Febuxostat is a novel non-purine selective xanthine oxidase inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of hyperuricemia in adults with gout. The UV spectroscopic method has been developed for the determination of febuxostat in Dimethylformamide (DMF) at the wavelength range between the 270-400 nm. Febuxostat showed an absorption peak at 317 nm. Linearity ranges were found as 2-10 microgram. Developed method was found to be validated and showed good precision and reproducibility.
Febuxostat is a novel, selective xanthine oxidase/dehydrogenase inhibitor that works by decreasing serum uric acid in a dose dependent manner. Febuxostat works by non-competitively blocking the molybdenum pterin centre, which is the active site of xanthine oxidase(1).
Drug Profile:
Febuxostat (TEI-6720, TMX-67), 2-(3-cyano-4-[2-methyl propoxyl]phenyl)-4-methylthiazole-5-carboxylic acid, is a thiazolecarboxylic acid derivative with the empirical formula C16H16N2O3S. Febuxostat has a molecular mass of 316.38 g/mol and is largely bound to albumin with volume of distribution at steady state of 0.7 mg/kg. Febuxostat is soluble in water, in methanol it is slightly soluble and freely soluble in N,N-dimethylformamide(2).
Structure of Febuxostat:-

PHARMACOKINETICS AND METABOLISM:
Route of administration:
Febuxostat is administrated by the oral route. Febuxostat is absorbed at a rate of 49%, 99.2% of which bound to albumin. Febuxostat absorption and efficacy is not affected by food or achlorhydria, but high fat foods can delay the absorption rate but show no significant effect on its efficacy.
Excretion:
Febuxostat is excreted in urine and feces. In urine 49% is excreted in form of metabolite and 3% excreted as unchanged compound, whereas in feces 12% is excreted as unchanged compound and 45% is excreted in the form of metabolite.
Half-life:
Febuxostat has the half-life of 5hr to 7hr.
Metabolism:
Febuxostat is metabolised via both conjugation by uridine diphosphate glucuronosyltransferase (UGT) enzymes (UGT1A1, UGT1A3, UGT1A9, UGT2B7) and oxidation by CYP enzymes (CYP1A2, CYP2C8 and CYP2C9), active metabolites are formed by oxidation of the isobutyl side chain(3).
METHOD DEVELOPED:
Method for the determination of the febuxostat in biological material which has been reported previously included High performance liquid chromatography (HPLC) with tandem-mass spectrometry, High performance thin layer chromatographic method, HPLC-MS. Although UV estimation of febuxostat in methanol and 0.1n NOAH was reported but estimation in Dimethylformamide (DMF) has not been reported.
In this present study simple, precise and accurate spectroscopic method has been developed for the estimation of febuxostat using DMF as a solvent(4).
MATERIALS AND METHOD:
A Shimadzu UV-1900i UV/Vis double beam spectrophotometer was used for the spectral measurement. Wensar AN ISO 9001 analytical balance was used for the weighing purposes. The APIs of febuxostat has been purchased from the Yarrow chem products (Mumbai) with 99.7% assay value. The DMF used is of analytical grade.
Selection of Analytical Wavelength:
Appropriate dilutions were prepared for drug from the standard stock solution and the solutions were scanned in the wavelength range of 270-400 nm.
Preparation of stock solutions:
10mg APIs of Febuxostat was weighed and transferred to a 10 ml volumetric flask and dissolved in DMF. The volumetric flask was shaken and volume was made up to the mark with DMF to give a dilution containing 1000 microgram/ml. from this stock solution pipette out 1ml in the 10 ml volumetric flask and make up the volume to 10 ml with DMF to give a solution containing 100 microgram/ml(5).
Selection of analytical concentration range:
From the standard stock solution of febuxostat appropriate amount of aliquots were pipetted out in 10 ml volumetric flask and dilution were made with the DMF to obtain the working standard solutions of concentration from 2 – 10 micrograms/ml. The absorbance were measured at 317nm(6).
Calibration curve for the Febuxostat (2 – 10micrograms/ml):
Appropriate volumes aliquots were transferred to different volumetric flask of capacity 10 ml from the standard stock solution. The volume was adjusted to 10 ml with DMF to obtain the concentration of 2, 4, 6, 8, 10, micrograms/ml. Absorbance spectra of each solution were measured against DMF as a blank at the wavelength 317nm. The regression equation and correlation coefficient were determined and presented(7).
Validation of the spectroscopic method:
Linearity and Range:
The linearity of a analytical method is its ability to produce results that are directly proportional to the concentration of analyte in the sample within a given range. Analytical method's range encompasses the separation of upper and lower levels of analyte that are accounted for within a reasonable range of Precision, Accuracy or Linearity(8).
Precision:
The precision of an analytical method is the degree of agreement among individual test results, when a analytical method is applied repeatedly to multiple samplings. It shows an indication of random error results and expressed as % relative standard deviation (% RSD). The % RSD value should be less than the 2%(9).
Intra and Inter-day precision:
Variations of results within the same day (intra – day), variation of results between days (inter – day) were analyzed. Intra – day precision was determined by analyzing Febuxostat for 6 times in the same day at different interval of time at 317 nm. Inter – day precision was determined by analyzing daily once for six days at 317nm. The % RSD was calculated and should be less than the 2%(10).
Ruggedness:
The dilutions were prepared and analyzed with change in the analytical condition like different laboratory conditions and different analyst and reported.
Robustness:
Robustness studies assumed that the small changes in any of the variables does not significantly affect the results(11). The robustness of the method is done by making small changes in the method like changing the wavelength(12)(13).
Accuracy:
Accuracy of the method was evaluated with the help of percentage recovery and standard deviation (SD). The three concentration of the drug ( 40%, 60%, 80%) were spiked individually and % recovery was calculated(14)(15).
LOD and LOQ:
LOD is the lowest detectable concentration of the analyte by the method and LOQ is the minimum quantifiable concentration(7). The LOD and LOQ was calculated by using the formula LOD = 3.3 ?/s and LOQ was calculated by using the formula LOQ = 10 ?/s(16).
RESULT AND DISCUSSION:
Linearity and range:
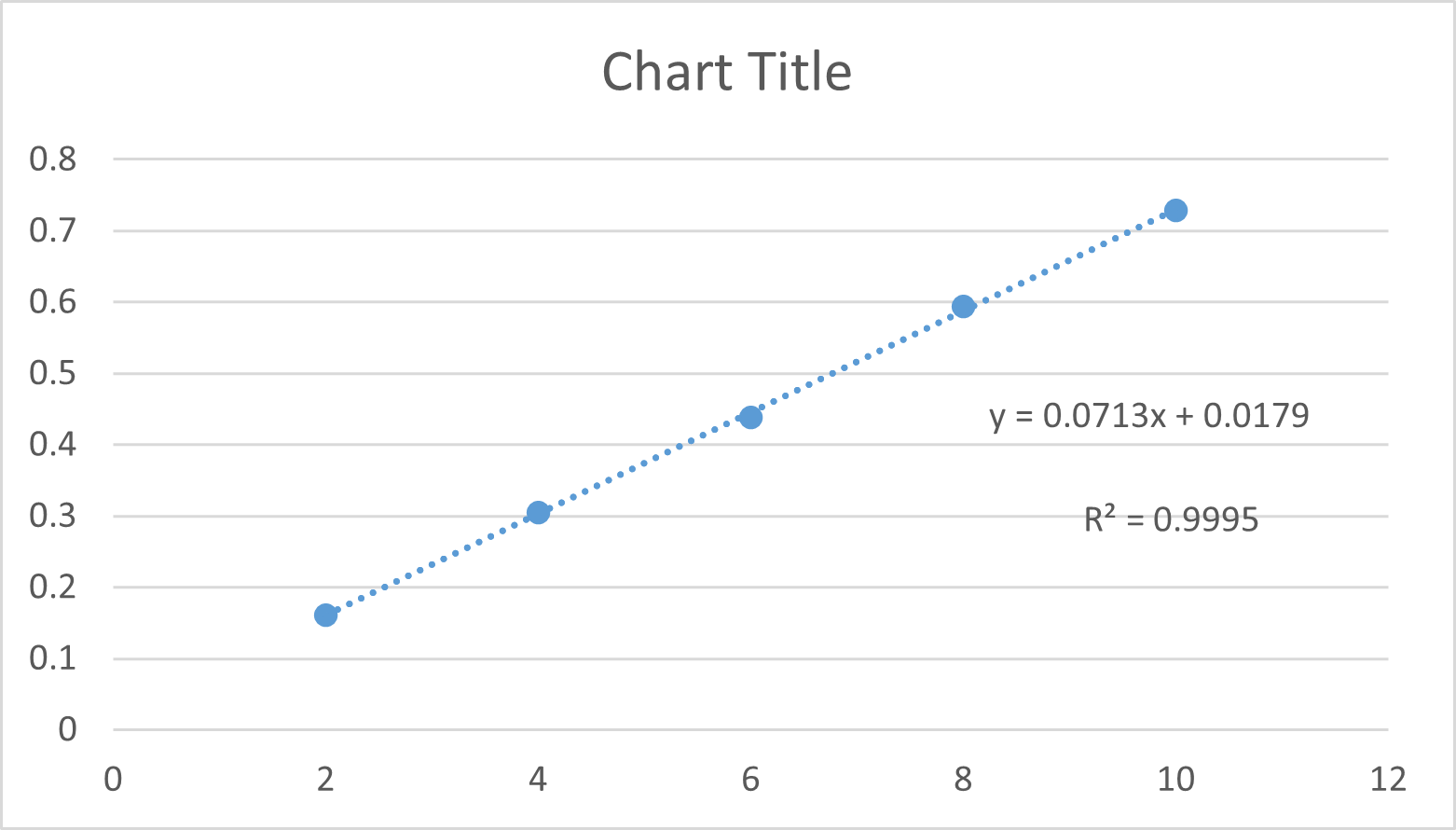
Febuxostat exhibits its maximum absorption at 317 nm and obeyed beer’s law in the range of 2-10 microgram/ml concentration. The linear regression of absorption vs concentration yield equation y=0.0713x + 0.0179 with a correlation coefficient of 0.9995. The results are summarized in Table 1.
Linearity curve for the febuxostat at 317 nm by spectroscopic method


 Akhil Sharma *
Akhil Sharma *






 10.5281/zenodo.10967854
10.5281/zenodo.10967854