Abstract
Alzheimer's disease (AD) remains one of the most challenging neurodegenerative disorders, affecting millions worldwide. Despite significant advances in understanding its pathology, effective treatment and early diagnosis remain elusive. This review explores the latest therapeutic approaches, including targeted drug therapies, immunotherapies, and novel interventions like gene editing and neurostimulation. Additionally, the review delves into emerging diagnostic tools, such as advanced imaging techniques, biomarkers, and AI-driven diagnostics, which are pushing the boundaries of early detection. By examining these advancements alongside the ongoing challenges in clinical application, this paper highlights the potential future prospects in Alzheimer's research. The synthesis of current knowledge aims to provide a comprehensive understanding of the landscape of Alzheimer's treatment and diagnostics, emphasizing the need for continued innovation and interdisciplinary collaboration to combat this devastating disease.
Keywords
Neurodegenerative disorders, Gene editing, Emerging therapies, Immunotherapies, Therapeutic approaches, Alzheimer's disease, Diagnostic innovations
Introduction
Alzheimer's disease (AD) is the most common cause of dementia, characterized by progressive cognitive decline, memory loss, and a myriad of neuropsychiatric symptoms. First described over a century ago, AD has since emerged as a significant public health concern, affecting over 55 million people globally. [1] As the aging population grows, the incidence of Alzheimer's is expected to rise dramatically, placing an increasing burden on healthcare systems worldwide.
DEFINITION:
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by the gradual decline in cognitive function, memory, and behavioral abilities, ultimately leading to a loss of independence in daily activities. It is the most common cause of dementia, accounting for approximately 60-80% of all dementia cases [2].
ADVANTAGES AND DISADVANTAGES
Advantages:
- Symptomatic Relief
- Targeted Therapies
- Research and Development
Disadvantages:
- High Cost and Accessibility
- Limited Efficacy
- Side Effects
ANATOMY AND PHYSIOLOGY OF ALZHIMER’S DISEASE

Alzheimer's disease (AD) is a complex neurodegenerative disorder that primarily affects the brain's anatomy and physiology, leading to progressive cognitive decline and memory impairment.[3] Understanding the anatomical and physiological changes associated with Alzheimer's is crucial for grasping how the disease progresses and affects individuals.
Layers of Brain:
Meninges:
The meninges are three protective membranes that surround the brain and spinal cord, providing a barrier against physical impacts and infections.[4]
Cerebral Cortex:
The cerebral cortex is the outer layer of the brain, often referred to as the "gray matter" due to its appearance
Four layers of brain:
-
- Molecular Layer (Layer I)
- External Granular Layer (Layer II)
- External Pyramidal Layer (Layer III)
- Internal Granular Layer (Layer IV)
Brain stem:
The brainstem, consisting of the midbrain, pons, and medulla oblongata, is responsible for regulating vital functions such as heart rate, breathing, and blood pressure.[5]
Cerebellum:
The cerebellum is located at the back of the brain and is primarily responsible for coordinating movement, balance, and posture. It also plays a role in motor learning and cognitive functions
MECHANISM OF ALZHEMIER’S DISEASE
- Amyloid Beta (A?) Accumulation.
- Formation of Amyloid Plaques
- Hyperphosphorylation of Tau Protein
- Release of Pro-inflammatory Cytokines
- Impairment of Synaptic Transmission
Function of brain:
Thinking and Reasoning:
The brain processes information, enabling logical reasoning, problem-solving, and decision-making.
Memory:
The brain encodes, stores, and retrieves memories, allowing individuals to learn from past experiences.
Vision:
The occipital lobe processes visual information received from the eyes, allowing for the perception of light, color, and motion.
Coordination and Balance:
The cerebellum coordinates movements, ensuring smooth, balanced, and precise actions Whereas, exocrine activity releases water, urea & ammonia & skin secretes like sweat & pheromones, etc.
Emotion Processing:
The limbic system, including the amygdala and hippocampus.
Social Behaviour:
The brain is involved in social interactions, empathy, and understanding social cues, largely relying on the prefrontal cortex and limbic structures.
ALZHEMIER’S DISEASE DRUG DELIVERY SYSTEM
Nanoparticle-Based Drug Delivery:
Polymer Nanoparticles: Biodegradable polymeric nanoparticles can be designed to encapsulate therapeutic agents, allowing for controlled release. These nanoparticles can cross the blood-brain barrier (BBB) more effectively than larger molecules.[6]
1. Certain ligands (e.g., transferrin) can bind to receptors on the endothelial cells of the BBB, promoting transcytosis
2. This technique temporarily disrupts the BBB using ultrasound waves, allowing larger drug molecules or nanoparticles to enter the brain
3. Administering drugs via the nasal route allows for direct access to the CNS, bypassing the BBB.
Drug-:
These drugs increase the levels of acetylcholine, a neurotransmitter that is deficient in Alzheimer's patients Donepezil (Aricept), Rivastigmine (Exelon), Galantamine (Razadyne) [7].
Permeation Enhancer:
Permeation enhancers are substances that facilitate the transport of drugs across biological barriers, such as the skin or the blood-brain barrier (BBB). [8] They are particularly important in drug delivery systems for conditions like Alzheimer's disease, where effective brain penetration is crucial for therapeutic efficacy. Here’s an overview of the types of permeation enhancers, their mechanisms, and applications:
Properties of permeation enhancer:
- Chemical Permeation Enhancers
- Physical Permeation Enhancers
- Biological Permeation Enhancers
- Peptides and Proteins
- Nanocarriers
- Odorless, colorless, economical & cosmetically acceptable
Other excipients:
Excipients are inactive substances that serve as vehicles or carriers for active pharmaceutical ingredients (APIs) in drug formulations. They play various roles in drug delivery systems, including enhancing stability, bioavailability, and patient acceptability. [9] Here’s an overview of some common excipients and their functions:
Fillers (Diluents):
Microcrystalline cellulose
Binders:
Polyvinylpyrrolidone (PVP)
Disintegrants:
Sodium starch glycolate
Preservatives:
Parabens (e.g., methylparaben, propylparaben)
Excipients play a crucial role in pharmaceutical formulations by enhancing the performance, stability, and acceptability of drug products. Their selection and optimization are essential for developing effective drug delivery systems tailored to specific therapeutic needs, including those for diseases like Alzheimer’s. Understanding the functions and properties of different excipients is vital for pharmaceutical scientists in formulating safe and effective medications.
Diagnosis:
The diagnosis of Alzheimer's disease (AD) involves a comprehensive evaluation that includes clinical assessments, cognitive testing, medical history, and various diagnostic tools.
- Clinical Evaluation
- Cognitive Testing
- Physical and Neurological Examination
- Diagnostic Imaging
- Biomarker Testing
- Differential Diagnosis
Diagnosing Alzheimer’s disease is a multifaceted process that requires a careful and thorough assessment by healthcare professionals. Early and accurate diagnosis is crucial for effective management and treatment planning, as it allows for timely intervention and support for patients and their families. Ongoing research continues to refine diagnostic criteria and develop new tools to enhance the accuracy of Alzheimer’s disease diagnosis.
ROUTE OF DRUG PENETRATION ACROSS BBB:
The blood-brain barrier (BBB) is a highly selective permeability barrier that protects the brain from potentially harmful substances while allowing essential nutrients to pass through. The delivery of drugs to the central nervous system (CNS) is a significant challenge due to this barrier. Various routes of drug penetration across the BBB have been identified, including the following: Via sweat gland [10].
- Paracellular Route
- Transcellular Route
- Endocytosis and Transcytosis
- Carrier-Mediated Transport
The delivery of drugs across the blood-brain barrier remains a significant challenge in treating central nervous system disorders. Understanding the various routes of drug penetration can aid in the development of more effective drug delivery systems. Ongoing research is focused on improving drug design and delivery methods to enhance brain penetration and therapeutic outcomes.
ALZHEIMER'S DISEASE TREATMENT METHODS:
Alzheimer’s disease (AD) treatment methods encompass a variety of approaches aimed at managing symptoms, slowing disease progression, and improving quality of life for patients and their caregivers. Here’s a detailed overview of current treatment methods for Alzheimer’s disease [11]
1.Pharmacological Treatments
A. Cholinesterase Inhibitors
B. NMDA Receptor Antagonists.
2. Non-Pharmacological Treatments
A. Cognitive Stimulation Therapy (CST)
B. Reminiscence Therapy
C. Occupational Therapy
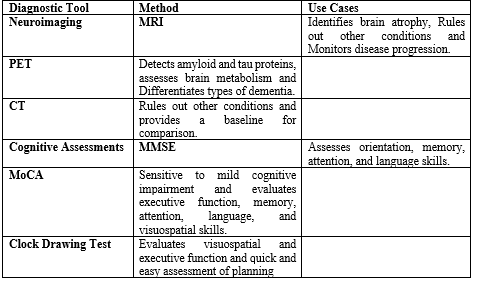
Diagnostic tool of AD:
The diagnosis of Alzheimer’s disease (AD) relies on a combination of clinical evaluations, cognitive assessments, medical history, and various diagnostic tools. While there is no definitive test for AD, several diagnostic tools are commonly used to aid in the assessment of the disease.
- Clinical Dementia Rating Scale (CDR)
- Mini-Mental State Examination (MMSE)
- Montreal Cognitive Assessment (MoCA)
- Magnetic Resonance Imaging (MRI)
- Computed Tomography (CT)
- Positron Emission Tomography (PET)
- Cerebrospinal Fluid (CSF) Analysis
- Blood Tests
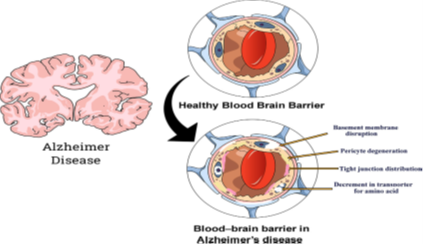
Blood-brain barrier: (A) healthy blood-brain barrier; (B) blood-brain barrier in case of Alzheimer’s disease
FORMULATION OF ALZHEMIER’S DISEASE
Formulating treatments for Alzheimer’s disease (AD) involves creating pharmaceutical products that effectively deliver therapeutic agents to manage symptoms, slow disease progression, and improve the quality of life for patients. Below is an overview of key considerations and approaches in the formulation of Alzheimer’s disease treatments:[12]
A. Cholinesterase Inhibitors
Examples:
Donepezil, Rivastigmine, Galantamine.
Mechanism:
These drugs inhibit the enzyme acetylcholinesterase, which breaks down acetylcholine, thereby increasing its levels in the brain to improve cognitive function.
Formulation Considerations:
Dosage Forms:
Tablets, oral solutions, and transdermal patches (e.g., Rivastigmine patch).
B. NMDA Receptor Antagonists
Example:
Memantine.
Mechanism:
Regulates glutamate activity to prevent excitotoxicity, which contributes to neurodegeneration.
Formulation Considerations:
Dosage Forms:
Extended-release formulations to maintain stable drug levels over time.
Bioavailability:
Enhancing solubility and absorption.
C. Disease-Modifying Therapies
Examples:
Aducanumab, Lecanemab.
Mechanism:
Target amyloid plaques and tau tangles associated with Alzheimer's pathology.
Formulation Considerations Administration Route:
Intravenous infusion for monoclonal antibodies.
Stability and Storage:
Ensuring proper storage conditions to maintain efficacy.
D. Dosage Forms
Oral Dosage Forms:
Tablets, capsules, and oral solutions for cholinesterase inhibitors and memantine.
Controlled-release formulations to ensure consistent drug levels.
Transdermal Patches:
For drugs like Rivastigmine to provide continuous drug delivery over an extended period.
Injectable Formulations:
Intravenous formulations for monoclonal antibodies targeting amyloid plaques.[13]
The formulation of Alzheimer's disease treatments is a complex process that requires careful consideration of drug properties, delivery methods, and patient needs. Ongoing research into innovative formulation strategies continues to improve the effectiveness of treatments and enhance the quality of life for individuals affected by Alzheimer’s disease. Collaboration between pharmaceutical scientists, clinicians, and researchers is essential to develop safe and effective therapies for this challenging condition.
FUTURE DIRECTIONS IN ALZHEIMER'S DISEASE TREATMENT:
Future directions in Alzheimer’s disease (AD) treatment focus on developing more effective therapies, improving early diagnosis, and understanding the underlying mechanisms of the disease. Here are some key areas of research and potential advancements in the treatment of Alzheimer’s disease:[14]
1.Disease-Modifying Therapies
Targeting Amyloid and Tau Proteins: Continued development of monoclonal antibodies and small molecules aimed at reducing amyloid-beta plaques and tau tangles in the brain is a significant focus. New therapies may offer improved efficacy and safety profiles.[15]
Combination Therapies:
Exploring the synergistic effects of combining existing treatments (e.g., cholinesterase inhibitors with disease-modifying agents) to enhance overall therapeutic outcomes. Personalized Medicine
2.Biomarker-Driven Approaches:
Utilizing biomarkers for early diagnosis and patient stratification can help tailor treatments to individual patients based on their unique biological profiles. This includes genetic testing and analysis of cerebrospinal fluid (CSF) and blood biomarkers.
Genetic Targeting:
Advances in genetic research may lead to therapies targeting specific genetic risk factors (e.g., APOE ?4 allele) and personalizing treatment strategies.
3.Neuroinflammation Modulation
Targeting Inflammatory Pathways:
Research into the role of neuroinflammation in Alzheimer’s disease has gained traction. Future therapies may aim to modulate inflammatory responses in the brain to slow disease progression.
4.Neuroprotective Agents
Development of Neuroprotective Compounds:
Research into compounds that can protect neurons from damage and promote neuronal health, potentially preventing or slowing the onset of Alzheimer’s disease.
5.Enhancing Drug Delivery Systems
Nanoparticle and Liposome Technologies:
Innovative drug delivery systems that improve the permeability of therapeutic agents across the blood-brain barrier (BBB) are being explored. This includes the use of nanoparticles and liposomes to enhance drug stability and bioavailability.
6. Cognitive Rehabilitation and Non-Pharmacological Interventions
Cognitive Training and Rehabilitation: Research into cognitive training programs and interventions designed to enhance cognitive function and improve quality of life for patients may become integral to comprehensive treatment plans.[16]
7. New Diagnostic Tools
Advanced Imaging Techniques:
Development of more sophisticated imaging techniques (e.g., PET scans with novel tracers) for earlier detection of Alzheimer's disease and monitoring treatment responses.
Blood Biomarkers:
Continued research into identifying blood-based biomarkers for early detection and monitoring of disease progression, potentially leading to easier and more accessible diagnostic methods.
8.Clinical Trials and Research Initiatives
Expanded Clinical Trials:
Encouraging participation in clinical trials for novel therapies and interventions to gather data on efficacy and safety, ultimately leading to more treatment options.[18]
Collaborative Research Efforts:
Increased collaboration between academia, industry, and regulatory bodies to expedite the development of new treatments and facilitate their approval.[17]
9.Digital Health Technologies
Telemedicine and Remote Monitoring:
The use of digital health technologies to enhance patient care, including remote cognitive assessments and telehealth services for improved access to care.
Artificial Intelligence:
Leveraging AI and machine learning to analyse data from clinical trials, predict patient outcomes, and identify potential therapeutic targets.[19]
NOTE:
The future of Alzheimer’s disease treatment lies in a multi-faceted approach that combines advancements in drug development, personalized medicine, non-pharmacological interventions, and innovative diagnostic tools. [20] Continued research and collaboration will be crucial in developing effective therapies to manage and ultimately prevent Alzheimer’s disease, improving the quality of life for patients and their caregivers
CONCLUSION:
Alzheimer's disease (AD) remains a significant global health challenge, affecting millions of individuals and their families. The complexity of the disease necessitates a comprehensive approach to treatment and management, incorporating advancements in both pharmacological and non-pharmacological interventions. Emerging therapies targeting key pathological features, such as amyloid-beta plaques and tau tangles, offer promising avenues for modifying the disease's progression and improving cognitive function..
REFERENCES
- Selkoe, D. J., & Hardy, J. (2016). The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Molecular Medicine, 8(6), 595-608.
- Alzheimer's Association. (2023). 2023 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 19(4), 434-490.
- Cummings, J., Lee, G., Ritter, A., Sabbagh, M., & Zhong, K. (2020). Alzheimer's disease drug development pipeline: 2020. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 6(1), e12050.
- Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., ... & Silverberg, N. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia, 14(4), 535-562.
- Hampel, H., O'Bryant, S. E., Castrillo, J. I., Ritchie, C., Rojkova, K., Broich, K., ... & Benda, N. (2018). Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nature Reviews Neurology, 14(11), 639-652.
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., ... & Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413-446.
- Mullard, A. (2021). Landmark Alzheimer's drug approval confounds research community. Nature, 594(7863), 309-310.
- Teipel, S. J., Grothe, M. J., Zhou, J., Sepulcre, J., Dyrba, M., Chiesa, P. A., ... & Alzheimer's Disease Neuroimaging Initiative. (2016). Measuring cortical connectivity in Alzheimer's disease as a brain neural network pathology: Toward clinical applications. Journal of International Neuropsychological Society, 22(2), 138-163.
- Katsuno, M., Tanaka, F., Sobue, G. (2022). Clinical trials of emerging disease-modifying therapies for Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry, 93(8), 873-879
- Sperling, R. A., Mormino, E. C., & Johnson, K. A. (2014). The evolution of preclinical Alzheimer's disease: Implications for prevention trials. Neuron, 84(3), 608-622.
- Querfurth, H. W., & LaFerla, F. M. (2010). Alzheimer's disease. New England Journal of Medicine, 362(4), 329-344.
- Karran, E., & Walker, L. (2013). The amyloid cascade hypothesis: Are we poised for success? Nature Reviews Drug Discovery, 12(10), 751-752.
- Holmes, C., et al. (2008). Systemic inflammation and disease progression in Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 79(1), 66-70.
- Wang, Y., & Mandelkow, E. M. (2016). Tau in physiology and pathology. Nature Reviews Neuroscience, 17(1), 22-35.
- Zhao, Y., & Huo, Y. (2018). Alzheimer's disease: A review of the current concepts and therapeutic options. Journal of Alzheimer's Disease, 66(1), 15-30.
- De Strooper, B., & Karran, E. (2016). The cellular phase of Alzheimer's disease. Cell, 164(4), 603-615.
- Hardy, J., & Higgins, G. A. (1992). Alzheimer's disease: The amyloid cascade hypothesis. Science, 256(5054), 184-185.
- Selkoe, D. J. (2001). Alzheimer's disease is a synaptic failure. Science, 298(5594), 789-791.
- Feng, X., & Wang, H. (2020). Nanoparticle-based drug delivery systems for the treatment of Alzheimer’s disease: An update. Frontiers in Neuroscience, 14, 284.
- Huang, Y., & Liu, Y. (2021). Intranasal drug delivery for Alzheimer’s disease: A promising approach for therapeutic development. Frontiers in Aging Neuroscience, 13, 675141.


 Sushma*
Sushma*
 Vikas Kumar Srivastava
Vikas Kumar Srivastava



 10.5281/zenodo.13346603
10.5281/zenodo.13346603