Abstract
Historical background of epilepsy The word epilepsy is derived from Greek word Epilambane in and means to seizure upon or to taking hold of or to take over. Epilepsy is a chronic neurological disorder, with a prevalence of about 1%, which is characterized by the recurrent appearance of spontaneous seizures due to neuronal hyperactivity in the brain (Dell;1986) Whitman S, 01 Definition of epilepsy Epilepsy is a chronic neurological disorder, with a prevalence of about 1%, which is characterized by the recurrent appearance of spontaneous seizures due to neuronal hyperactivity in the brain In 2005, a Task Force of the International League against Epilepsy (ILAE) formulated conceptual and operational definitions of “seizure” and “epilepsy” Conceptual Definition of Seizure and Epilepsy – 2005 Report An epileptic seizure is a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain. Epilepsy is a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures, and by the neurobiological, cognitive, psychological, and social consequences of this condition. The definition of epilepsy requires the occurrence of at least one epileptic seizure.
Keywords
epilepsy, GABA Areceptors, tonic, clonic, tonic- clonic, myoclonic.
Introduction
The Changes in ionic concentrations observed during hyperexcitation—increased extracellular K+or decreased extracellular Ca2+, for example—may be caused by decreases in extracellular size or volume. Failure of Na-K- pumps due to hypoxia or ischemia is known to promote epileptogenesis in animal models, and interference with Cl--K+ transport, which controls intracellular Cland regulates GABA-activated inhibitory Cl currents, may lead to enhanced excitation. Excitability of synaptic terminals depends on the extent of depolarization and the amount of neurotransmitter released. Synchronization following abnormal bursts of spikes in the axonal branching of thalamocortical relay cells plays a key role in epileptogenesis (Engelborghset al; 2010) Synaptic Mechanisms: Synaptic pathophysiology of epilepsy and epileptic disorders primarily involves reduced GABAergic inhibitionor enhanced glutamatergic excitation. 2-5
Gaba
GABA levels have been shown to be reduced in the cerebrospinal fluid (CSF) of patients with certain kinds of epilepsy, such as infantile spasms and untreated generalized tonic-clonic seizures, and in excised epileptic tissue from patients with drug-resistant epilepsy, suggesting that these patients have decreased inhibition.
Dogs with epilepsy have been shown to have low CSF levels of GABA, and mice genetically susceptible to audiogenic seizures have a lower number of GABA receptors than non-seizure.
- MATERIAL AND METHODS:
-
- Animals:
Swiss albino mice weighing 18-22 gm were purchased from Global Bioresearch Solutions Private Limited, H No 251 Nhavi, Tal - Bhor, Dist- Pune, Pune. The animals were housed in polypropylene cages and maintained under the environmental condition of temperature 25±1 ºC and relative humidity of 45-55 % under a 12h light: 12 dark cycles. The animals had free access to food pellets (Nav Maharashtra Chakan oil mills Ltd., Pune) and water ad libitum. The Institutional Animal Ethics Committee (IAEC) of Loknete shri dadapatil Pharate College of pharmacy, Mandavgan pharata approved all the experimental protocols under the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA). The protocol approval number is 2168/PO/Re/S/22/CPCSEA.
Chemicals:
|
Name of
chemical
|
specification
|
Manufacturer’
s name
|
quantity
purchased
|
batch
number
|
storage
conditions
|
|
Kaempferol
|
I.P.
|
Sigma-Aldrich
Chemicals Private Limited
|
5 gm
|
60010-
25G
|
2-8 ºC
|
|
Phenytoin
|
I.P.
|
5 gm
|
57-41-0
|
2-8 ºC
|
|
Potassium
hydroxide
|
I.P.
|
Merck Specialities Pvt. Ltd., Mumbai, India
|
500 gm
|
MH9M
591251
|
R.T.
|
|
Potassium
chloride
|
I.P.
|
500 gm
|
ML9M5
93064
|
R.T.
|
|
Folin phenol
reagent
|
I.P.
|
100 ml
|
AK0A6
00984
|
R.T.
|
|
Chloroform
|
I.P.
|
2.5 lit
|
ll1lf615
35
|
R.T.
|
|
Acetic acid
|
I.P.
|
500 ml
|
AD4A5
40152
|
R.T.
|
|
EDTA
|
I.P.
|
100 gm
|
QC2Q6
20407
|
R.T.
|
|
Sodium
chloride
|
I.P.
|
|
500 g
|
ML9M5
93000
|
R.T.
|
|
Sodium
Phosphate (Dibasic)
|
I.P.
|
Himedia Lab. Pvt. Ltd., Mumbai-
400 806, India
|
500 gm
|
T- 835005
|
R.T.
|
|
Adenosine
triphosphate
|
I.P.
|
5 gm
|
000006
4674
|
R.T.
|
|
Tris Free
Base
|
I.P.
|
100 gm
|
MB029
|
R.T.
|
|
Boric Acid
|
I.P.
|
100 gm
|
MB007
|
R.T.
|
|
Epinephrine
|
I.P.
|
5 gm
|
000006
6488
|
R.T
|
|
Tris HCl
|
I.P.
|
100 gm
|
000004
9048
|
R.T.
|
|
Adenosinetr
iphosphate
|
I.P.
|
5 gm
|
000006
4674
|
R.T
|
|
Strychnine
|
I.P.
|
5 gm
|
S0532-
5G
|
2-8 ºC
|
|
Sulphanilam
ide
|
I.P.
|
LobaChemi Pvt. Ltd., Mumbai – 400 005
|
100 gm
|
GM012
210
|
R.T
|
|
Phosphoric
acid
|
I.P.
|
500 ml
|
LG0120
10
|
R.T
|
|
Naphthalam ine Diamine
HCl
|
I.P.
|
10 gm
|
LB2245 09
|
R.T
|
|
Magnesium
sulphate
|
I.P.
|
500 gm
|
v
209205
|
R.T
|
|
Sodium
carbonate
|
I.P.
|
500 gm
|
A
283807
|
R.T
|
|
Sodium
|
I.P.
|
500 gm
|
A
|
R.T
|
|
pottasium
tartrate
|
|
|
|
566809
|
|
|
Formaldehy
de
|
I.P.
|
500 ml
|
LB
241809
|
R.T
|
|
Ammonium
molybdate
|
I.P.
|
100 gm
|
SL2947
1205
|
R.T.
|
|
Pottasium dihydrogen O-
phosphate
|
I.P.
|
500 g
|
GB 276911
09
|
R.T.
|
|
Potassium dihydrogen orthophosph
ate
|
I.P.
|
500 gm
|
GB2769 1109
|
R.T.
|
|
Methanol
|
I.P.
|
Molychem B-9, MIDC industrial area, Badlapur, dist Thane 421 503, India
Research lab fine Mumbai 400(002), India
|
2.5 lit
|
MCRT-
5162
|
R.T.
|
|
Sodium sulphite
|
I.P.
|
500 g
|
014250
90612
|
R.T.
|
|
Hydrochlori
c acid
|
I.P.
|
MP
Biomedicals India Private Limited, India
|
AS003
|
500 gm
|
R.T
|
|
Sodium
hydroxide
|
I.P.
|
---
|
500 gm
|
R.T
|
|
Copper
sulphate
|
I.P.
|
PCT0104-
500G
|
500 gm
|
R.T.
|
|
Sulphuric
acid
|
I.P.
|
AS016
|
500 ml
|
R.T
|
|
O –
Pthalaldehy de
|
I.P.
|
|
---
|
5 gm
|
R.T
|
|
Ninhydrin
|
I.P.
|
491200010
|
10 gm
|
R.T
|
|
n-Heptane
|
I.P.
|
3B Black Bio Biotech India Ltd.
|
3B1159
|
2.5 lit
|
R.T
|
|
n-butanol
|
I.P.
|
3B1102
|
2.5 lit
|
R.T
|
|
thiobarbituri
c acid
|
I.P.
|
3B1154
|
100 gm
|
R.T
|
|
Trichloroace
tic
|
I.P.
|
3B1155
|
100 gm
|
R.T
|
|
Sucrose
|
I.P.
|
Fisher scientific
Powai, Mumbai
|
500 gm
|
1043/1
|
R.T.
|
|
Sodium
bicarbonate
|
I.P.
|
Analab fine chemicals Mumbai - 400083 (India)
|
500 gm
|
3094
6502-1
|
R.T.
|
|
Sodium
metabisulph ite
|
I.P.
|
500 gm
|
|
R.T
|
|
Name of equipment
|
Model and make
|
Manufacturer’s
name
|
Address, city,
country
|
|
Spectrofluorometer
|
Jasco F-8200
|
JASCO Benelux B.V.
|
Veldzigt 2a, 3454
PW de Meern
|
|
UV Spectrophotometer
|
V-630
Sr. No. B157261148
|
Jasco
|
Japan
|
|
Centrifuge
|
Remi RC4 Lab.
Centrifuge
|
Remi Motors Ltd.
|
Mumbai – 400
058, India
|
|
Animal weighing
electronic balance
|
CB-220
|
Contech Instruments
Co.
|
Delhi
|
|
Chemical weighing
balance
|
AB-204-S,
Metler Tolledo
|
Classic made
|
Switzerland
|
|
Tissue Homogenizer
|
RQ-127A
|
Remi Equipment Pvt.
Ltd.
|
Mumbai, India
|
|
Actophotometer
|
MSW-013
|
Mohit Scientific
Works
|
Ambala, Haryana,
India
|
1.1.4.Preparation of drug solution, storage, volume, and route of administration:
1.1.4.1.Kaempferol:
?Preparation of test drug solution:
Drug solution of Kaempferol was prepared by using distilled water a vehicle
?Storage of drug solution:
Kaempferol powder was stored in a desiccator. A fresh drug solution was prepared for each day’s work. The solution was kept in airtight amber-colored bottles and stored at room temperature until ready for use.
?The volume of drug administration:
The volume of Kaempferol solution to be administered was calculated based upon the body weight of animals.
?Route of administration:
Kaempferol solution was administered per oral (p.o.) route.
1.1.4.2.Phenytoin:
?Preparation of standard drug solution:
Solution of Phenytoin was prepared with 1% Sodium-carboxy methylcellulose as the vehicle.
?Storage of drug solution:
Phenytoin powder was stored in a refrigerator below 25 ºC. A fresh drug solution was prepared for each day’s work.
?The volume of drug administration:
The volume of Phenytoin solution to be administered was calculated based upon the body weight of animals.
?Route of administration:
Phenytoin solution was administered through per oral (p.o.) route.
1.1.5.STR-induced convulsions in laboratory animals:
1.1.5.1.Experimental designs:
The animals were divided randomly into groups with six mice per group as follows:
?Group I: Normal group
The mice received only vehicle (Distilled water).
?Group II: STR control
The mice receive STR (5 mg/kg, i.p.) and only vehicle (Distilled water, 10 mg/kg)
?Group III: Phenytoin (25) group
The mice have received STR (5 mg/kg, i.p.). They were pre-treated with Phenytoin at a dose of 25 mg/kg, p.o., for 7 days.
Group IV: Kaempferol (25) group
The mice have received STR (5 mg/kg, i.p.). They were pre-treated with Kaempferol at a low dose of 25 mg/kg, p.o for 7 days.
?Group V: Kaempferol (50) group
The mice have received STR (5 mg/kg, i.p.). They were pre-treated with Kaempferol at a medium dose of 50 mg/kg, p.o for 7 days.
?Group VI: Kaempferol (100) group
The mice have received STR (5 mg/kg, i.p.). They were pre-treated with Kaempferol at a high dose of 100 mg/kg, p.o for 7 days.
1.1.5.2. Induction of STR-induced convulsions:
?Mice were divided into various groups as mentioned above.
?On 0 day all the behavioural parameter were evaluated before drug administration from 1 to 7 day all animal except normal.
?Pre-treatment was given to all the treatment groups with Kaempferol (25, 50, and 100 mg/kg) and Phenytoin (25 mg/kg) daily for 7 days.
?On 7th days convulsions were induced by administration of STR (5 mg/kg, i.p.) 45 min after drug treatment.
?Onset of convulsion, duration of convulsion was observed was observed post STR administration.
?Post behavioral parameter assessment mice were sacrificed and brain was removed immediately for biochemical analysis. 7-9
1.1.5.3.Treatment of Kaempferol and Phenytoin:
Kaempferol (25, 50, and 100 mg/kg) and Phenytoin (25 mg/kg) with different calculated doses based on the animal’s body weight were administered per oral for 7 days.
The observations were recorded on various days in the morning, and doses were administrated immediately afterward.
1.2.Parameter for assessment of the effect of Kaempferol on STR-induced convulsions in mice:
1.2.1.In-vivo parameters:
1.2.1.1.Body weight
?Mice were weighed daily using animal weighing balance.
1.2.1.2.Determination of tonic-clonic convulsions
?The convulsive behavior of each mice for onset and duration of clonic and tonic seizures was observed for 30 min for signs of neurological deficits, especially hind- limb tonic seizures or convulsions, and the resultant seizures were scored as follows:
- unresponsiveness = 0
- mild contractions = 1
- clonic seizures = 2
- tonic seizures = 3 (forelimb and then hindlimb rigidly extended to rear)
- death = 4
?Mice experiencing lethal convulsions were excluded from the study. Mice that exhibited at least three consecutive stage 4 or stage 5 seizures were considered convulsed, and used in this study
1.2.1.3.Locomotor activity:
?The animal locomotor behavior was monitored using Actophotometer. Actophotometer provided with a digital counter, photocell and a light source were used to measure locomotor activity (horizontal movement) of animals.
?Each animal was placed in Actophotometer for 5 minutes and basal activity score was recorded for all animals.
?Each animal was treated with respective drug and activity score was recorded after 30 min and 1hr.
?Deceased activity score was taken as index of CNS depression. 10-14
•Results
-
- Effect of kaempferol on body weight:
|
Body weight (gm) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin
(25 mg/kg)
|
Kaempferol
(25 mg/kg)
|
Kaempferol
(50 mg/kg)
|
Kaempferol
(100 mg/kg)
|
|
20.67 ±
1.12
|
21.67 ±
0.71
|
20.50 ± 0.67
|
21.17 ± 0.54
|
21.67 ± 1.12
|
19.00 ± 0.97
|

Fig. No. 7.1. Graphical representation of effect of kaempferol on body weight

Effect of kaempferol on onset and duration of convulsion in STR-induced epilepsy:
|
Parameter
|
Onset and duration of convulsion Mean ± SEM
|
|
Nor
mal
|
STR
control
|
Phenytoin
(25 mg/kg)
|
Kaempferol
(25 mg/kg)
|
Kaempferol
(50 mg/kg)
|
Kaempferol
(100 mg/kg)
|
|
Onset of
convulsion
|
---
|
3.33 ±
0.80
|
44.67 ±
0.80
|
12.67 ± 0.56
|
24.50 ± 0.56
|
38.33 ± 0.67
|
|
Duration of
clonic
|
---
|
155.67
± 4.75
|
54.83 ±
4.06
|
153.67 ±
5.05
|
116.33 ±
5.52
|
103.00 ±
4.60
|
|
Duration of
tonic
|
---
|
117.33
± 4.43
|
22.67 ±
3.50
|
103.67 ±
3.22
|
73.83 ± 4.09
|
26.67 ± 3.89
|

Fig. No. 7.2. Graphical representation of effect of kaempferol on onset and duration of convulsion in STR-induced epilepsy.
Data were analyzed by one-way ANOVA followed by Dunnett’s test **P < 0>
-
- Effect of kaempferol on locomotor activity during STR-induced post-ictal depression:
|
Locomotor activity (Counts / 5 mins) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol (50 mg/kg)
|
Kaempferol (100 mg/kg)
|
|
531.70 ±
3.70
|
--
|
60.00 ± 5.83
|
423.80 ±
8.33
|
347.80 ± 6.99
|
180.80 ±
6.33
|

Fig. No. 7.3. Graphical representation of effect of kaempferol on locomotor activity during STR-induced post-ictal depression
Data were analyzed by one-way ANOVA followed by Dunnett’s test. **P < 0>
1.3.Effect of kaempferol on STR-induced alteration in brain noradrenaline levels:
|
Brain NA (ng/g of brain tissue) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol (50 mg/kg)
|
Kaempferol (100 mg/kg)
|
|
21.02 ±
0.42
|
5.25 ±
0.63
|
14.89 ± 0.77
|
6.46 ± 0.78
|
10.85 ± 0.47
|
12.30 ± 0.86
|

Fig. No. 7.4. Graphical representation of effect of kaempferol on STR-induced alteration in brain NA levels
Data were analyzed by one-way ANOVA followed by Dunnett’s test. ###P < 0>P < 0>P < 0>
brain NA levels compared to STR control rats. Phenytoin (25 mg/kg, p.o.) treatment also showed significantly (P < 0>
Effect of kaempferol on STR-induced alteration in brain dopamine levels:
|
Brain DA (ng/g of brain tissue) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol (50 mg/kg)
|
Kaempferol (100 mg/kg)
|
|
72.93 ±
2.28
|
46.58 ±
1.65
|
59.16 ± 2.26
|
49.06 ± 0.91
|
51.95 ± 0.97
|
57.63 ± 2.09
|

Fig. No. 7.5. Graphical representation of effect of kaempferol on STR-induced alteration in brain DA levels (25 mg/kg, p.o.)


significantly elevated (P < 0>P < 0>
Effect of kaempferol on STR-induced alteration in brain total protein level:
|
Brain Total protein (mg/gm) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol (50 mg/kg)
|
Kaempferol (100 mg/kg)
|
|
3.65 ±
0.36
|
11.60 ±
0.28
|
5.28 ± 0.29
|
10.91 ± 0.37
|
8.67 ± 0.35
|
6.08 ± 0.44
|
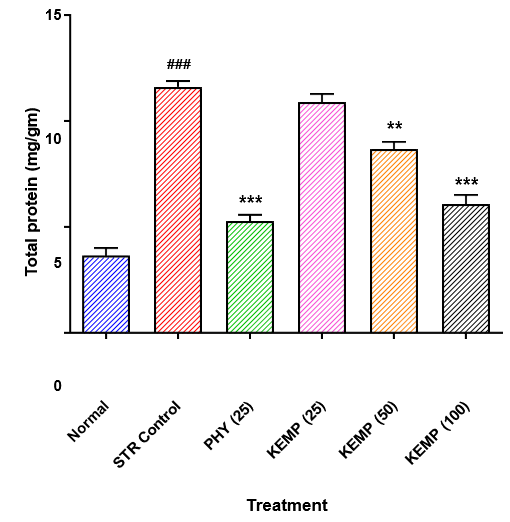
Fig. No. 7.6. Graphical representation of effect of kaempferol on STR-induced alteration in
Compared with normal rats, STR control rats show a significant increase (P < 0>
1.6.Effect of kaempferol on STR-induced alteration in brain SOD and GSH level:
|
Parameter
|
Brain SOD (U /mg of protein) and GSH ?g/mg of protein) levels Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin
(25 mg/kg)
|
Kaempferol
(25 mg/kg)
|
Kaempferol
(50 mg/kg)
|
Kaempferol
(100 mg/kg)
|
|
SOD
|
|
|
|
|
|
|
|
(U/mg of
protein)
|
14.48 ±
0.37
|
4.94 ±
0.38
|
10.68 ± 0.40
|
5.00 ± 0.40
|
8.37 ± 0.41
|
9.97 ± 0.36
|
|
GSH
|
|
|
|
|
|
|
|
(?g/mg
of
|
2.61 ±
0.25
|
0.51 ±
0.24
|
2.05 ± 0.29
|
0.94 ± 0.24
|
1.59 ± 0.28
|
1.70 ± 0.29
|
|
protein)
|
|
|
|
|
|
|

Data were analyzed by one-way ANOVA followed by Dunnett’s test. ###P < 0>
There was a significant decrease (P < 0>
1.7. Effect of kaempferol on STR-induced alteration in brain MDA and nitric oxide level:
|
Parameter
|
Brain MDA (nM/mg of protein), nitric oxide (?g/mL) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol(50mg/kg)
|
Kaempfer ol
(100
mg/kg)
|
|
MDA
|
|
|
|
|
|
|
|
(nM/m
|
|
|
|
|
|
|
|
g of
protein)
|
2.93 ±
0.36
|
9.30 ±
0.24
|
4.30 ± 0.17
|
7.63 ± 0.25
|
5.93 ± 0.21
|
5.20 ± 0.30
|
|
|
|
|
|
|
|
|
|
Nitric
|
|
|
|
|
|
|
|
oxide
(?g/m
|
0.158 ±
0.007
|
0.268 ±
0.004
|
0.165 ± 0.003
|
0.252 ±
0.005
|
0.225 ± 0.006
|
0.187 ±
0.005
|
|
L)
|
|
|
|
|
|
|

Fig. No. 7.8. Graphical representation of effect of kaempferol on STR-induced alteration in brain MDA and NO levels
Data were analyzed by one-way ANOVA followed by Dunnett’s test. ###P < 0>
1.8.Effect of kaempferol on STR-induced alteration in brain Na-K-ATPase level:
|
Na-K-ATPase level (?mol/mg of protein) Mean ± SEM
|
|
Normal
|
STR
control
|
Phenytoin (25 mg/kg)
|
Kaempferol (25 mg/kg)
|
Kaempferol (50 mg/kg)
|
Kaempferol (100 mg/kg)
|
|
20.60 ±
0.44
|
2.56 ±
0.4
|
14.51 ± 0.65
|
4.49 ± 0.59
|
10.18 ± 0.54
|
12.92 ± 0.57
|

Fig. 7.9. Effect of kaempferol on STR-induced alteration in brain Na-K-ATPase level
Data were analyzed by one-way ANOVA followed by Dunnett’s test. ###P < 0>P < 0>P < 0>P < 0>P < 0>P < 0>P < 0> 18
•SUMMARY AND CONCLUSION:
Kaempferol treatment demonstrated significant effects on various neurochemical parameters in the study. Notably, brain noradrenaline (NA) levels increased significantly, while dopamine (DA) levels also showed a significant rise at higher doses. Additionally, kaempferol enhanced brain antioxidant activity, with substantial increases in superoxide dismutase (SOD) and glutathione (GSH) levels. Furthermore, these treatments effectively reduced the onset and duration of convulsions induced by STR, as well as significantly decreasing brain total protein, malondialdehyde (MDA), and nitric oxide levels. Overall, kaempferol exhibited promising neuroprotective and anticonvulsant properties, highlighting its potential therapeutic benefits. 19-20
REFERENCES
- Dell J L. Social dimensions of epilepsy: stigma and response. In: Hermann BP, eds. Psychopathology in epilepsy: social dimensions 1986: 185-210.
- Acharya J N. Recent advances in epileptogenesis. CurrSci 2002; 82:10
- Fisher R S, Van W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia2005; 46:470-2
- Frucht M M, Quigg M, Schwaner C, Fountain N B. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia41 (12): 1534–1539.
- Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia 1999; 40:631–6.
- Shorvon S. The etiologic classification of epilepsy.Epilepsia 2011; 52(6):1052–1057
- Fisher R S, Van W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia2005; 46:470-2
- Engelborghs S, Hooge R, Deyn P. Pathophysiology of epilepsy. Acta neurol. Belg2000; 100 (4), 201-213
- Rogawski M A. Astrocytes get in the act in epilepsy. Nat Med 2005; 11: 919-20.
- Rogawski M A, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci 2004; 5: 553-64.
- Rogawski M A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr 2011; 11:56-63
- Ghani A. Changes in ?-aminobutyric acid during different stages of picrotoxininduced seizure, and the effect of pretreatment with ?-acetylenic GABA and phenobarbital. J Biosci 1989; 14 (1): 63–67.
- Sirven J, Noe K. Antiepileptic Drugs 2012: Recent Advances and Trends. Mayo Clin Proc. 2012; 87(9): 879- 889.
- Das A. Review on Nutritional, Medicinal and Pharmacological Properties of Centella asiatica (Indian pennywort).Journal of Biologically Active Products from Nature 2013; 1(4):216 – 228.
- Mamtha B, Kavitha K, Srinivasan K., Shivananda. P. An in vitro study of the effect of Centella asiatica [Indian pennywort] on enteric pathogens. Indian J. of pharmacology 2004; 36 (1): 41.
- Oyedeji O and Afolayan A. Chemical composition and anti bacterial activity of the essential oil of Centella asiatica growing in South Africa. Pharmaceutical Biology 2005; 43(3): 249-252.
- Namara J. Emerging insights into the genesis of epilepsy.NATURE 1999; 399 (24):15-22
- McNamara J. Kindling: an animal model of complex partial epilepsy. Annals of neurology 1984; 16: 72-76.
- Stanley N. Phytochemical analysis of Centella asiatica 2011; http//www.Nina Stanley
/eHow.com
Mostafa RM, Moustafa YM, Mirghani Z. Thymoquinone alone or in combination with Phenobarbital reduces the seizure score and the oxidative burden in pentylenetetrazole-kindled rats. Oxid Antioxid Med Sci 2012; 1(3): 185-19


 Kantode Dipti*
Kantode Dipti*
 Kavitake Dhiraj
Kavitake Dhiraj










 10.5281/zenodo.14409093
10.5281/zenodo.14409093