Abstract
The development of novel drug delivery systems (NDDS) has revolutionized the pharmaceutical industry by enhancing therapeutic efficacy, reducing side effects, and improving patient compliance. This thesis provides an overview of NDDS, classifying them into targeted, controlled, and modulated release systems. The significance of polymers in NDDS is highlighted, with a focus on natural, synthetic, biodegradable, conducting, and smart polymers. The advantages of NDDS, including increased bioavailability, reduced toxicity, and improved patient outcomes, are discussed. The thesis also explores various applications of NDDS, such as cancer therapy, tissue engineering, and gene delivery. Furthermore, the limitations and challenges associated with NDDS are addressed, emphasizing the need for continued research and development. This study contributes to the understanding of NDDS and their potential to transform the field of pharmaceuticals.
Keywords
Novel Drug Delivery Systems, Polymers, Targeted Release, Controlled Release, Modulated Release, Biodegradable Polymers, Smart Polymers, Cancer Therapy, Tissue Engineering, Gene Delivery
Introduction
DRUG DELIVERY SYSTEM
Every drug molecule needs a delivery system to carry the drug to the site of action upon administration to the patient. Drug delivery is the method of administering pharmaceutical compound to achieve a therapeutic effect in humans or animals. Delivery of the drugs can be achieved using various type of dosage forms like tablets, capsules, creams, liquids, ointments etc.
Classification of Drug Delivery Systems:
- Administration routes: topical, parenteral, inhaled, and oral.
- Release mechanism: targeted, controlled, sustained, and immediate.
- Form of dosage: implants, injections, tablets, and capsules.
Oral (by mouth), topical (skin), trans-mucosal (nasal, buccal, sublingual, vaginal, ocular, and rectal), parenteral (injection into the systemic circulation), and inhalation routes are the most widely used drug delivery techniques.
Two primary categories can be used to further categorise the drug delivery system:
l. Conventional drug delivery system.
2. Novel drug delivery system. [1]
Cnventional drug delivery system
These include parenteral and oral drug delivery, where the drug is distributed throughout the body through systemic blood circulation. Most drugs only reach a small portion of the body, such as in chemotherapy, where about 99 percent of the drugs are administered do not reach the tumour site.
The Examples of these systems includes:
? Oral Delivery
? Buccal/Sublingual Delivery
? Rectal Delivery
? Intravenous Delivery
? Sub Cutaneous Delivery
? Intravenous Delivery
Most of these conventional drug delivery systems are known to provide immediate release of the drug with little or no control over delivery rate. To achieve & maintain therapeutically effective plasma conc. Several doses are needed daily which may cause significant fluctuations in plasma. Because of these fluctuations in plasma levels the drug level could fall below the MEC. Such fluctuations result in unwanted side effects or lack of intended therapeutic benefit. Sustained-release & controlled release drug delivery systems can reduce the undesired fluctuations of drug levels, reduce side effects, while improving the therapeutic outcome of the drug.
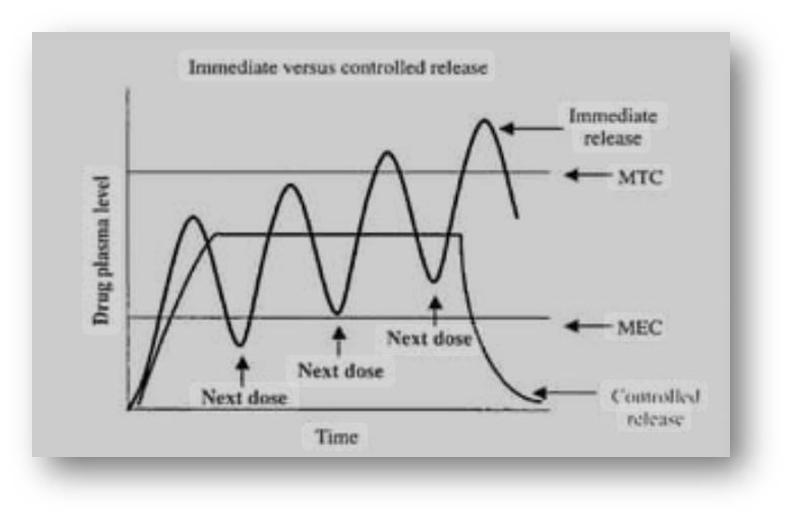
Figure:01: Therapeutic or toxic levels and immediate – versus controlled release dosage forms
NOVEL DRUG DELIVERY SYSTEM
Drugs are released from novel or targeted drug delivery systems at specific site and/or at a specific rate. Innovative drug delivery strategies improve pharmaceuticals' therapeutic efficacy.
Their objective is to enhance the solubility, durability, and bioavailability of medicinal substances. NDDS's targeted delivery methods can also lessen side effects. It is a combination of advance technique and new dosage forms which are far better than conventional dosage form and involves medical devices. It increases the potency of the drug, regulates its release to produce a long-lasting therapeutic effect, increases safety, and targets the drug precisely to the desired tissue.
These systems include:
- Targeted drug delivery systems
- Nanoparticle-based drug delivery systems
- Liposomal drug delivery systems
- Polymeric micelles and dendrimer-based systems
- Hydrogel-based systems and implantable devices
- Gene therapy-based and cell-based systems.[2]
ADVANTAGES:
- Increased bioavailability of some medications.
- Enhanced adherence by patients
- A decrease in blood drug level fluctuations
- A decrease in overall drug use when compared to traditional therapy
- Decreased drug accumulation during long-term treatment
- Decrease in systemic/local drug toxicity.
- Decrease in the number of times drugs are administered.[3]
DISADVANTAGES:
- Not all medications can be formulated into a dosage form.
- In the event that a poor formulation strategy is used, there is a chance of dose dumping.
- •A higher chance of first-pass metabolism
- •In certain circumstances, a less precise dose adjustment may be possible [4].
The following are some advantages of NDDS over conventional methods:
- It offers the right dose at the right time and place.
- It reduces production costs and makes efficient use of costly medications and excipients.
- ?Improving therapy, comfort, and standard of living for patients.
- Depending on the dosage form, the traditional dosage forms cause variations in the blood drug level and offer instant drug release. Therefore, a novel drug delivery system is required to keep the drug concentration within the therapeutically effective range.[5]
Difference between conventional and novel:
Table:1
|
Aspect
|
Conventional
|
Novel/Targeted
|
|
Release rate
|
Constant
|
Controlled/Targeted
|
|
Site of action
|
Systemic
|
Local/Specific
|
|
Efficacy
|
Lower
|
Higher
|
|
Toxicity
|
Higher
|
Lower
|
|
Patient compliance
|
Lower
|
Higher
|
Classification of Novel Drug Delivery Systems:
- First-generation novel drug delivery systems: Targeted and controlled release systems.
- Second-generation novel drug delivery systems: Nanoparticlebased and liposomal systems.
- Third-generation novel drug delivery systems: Gene therapy-based and cell-based systems.[6]
MODES OF NDDS
? Targeted Drug Delivery System
? Controlled Drug Delivery System
? Modulated Drug Delivery System
Targeted Drug Delivery System
The medication is administered to minimise systemic side effects and maximise therapeutic efficacy. It is only active in the targeted area of the body, which is the cancerous tissues, and is released gradually over time.
TDDS examples include:
- Delivery via monoclonal antibodies (e.g., Rituximab)
- Delivery via nanoparticles (e.g. Doxil)
Controlled drug delivery system:
A controlled drug delivery system is one that distributes the medication either locally or systemically for a predetermined amount of time at a set rate, or [Zero order]. The drug is released gradually. This allows for formulation changes to regulate the drug's release rate or speed.
CDDS examples include:
- Transdermal patches (nicotine, for example)
- Metformin and other oral controlled drug release tablets.
Modulated Drug Delivery System :
A modulated drug delivery system gives you exact control over how the drug is delivered by releasing the drug in response to particular physiological or environmental cues. Here, tools like nebulisers and metered dose inhalers (MDIs) regulate and control the rate of drug release.
Examples:
- PH-responsive nanoparticles as a treatment for cancer
- Hydrogels that respond to temperature to promote wound healing.
POLYMERS
A polymer is a large molecule composed of numerous tiny repeating units called monomers and joined together in a repeating pattern. [7]
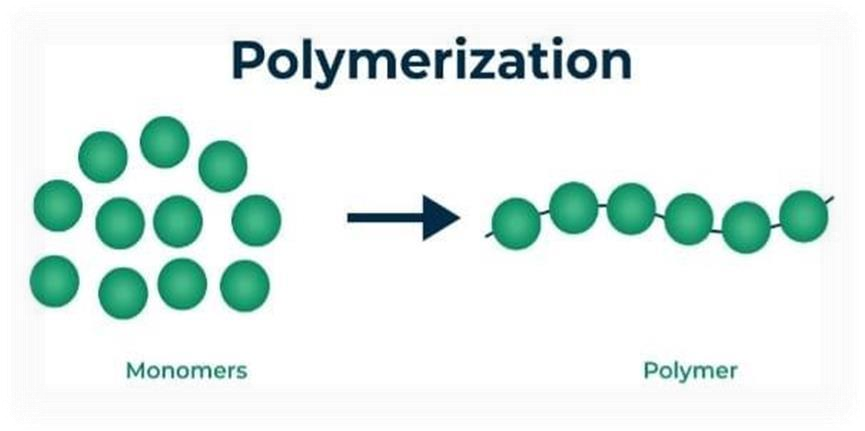
Figure:2 Schematic Representation of Polymerization Process.
SIGNIFICANCE
? Polymers are playing an increasingly important role in the field of drug delivery.
? Polymers are the major tool for controlling the medication release rate
from the formulation.
? Polymers can be used to conceal the flavour of a medicine, improve its stability, and change its release properties.
PROPERTIES:
The polymers used for medicine delivery are categorised based on the qualities listed below:
- A polymer can be either natural, synthetic, or a combination of the two.
- Chemical nature: derivatives of cellulose, polyester, polyanhydride, proteins, and so forth can all be utilised.
- Backbone stability: polymers can be biodegradable or nonbiodegradable.
- The polymer's solubility can be classified as hydrophilic or hydrophobic.
Because of their strong immunogenicity, synthetic polymers should not be used for extended periods of time.[8]
Types of polymers:
1.Natural polymers
2.Synthetic polymers
3.Biodegradable polymers
4.Conducting polymers
5.Smart polymers
1.Natural Polymer:
Natural polymers occur in nature and can be extracted. These are large molecules composed of repeating structural units, derived from natural sources such as plants, animals, and microorganisms. They are often water based.

Figure.3: Examples of natural polymers
Types of Natural Polymers:
- Polymers Based on Carbohydrates:
Cellulose (plant cell walls) Starch (plant storage) Glycogen (animal energy storage) Chitin (insect exoskeletons) Alginate (brown algae) Carrageenan (red algae) Pectin (plant cell walls) Xylan (plant cell walls)
Dextran (bacterial polysaccharide) Hyaluronic acid (connective tissue)
- Protein-based Polymers: Collagen (animal connective tissue) Gelatin (animal-derived protein) Silk (insect fibers) Wool (animal fibers) Zein (plant protein) Casein (milk protein) Soy protein (plant protein) Wheat gluten (plant protein)
- Lipid-based Polymers:
Waxes (plant and animal-derived) Cutin (plant cuticle) Suberin (plant cell walls) Natural rubber (plant-derived)
- Nucleic Acid-based Polymers:
DNA (genetic material) RNA (genetic material)
Lignin (plant cell walls) Tannins (plant-derived) Resins (plant-derived) Natural latex
(plant-derived)
- Biotechnology-derived Polymers:
Bacterial cellulose Xanthan gum (bacterial polysaccharide) Polyhydroxyalkanoates
(PHA, bacterial polyesters) Polylactic acid (PLA, bacterial-derived)
Agar (red algae) Carrageenan (red algae) Alginate (brown algae) Chitosan
(crustacean shells)
Properties:
- Biodegradability
- Equilibrium state
- Sustainable resources
- Non-toxic
- Minimal effect on the environment Applications:
- Pharmaceutical (drug delivery, excipients)
- Biomedical (tissue engineering, wound healing)
- Food industry (stabilizers, thickeners)
- Beauty (hair and skin care)
- Natural fibre textiles
- Production of paper
- Sticky Materials
- Finishes Benefits :
- Biodegradability
- Minimal toxicity
- Sustainable resources
- Economical
- Special qualities
Chitosan:
Chitosan, a polysaccharide derived from chitin, is widely used due to its mucoadhesive properties, biocompatibility, and ability to enhance drug absorption across biological barriers. Its positive charge allows for ionic interactions with negatively charged molecules, aiding in controlled drug release.
Applications:
Drug delivery through the mouth, nose, and eyes is accomplished by chitosan-based nanoparticles. Alginate:
Alginate, another polysaccharide, forms gels in the presence of divalent cations such as calcium. It is frequently used for encapsulating drugs and ensuring their sustained release.
Applications:
Alginate-based hydrogels are used in tissue engineering, oral medication delivery, and wound healing.
Gelatin:
It is a protein polymer that can be used to create hydrogels that can carry medications that are both hydrophilic and hydrophobic. Its biodegradability and simplicity of modification make it a widely used material.
Applications:
Gene delivery, cancer treatment, and vaccine formulations employ hydrogels and gelatin nanoparticles.
2.SYNTHETIC POLYMERS:
Man-made materials called synthetic polymers are made of monomers— repeating molecular units—that are chemically joined to form long chains. They go by the name "plastics" and find use in a wide range of products, including consumer electronics and medical equipment.
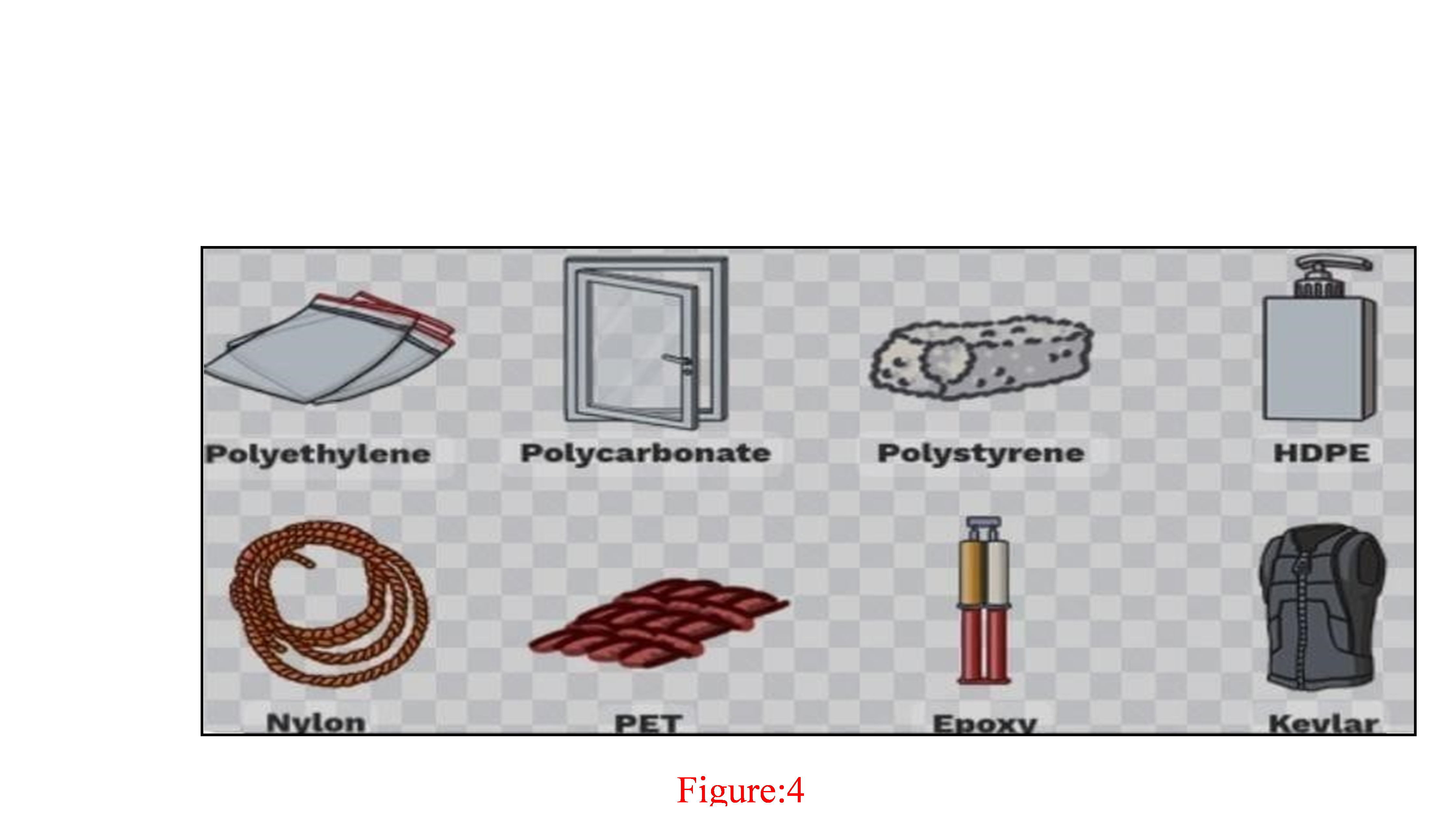
Examples of the synthetic polymers
Synthetic Polymer Types:
1.Thermoplastics: These materials, such as polyethylene and polypropylene, can be melted and reformed several times.
2.Thermosets: (e.g., epoxy, polyurethane): Once set, cannot be melted or reformed.
3.Rubber-like qualities: elastomers (silicone, polybutadiene). Synthetic Polymer Examples: Polythene (PE) Polypropylene (PP) PVC, or polyvinyl chloride PET, or polyethylene terephthalate Nylon Polycarbonate (PC) acrylonitrile However, butadiene ABS styrene
Polyurethane (PU) PTFE, or polytetrafluoroethylene
Applications:
- Materials for packaging
- Parts for automobiles
- Devices for medicine
- Fabrics
- Building supplies
- Consumer goods (appliances, toys)
- Adhesives
- Finishes
Benefits:
- Compact design
- Impervious to corrosion
- Poor upkeep
- Economical
- Adaptable
Drawbacks:
- Environmental issues (materials that don't biodegrade)
- Chemical leaching poses a health risk
- Limited ability to recycle
- Reliance on non-renewable materials
Application Of Man-Made Polymers:
A few applications are listed below:
- Plastic bags and film wraps are made of a polymer known as polyethylene.
- Toys, electrical insulation, bottles, and other items all contain polyethylene.
- Flooring, pipes, and siding are made of polyvinyl chloride, or PVC.
4.The man-made polymer polystyrene finds application in packaging and cabinets.
5. Latex paints and adhesives both contain polyvinyl acetate.[9] The FDA has approved poly(lactic-co-glycolic acid) (PLGA), a widely used biodegradable polymer. It breaks down into glycollic and lactic acids, which the body can easily metabolise. PLGA regulates the lactic acid to glycolic acid ratio, enabling controlled drug release.
Applications:
Depot injections, microparticles, and systems based on nanoparticles are among the long-term drug delivery methods that employ PLGA. In cancer therapy, where prolonged release is essential, it is especially helpful.
Polyethylene glycol (PEG)is a hydrophilic polymer that eludes immune system recognition due to its stealth characteristics. The process of attaching PEG to medications or drug carriers, known as PEGylation, lengthens the duration of circulation and lowers immunogenicity. Applications: PEG is utilised in the production of micelles, liposomes, and nanoparticles for the targeted delivery of medication in inflammatory, cardiovascular, and oncological disorders.
Polycaprolactone (PCL): PCL is a semi-crystalline biodegradable polymer that can be used for long-term drug delivery because it degrades more slowly than PLGA.
Applications: PCL-based nanoparticles are used to deliver contraceptives and deliver drugs with sustained release in tissue engineering and bone regeneration.
3.Biodegradable Polymer
Biodegradable polymers are materials that can break down naturally in the environment, typically through microbial degradation, into harmless products.[10]
Categories:
- PLA (polylactic acid)
- PHAs, or polyhydroxyalkanoates
- PGA, or polyglycolic acid
- PCL, or polycaprolactone
- Bioplastics based on starch
- Acrylamide of cellulose
- Chitin Polyesters that degrade naturally (PBAT, PBS, etc.)
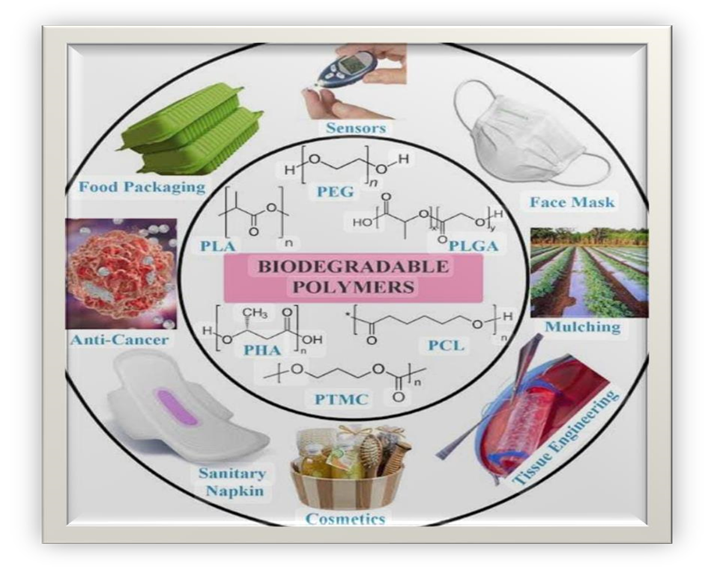
Figure 5:Examples of biodegradable polymers
Non-Biodegradable polymers
Synthetic polymers that are resistant to enzymatic or microbial breakdown in the environment are known as non-biodegradable polymers. These polymers cause environmental problems because they last for hundreds or even thousands of years. Their main drawback is that once the medication is finished, they must be surgically removed from the body. Therefore, the use of non-biodegradable polymers is limited to situations where the implant is easily removed.
Non-biodegradable polymer examples include: Polyvinyl chloride
(PVC), Polypropylene (PP), and Polyethylene (PE) PET, or polyethylene terephthalate Polytetrafluoroethylene (PTFE), Polyurethane (PU), and
Polystyrene (PS) Butadiene, Acrylonitrile, and Nylon Styrene (ABS) Effects on the Environment:
- Plastic waste in rivers and seas
- Marine life consuming microplastics
- Contamination of soil
- Issues with litter and aesthetics
- Emissions of greenhouse gases during production
- Depletion of non-renewable resources
4.Conducting Polymers:
Electrical conductivity is a property of organic materials known as conducting polymers (CPs), which makes them useful in a variety of
applications.[11]
Conducting Polymer Examples: Polyacetylene (PA) Polyaniline
(PANI) Phenylene vinylene polymer (PPV) PEDOT, or poly(3,4ethylenedioxythiophene), Polythiophene (PT) Polypyrrole (PPy)
5.Smart Polymers:
Materials that alter their characteristics in response to external stimuli, such as temperature, pH, light, or chemicals, are referred to as smart polymers, also known as stimuli-responsive polymers.
Smart Polymer Types:
- Thermoresponsive polymers, such as PVCL and PNIPAM
- Polymers that react to pH, such as poly (acrylic acid) and polybases
- Photoresponsive polymers, such as those containing azobenzene
- Polyelectrolytes and other electroresponsive polymers
- Biomimetic polymers, such as those modelled after proteins [12]
Mechanism of Drug release in controlled delivery system:
1. Diffusion-Controlled Release:
One of the most widely used methods for regulating drug release is diffusion, in which drug molecules migrate from an area of higher concentration to one of lower concentration.
Matrix Diffusion:
The drug diffuses throughout a polymer matrix in matrix-based systems. As bodily fluids permeate the matrix, the drug diffuses out due to the gradient in concentration. The longer the distance the drug must travel, the lower its concentration gets over time. In polymer-based systems and hydrogels, this kind of release is typical.
Reservoir Diffusion:
A polymeric membrane encapsulates the medication in reservoir systems. At a regulated pace, the medication diffuses through the membrane. The thickness and characteristics of the membrane can be changed to alter the rate of release.
2.Dissolution-Controlled Release:
This type of release relies on the drug's or its carrier matrix's slow dissolution in bodily fluids. The medication is then bioavailable for absorption after it has dissolved.
Matrix Dissolution:
In matrix systems, the medication is evenly distributed throughout a matrix that gradually dissolves. The drug is released into the surrounding fluid as the matrix dissolves.
Dissolution of Reservoir: In reservoir systems, the medication is enclosed in a coating that dissolves at a predetermined pace. The drug release profile is determined by the coating's rate of
dissolution.
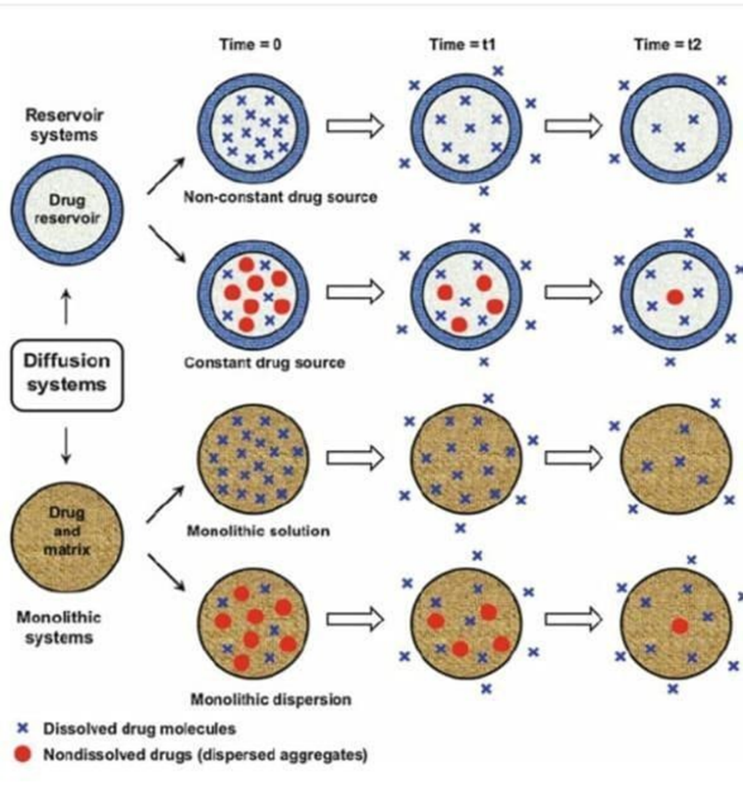
Figure 6:Schematic Representation of Reservoir and Monolithic drug delivery system

Figure:7 Comparison of drug release mechanism in Reservoir and Monolithic drug delivery system
Table :2
|
Types of controlled release
|
Preparation Method
|
|
Diffusion control Release
|
Reservior
Diffusion control
|
- Core tablet is prepared
- It is coated by insoluble polymers
|
|
Matrix Diffusion control
|
Tablet powder and insoluble polymer mixed together
• Compressed into
tablet
|
|
Dissolution control release
|
Reservior
Dissolution control
|
- Drug granules are prepared
- They coated with
different concentrations of slowly soluble polymer
- Granules packed into capsule
|
|
Matrix Dissolution control
|
- Tablet powder and slowly soluble polymer mixed together
- Compressed into a
tablet
|
3. Release Controlled by Erosion:
Drug release in erosion-based systems occurs when the polymeric carrier erodes or breaks down. This process is especially crucial in biodegradable systems because the matrix breaks down gradually as a result of external variables like pH or enzymatic activity.
Bulk Erosion:
Drug release results from the uniform degradation of the whole polymer matrix in bulk-eroding systems. Surface Erosion:
From the outside inward, surface-eroding systems deteriorate layer by layer. The rate at which drugs release is directly correlated with the polymer's rate of erosion.
4.Osmotically Controlled Release:
Drug release is governed by osmotic pressure in osmotically controlled systems. Osmotic pressure is created in these systems when water passes through a semi-permeable membrane and into the drug delivery device. The drug solution is forced through a tiny opening at a predetermined pace by this pressure.
A steady, zero-order release is produced by osmotic pumps, which means that the drug is released gradually and at a constant rate regardless of the surrounding conditions.
- Swelling-Controlled Release:
Systems that use this technique make use of hydrophilic polymers, which draw in water and cause the matrix to swell. The swelling of the polymer results in an increase in its permeability or the creation of channels that allow the drug to diffuse. Hydrogels, whose swelling kinetics can be precisely controlled to control drug release, are frequently found to exhibit swelling-controlled release.
- Release Sensitive to pH:
Changes in the pH environment cause the release of drugs in pH-sensitive drug delivery systems. These systems are made of polymers that, depending on the pH, either dissolve or swell. For instance, a medication intended for intestinal release could be enclosed in a polymer that solidifies in the stomach's acidic environment but dissolves in the intestines' basic pH.
- Targeted Release (Ligand-Mediated):
Drugs can be delivered to particular tissues or cells using targeted delivery methods, which lowers systemic side effects and increases therapeutic efficacy. These systems are frequently designed to bind to particular receptors on the target cells using targeting ligands, which can be small molecules, peptides, or antibodies.
The drug is released from the target site once the drug delivery system binds to it, either by endocytosis or through other mechanisms that are triggered by the binding.
- Stimuli-Responsive Release:
Drug delivery systems with this feature are made to release the medication in response to certain outside stimuli, like light, magnetic fields, temperature, or ultrasound.
Systems that respond to temperature: These systems release the medication when exposed to specific temperatures. Temperature variations may cause the polymer matrix to expand or contract, releasing the medication.
Systems that react to light: These systems release the medication in response to certain light wavelengths. Drug release occurs as a result of a chemical or physical change in the matrix brought on by the light. Magnetically triggered systems: The drug delivery system contains magnetic nanoparticles that are triggered by an external magnetic field.
- Ion-Exchange Controlled Release:
In ion-exchange systems, the drug-loaded polymer and the surrounding biological fluids exchange ions, which controls the release of the drug. A controlled release of the medication occurs when counter-ions in the bodily fluid displace the drug, which is bound to an ion-exchange resin.
- Release Triggered by Ultrasound:
In drug delivery systems, ultrasound waves can cause mechanical vibrations or heating that can lead to the drug carrier breaking down or the matrix becoming more permeable. This makes it easier for the medication to release in response to ultrasonic exposure, allowing it to target particular tissues or regions.
List of drug carrier in NDDS:
Pytosomes liposomes Nanoparticals
Emulsions
Microsphere
Ethosomes
Solid lipid particles
Niosomes
Proniosomes
Dendrimers
Liquid crystals
Hydrogels
Phytosome:
Phytosomes are lipid-compatible molecular complexes made up of the terms "some," which denotes cell-like, and "phyto," which means plant. The molar ratio of polyphenolic phytoconstituents and phosphatidyl choline can be complexed to create a novel herbal drug delivery system called a "Phytosome." Phytosomes are enhanced herbal products that are more effectively absorbed and used to achieve superior outcomes compared to traditional herbal extracts. The pharmacokinetic and therapeutic profiles of phytosomes are superior to traditional herbal extracts.
Benefits of phytosome:
Because phytosome enhances the absorption of active ingredients, a small dose is needed to achieve the desired effects.
There is a noticeable improvement in bile's solubility and drug entrapment with herbal constituents, and it can specifically target the liver.
Phytosome exhibits good stability because it forms chemical bonds between phosphatidylcholine molecules.
The percutaneous absorption of herbal phytoconstituents is enhanced by phytosomes.[13]
Liposomes:
Liposomes are vesicles made of many, few, or just one phospholipid bilayer; the polar character of the liposomal core allows polar drug molecules to be encapsulated; amphiphilic and lipophilic molecules are solubilised within phospholipid bilayer according to their affinity towards phospholipids. Liposomes are used against infectious diseases and can deliver certain vaccines. During cancer treatment they encapsulate drugs, shielding healthy cells from their toxicity and preventing their concentration in vulnerable tissues like those of patient kidneys and livers. Liposomes can also reduce or eliminate certain common side effects of cancer treatment such as nausea and hair loss.
Benefits: By altering the surface with ligands or antibodies, liposomes improve the solubility of poorly soluble drugs, lower drug toxicity, and enable targeted delivery.
Applications:
Liposomal formulations find use in vaccinations, antifungal therapies, and cancer therapy (such as Doxil® for the delivery of doxorubicin). Challenges include high production costs, rapid clearance from the bloodstream by the reticuloendothelial system (RES), and stability issues.
Use of Liposomes:
By using liposomes to transport the drugs to the site of action, innovative drug delivery systems have made a significant and noteworthy advancement. Both modified and unmodified liposomes have the power to alter the pharmacokinetic parameters of the drugs. These are frequently employed to prevent adverse effects like myelosuppression and to deliver cytotoxic agents to the tumour tissue. They are also employed in receptormediated endocytosis targeting. The ability to target different medications to organs such as the heart, liver, kidney, lungs, and bones is another huge use for modified liposomes.[14]
Nanoparticles:
Nanoparticles, which can be either amorphous or crystalline, are solid particles that include nanospheres and nanocapsules with sizes ranging from 10 to 200 nm. They can encapsulate and/or adsorb a medication, shielding it from enzymatic and chemical deterioration. Biodegradable polymeric nanoparticles have garnered significant interest as possible drug delivery agents in recent times due to their potential uses in controlled drug release, targeting specific organs or tissues, acting as DNA carriers in gene therapy, and delivering proteins, peptides, and genes orally. [15]
Uses:
- Cancer treatment.
- Genetic modification.
- Delivery of vaccines.
- Disorders of the nervous system.
- Diagnostics and imaging.
Benefits of the delivery system for nanoparticles
- The herbal formulation is delivered straight to the site of action by the nanoparticulate system.
- A higher therapeutic index and efficacy.
- More stability through encapsulation
- Enhanced impact of pharmacokinetics
5.Producible in a range of sizes and with complex surface characteristics.
Emulsions:
Emulsion is a biphasic system in which minute droplets with diameters ranging from 0.1 ?m to 100 ?m are intimately distributed throughout the other phase. An emulsion always consists of two phases: an oily liquid, or non-aqueous phase, and water, or the aqueous phase.
Among these, the sub-micro-emulsion is referred to as liquid emulsion, and the microemulsion is also known as nano emulsion. Microemulsions are transparent, thermodynamically stable substances that are often combined with co-surfactants.
Benefits of formulation based on emulsions:
- Because it is packed in the inner phase and makes direct, the drug can be released for an extended period of time.
- Interaction with other tissues and the body.
3.When the lipophilic medications are formulated into an o/w/o emulsion, the oil droplets are phagocytosed by macrophages, which raises the concentration of the drug in the kidney, spleen, and liver.
4.The emulsion's herbal formulation will strengthen the hydrolysed formulated material's stability and enhance the drug's absorption into the skin and mucous membranes.
5.The novel kind, namely elemenum emulsion, is safe and effective as an anti-cancer medication to the liver and heart.[16]
Microsphere:
Small spherical particles, typically ranging in diameter from 1 ?m to 1000 ?m (1 mm), make up a microsphere. Micro-particles are another name for microspheres. A wide range of synthetic and natural materials can be used to create microspheres. Commercially available microspheres include glass, polymer, and ceramic materials. There are two types of microspheres: biodegradable and non-biodegradable. Albumin microspheres, modified starch microspheres, gelatin microspheres, polypropylene dextran microspheres, polylactic acid microspheres, and so on are examples of biodegradable microspheres. As per the extant literature reports on non-biodegradable microspheres, the only polymer that has been authorised for human use is polylactic acid, which is utilised as a controlled-release agent. Since the densities of solid and hollow microspheres differ greatly, they have different uses.
Uses:
- Cancer treatment.
- Delivery of vaccines.
- Hormone substitute medication.
4.Pain management.
5. Delivery to the eyes.
Ethosomes:
A combination of phospholipids and a high ethanol concentration forms ethosomes. Because this carrier can deeply penetrate the skin, medication delivery to the skin's deeper layers and blood circulation can both be improved. These formulations are helpful for delivering alkaloids topically to patients in the comforting forms of gel and cream. Due to the skin's lipid domain becoming more fluid, they exhibit an increase in permeability through the skin. The tropical delivery of ethosomes has limitations due to its unstable nature and low skin penetration. Tetrandine's topical absorption through dermal delivery was tested using ethosomes, and the relationship between formulations and the pharmacological activity of Tetrandine loaded in the formulation was also investigated obtained. Tetrandrine-loaded ethosomes were topically applied to rats, but the drug level was too low to be detected in the rat plasma, according to the results of the drug levels in the plasma. Consequently, ethosomes were shown to be a promising carrier for enhancing tetrandine topical delivery via skin.
Benefits of ethosomes:
- Ethosomes improve a drug's transdermal skin penetration.
- The delivery of numerous different types of medications in large quantities is made possible by etheromes. 3. Patients' compliance improves when the ethosomal medication is given in semisolid form.[17]
Solid lipid nanoparticles:
(SLNs) represent a novel approach to pharmaceutical formulation or delivery. For the delivery of medications to intestinal lymphatics, conventional methods including surface modification, prodrug synthesis, complex formation, and the use of permeability enhancers have been developed. In addition, a number of potential carriers for oral intestinal lymphatic delivery have been explored, including polymeric nanoparticles, self-emulsifying delivery systems, liposomes, microemulsions, micellar solutions, and most recently, solid lipid nanoparticles (SLN).
An average solid lipid nanoparticle's diameter ranges from 10 to 1000 nanometres, making it spherical in shape. Lipophilic molecules can be dissolved by the solid lipid core matrix found in solid lipid nanoparticles. Emulsifiers, or surfactants, stabilise the lipid core. Triglycerides (like tristearin), diglycerides (like glycerol bahenate), monoglycerides (like glycerol monostearate), fatty acids (like stearic acid), steroids (like cholesterol), and waxes (like acetyl palmitate) are all included in the broader definition of "lipid" used here. To stabilise the lipid dispersion, emulsifiers of all classes (in terms of charge and molecular weight) have been employed. Emulsifier combinations have been found to have a potentially more effective effect on preventing particle agglomeration.[18]
Niosomes:
Multilamellar vesicles called niosomes are created when cholesterol and non-ionic surfactants of the alkyl or dialkyl poly-glycerol ether class combine. Previous research conducted in collaboration with L'Oreal has demonstrated that niosomes share many characteristics with liposomes when it comes to their potential as drug carriers. Niosomes differ from liposomes in that they have a few benefits over the latter.[19]
Proniosomes:
Proniosomes gel is an advancement over noisome and has a multitude of uses in the delivery of active ingredients to desired locations. Gels containing proniosomes are those that, when hydrated in situ with skin moisture, transform into niosomes.
Dendrimers:
Dendrimers are well-defined, man-made nanoparticles with a diameter of roughly 5–10 nm. They consist of a control core surrounded by polymer layers. The surface of dendrimers has a variety of locations where drugs can be attached, as well as places where materials like PEG can be attached to change the way dendrimers interact with the body. Dendrimers can have PEG attached to them in order to "disguise" them and hinder the body's defence mechanism from identifying them, which will slow down the breakdown process. This intriguing particle has great potential for treating cancer. Other molecules can readily cling to its surface thanks to its numerous branches.
Dendrimers have been engineered by researchers into complex anticancer machines that carry five chemical tools: a molecule that binds to cancer cells; a second that fluoresces when it detects genetic mutations; a third that helps with x-ray tumour shape imaging; a fourth that carries drugs that are released when needed; and a fifth that would signal the death of cancerous cells. The inventors of these dendrimers tested them successfully on cancer cells in culture and soon hope to test them on live animals.
Liquid Crystals:
The characteristics of both liquid and solid states are combined in liquid crystals. They can have various geometries and alternative polar and nonpolar layers (a lamellar phase) that allow the inclusion of aqueous medication solutions.
Hydrogels:
Hydrogels are three-dimensional, hydrophilic polymeric networks that can absorb a lot of water or biological fluids. They function as carriers in swellable and swelling-controlled release systems or as regulators of drug release in reservoir-based controlled release systems.[20]
NOVEL DRUG DELIVERY SYSTEM FOR
DIABETES MANAGEMENT
Recent developments in drug delivery systems, such as oral, inhalable, and transdermal techniques, as well as the use of nanotechnology and glucoseresponsive smart systems in diabetes treatment, is given in this article.
1.Oral Insulin Delivery Systems:
Because it is non-invasive and can mimic the physiological pathway of endogenous insulin secretion, oral insulin administration is regarded as the "holy grail" of diabetes treatment. The main problems with oral insulin are low intestinal epithelium permeability and its breakdown by digestive enzymes in the gastrointestinal tract.
Current Developments:
1. Oramed Pharmaceuticals has made significant strides with their oral insulin capsule, which uses a protective coating to prevent degradation in the stomach and an absorption enhancer to facilitate insulin uptake in the small intestine.
2.Novo Nordisk (NN1953/OI338GT):
Novo Nordisk is a leader in insulin therapies and has been working on oral insulin formulations for many years. OI338GT is one of their investigational products. The drug aims to protect insulin from stomach acid and deliver it effectively into the bloodstream. However, none of their oral insulin formulations have yet reached the market.
- Biocon:
Biocon is working on oral insulin formulations, including a tablet form of insulin, but their product has not yet reached the market. They are in collaboration with partners and are conducting clinical trials.
- Capsulin by Diabetology Ltd.:
Diabetology is another company exploring oral insulin, with their product Capsulin. They are developing oral insulin using a proprietary gel capsule that protects insulin through the digestive system. Though Capsulin has shown promise in early trials, it is still not available commercially. Biotechnological Approaches: Encasing nanoparticles and using polymeric systems to shield insulin molecules from deterioration have demonstrated encouraging outcomes.
Clinical Implications: By reducing the need for injections, oral insulin can increase patient compliance. The long-term safety and effectiveness of these systems are being evaluated in ongoing clinical trials.[21]
2. Systems for Transdermal Drug Delivery:
Transdermal patches present a novel, non-invasive insulin delivery option. They can do away with the requirement for numerous daily injections and are made to deliver regulated insulin release over an extended period of time.
Microneedle Patches: These devices use tiny needles to gently pierce the skin and administer insulin or other diabetic drugs. One such example that is being developed is the microneedle patch from Zosano Pharma. Benefits: The ease of use of these patches increases patient adherence, and they may lead to more reliable glucose control.
Challenges: Concerns about skin irritation, variations in absorption rates according to skin type, and the necessity of additional research on longterm use still need to be addressed.[22] 3. Inhalable Insulin Delivery:
This non-invasive technique provides quick insulin delivery to the lungs.
One well-known FDA-approved product is MannKind Corporation's
Afrezza, which delivers fast-acting insulin absorbed through pulmonary tissues.
Mechanism of Action: Compared to subcutaneous insulin, Afrezza's action begins sooner because it is delivered through an inhaler device that absorbs insulin in the alveoli.
Clinical Benefits: Inhalable insulin provides faster glycaemic control, making it especially helpful for controlling blood sugar levels after meals. Also, by removing the discomfort associated with injections, it enhances the quality of life for patients.
Limitations: Compared to injectable forms, inhalable insulin has a lower bioavailability and is not recommended for patients with respiratory conditions like COPD or asthma.[23]
- The Use of Nanotechnology in the Delivery of Diabetes
Medications: Using nanoparticles, diabetes medications can be delivered with better targeting, increased stability, and controlled release. These devices can be programmed to release insulin or other medications that control blood sugar in response to physiological cues like blood sugar levels.
Oral Insulin Based on Nanoparticles: By using nanocarriers, such as liposomes or PLGA (polylactic-co-glycolic acid) nanoparticles, insulin can be protected from GI tract degradation and absorbed more easily. Combination Therapies: Insulin and GLP-1 agonists can be co-delivered by nanoparticles, enabling more thorough control of glucose levels and insulin resistance.
Future Directions: By enabling targeted and controlled drug release, nanoparticle-based delivery systems present a promising avenue for personalised diabetes treatment. Refining these systems to increase bioavailability and decrease side effects is the main focus of current research.[24]
- Intelligent Medication Administration Systems:
Intelligent medication delivery systems are starting to emerge as a groundbreaking method of managing diabetes. In order to replicate the body's natural regulation of insulin, these systems use stimuli-responsive materials to release insulin in response to changes in blood glucose levels. Glucose-Responsive Systems: Providing a closed-loop insulin delivery system, glucose-responsive hydrogels, nanoparticles, and microneedles can release insulin when blood sugar levels rise. Smart Insulin Patch: Based on glucose concentrations in the interstitial fluid of the skin, Zhen Gu's lab has created a smart insulin patch that releases insulin through the use of microneedles.
Benefits: These systems are a major advancement in the treatment of diabetes because they offer more accurate glucose control, lower the risk of hypoglycemia, and require fewer dosing interventions.[25]
- Artificial Pancreas with Closed-Loop Insulin Delivery Systems:
The creation of the artificial pancreas, which combines insulin pumps and continuous glucose monitoring, is a major advancement in the treatment of diabetes. One of the first hybrid closed-loop systems to receive FDA approval is Medtronic's MiniMed 670G system, which automatically modifies insulin delivery in response to real-time glucose readings.
Benefits: By lessening the burden of managing diabetes on a daily basis, these systems enhance glycaemic control and lower the risk of complications related to diabetes. challenges: Present barriers to widespread adoption include high costs and the requirement for additional algorithmic accuracy and reliability tuning.[26]
CONCLUSION:
In conclusion, the exploration of Novel Drug Delivery Systems (NDDS) highlights significant advancements in the pharmaceutical field that enhance drug efficacy, reduce side effects, and improve patient compliance. These systems, which include targeted, controlled, and modulated release mechanisms, offer innovative solutions that address limitations of conventional drug delivery. The application of natural, synthetic, and biodegradable polymers plays a critical role in optimizing drug release, targeting, and therapeutic outcomes. While NDDS offer considerable benefits, challenges such as formulation complexity and regulatory hurdles underscore the need for ongoing research and development. By advancing these systems, the potential to transform treatment modalities and patient care continues to grow, positioning NDDS as a cornerstone of modern and future pharmaceutical innovations
REFERENCES
- Journal of Controlled Release. Drug delivery systems: An overview. 2014;190:3-11.
- Junagade VB, et al., editors. Nanotechnology in Drug Delivery. 1st ed. Wiley-Blackwell; 2014. Chapters 1-6.
- Controlled release drug delivery systems reduce drug level fluctuation. Eur J Pharm Biopharm. 2017;115:51-60.
- Challenges in dose adjustment with controlled release drug delivery systems. J Clin Pharmacol. 2020;60(3):281-289.
- Efficient use of expensive drugs through novel drug delivery systems. Eur J Pharm Biopharm. 2016;107:1-10.
- Nanoparticle-based and liposomal systems: Second-generation novel drug delivery systems. Eur J Pharm Biopharm. 2016;108:24-32.
- Fried JR. Polymer Science and Technology. 3rd ed. Prentice Hall; 2014. Chapter 1: Introduction to Polymers.
- International Conference on Pharmaceutics and Novel Drug Delivery
- Systems. Polymeric Drug Delivery Systems. Conference proceedings; 2019. Synthetic polymers for advanced applications. Polymer Chem. 2020;11(18):2905-2916.
- Biodegradable polymers for sustainable applications. Biomater Sci. 2019;7(12):4912-4930.
- Conducting polymers for electronic applications. J Mater Chem. 2020;8(15):7196-7205.
- Dissolution-controlled release systems. J Pharm Sci. 2015;104(2):281291. Encapsulation and dissolution control. Int J Pharm. 2016;507(1-2):55- 62. Microencapsulation: A review. J Control Release. 2017;261:22-29.
- Hikino H, Kiso Y, Wagner H, Fiebig M. Antihepatotoxic actions of flavonolignans from Silybum marianum fruits. Planta Med. 1984;50(3):248-250.
- Chaturvedi M, Kumar M, Sinhal A, Saifi A. Recent development in novel drug delivery systems of herbal drugs. Int J Green Pharm. 2011;5(2):87-94.
- Kharat A, Pawar P. Novel drug delivery system in herbals. Int J Pharm Chem Biol Sci. 2014;4(4):910-930.
- Jumaa M, Muller BW. Lipid emulsions as a novel system to reduce the hemolytic activity of lytic agents: Mechanism of protective effect. Eur J Pharm Sci. 2009;9(4):285-290.
- Scarfato P, Avallone E, Iannelli P, Aquino RP. Quercetin microsphere by solvent evaporation: preparation, characterization, and release behavior. J Appl Polym Sci. 2008;109(5):2994-3001.
- Chao F, et al. Enhanced topical delivery of Tetrandrine by ethosomes for treatment of arthritis. Biomed Res Int. 2013;2013:161943.
- Jenning V, Thünemann AF, Gohla SH. Characterization of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167-177. PMID: 10802410.
- Yoshio K, Masazumi K, Shuichi A, Hiroaki N. J Control Release. 2005;105(1-2):16-21.
- Heinemann L, Jacques Y. Oral insulin and buccal insulin: A critical reappraisal. J Diabetes Sci Technol. 2009;3(3):568-584.
- Yu J, Zhang Y, Gu Z. Glucose-responsive insulin delivery based on micro/nanotechnology. Adv Healthc Mater. 2017;6(10):1600913.
- Rave K, Heinemann L, Bernhardt E. The feasibility of inhaled insulin therapy in type 1 diabetes. Diabetes Care. 2004;27(4):895-899.
- Yin H, Zhang Y. Nanotechnology advancements in oral delivery of diabetes medications. Adv Drug Deliv Rev. 2018;130:75-89.
- Wang J, Ye Y, Yu J, Gu Z. Stimuli-responsive insulin delivery systems: Strategies and applications. Adv Healthc Mater. 2018;7(6):1700887
- Forlenza GP, Deshpande S, Buckingham B. Application of closed-loop insulin delivery in pediatric patients. Curr Diab Rep. 2016;16(8):74.


 Dr. C. S. Parameswari*
Dr. C. S. Parameswari*
 K.Mounika
K.Mounika
 B Manasa
B Manasa
 N.Navya sree
N.Navya sree
 M.Sreya
M.Sreya
 M.Rajeswari
M.Rajeswari
 E. Anusha
E. Anusha







 10.5281/zenodo.14355738
10.5281/zenodo.14355738