;2(1):1-3.
- Satinder Kakar, Anurekha Jain. Magnetic Microspheres: An Overview. Asian Pac. J. Health Sci., 2019; 6(1):81-89.
- Meenu Bhatt, Satinder Kakar, Ramandeep Singh. A Review On Floating Drug Delivery System.IJRAPR.2015;5(2):57-67.
- Abbaraju Krishna shailaja, et. Al., Biomedical applications of microspheres, Journal of Modern Drug Discovery and Drug Delivery Research, 2015, 4 (2), Page 1-5.
- Kazi M. Zakir Hossain, et. Al., Development of microspheres for biomedical applications: a review, Prog Biomater, 2014, Page 1-19. 40. Kataria Sahil, et.
- Kazi M. Zakir Hossain, et. Al., Development of Microspheres for biomedical applications: a review, Prog Biomater, 2014;8(4):1-19.
- Tarun Virman, et. Al., Pharmaceutical Application Of Microspheres: An Approach For The Treatment Of Various Diseases, International Journal of Pharmaceutical Science and Reaserch, 3252-3260
- Saralidze K, Koole LH and Knetsch MLW: Polymeric microspheres for medical applications, Materials 2010; 3: 3537-64.
- Hafeli U: Physics and chemistry Basics of biotechnology, Focus on biotechnology: Review: Radioacttive microspheres for medica application 2002: 7: 213-48.
- Lokwani P, Goyal A, Gupta S, Songara R, Singh N and Rathore K: Pharmaceutical Applications of Magnetic Particles in Drug Delivery System IJPRD 2011; 147 –156


 Dr. C. S. Parameswari*
Dr. C. S. Parameswari*
 B.Manasa
B.Manasa
 K.Mounika
K.Mounika
 N.Navya sree
N.Navya sree
 M.Rajeswari
M.Rajeswari
 E.Anusha
E.Anusha
















 10.5281/zenodo.14276500
10.5281/zenodo.14276500