Abstract
Enzyme-linked immunosorbent measure (ELISA) is broadly utilized as symptomatic devices in pharmaceutical and as quality control measures in different businesses; it has too utilized as expository devices in biomedical investigate for the discovery and measurement of particular antigens or antibodies in a given test. These method share comparable essential standards and are inferred from the radioimmunoassay (RIA). RIA was to begin with depicted by Berson and Yalow (Yalow and Berson, 1960), for which Yalow was granted the Nobel Prize in 1977, to degree endogenous plasma affront. RIA was at that point created into a novel procedure to distinguish and degree natural atoms show in exceptionally little amounts, clearing the way for the investigation and discovery of incalculable other organic particles, counting hormones, peptides, and proteins. Since of the security concern with respect to its utilize of radioactivity, RIA measures were adjusted by supplanting the radioisotope with an protein, in this way making the modern-day ELISA.
Keywords
ELISA, pharmacological screening, enzyme-linked immunosorbent assay, drug discovery.
Introduction
ELISA
- In spite of the fact that the fundamental rule of ELISA and radioimmunoassay (RIA) strategies dates back to 1941 [1], RIA strategy was to begin with utilized by Yalow and Berson in 1960s to degree the endogenous plasma affront level [2]. In truth, ELISA strategy was concocted simulta neously by two investigate groups at the same time [3,4]. In any case, ELISA strategy was spearheaded to a great extent by the Swiss researchers Engvall, and Perlmann who kicked the bucket in 2005 [3]. These two analysts devel oped the ELISA strategy in 1971 by adjusting the RIA strategy [3]. In other words, they formulated the immunological ELISA strategy by conjugating the labeled antigen and counter acting agent radioisotopes in RIA with proteins instead of radioactive iodine 125. They utilized this unused strategy to determine the levels of IgG in rabbit serum [3]. Within the same year, a distinctive investigate group succeeded in evaluate ing human chorionic gonadotropin sums within the pee by utilizing horseradish peroxidase (EC 1.11.17) enzyme with the EIA strategy [4]
- How does the ELISA strategy work?
The antigen utilized within the ELISA strategy is bound to a strong stage. Tubes and microplates made of unbending polystyrene, polyvinyl and polypropylene are utilized as the strong stage. The microplates utilized must be able to appropriately adsorb the antigen and the hostile to body, but not adsorb the components within the other stages [3,5]. The proteins that can be utilized in ELISA incorporate beta galac tosidase, glucose oxidase, peroxidase, and antacid phosphatase. Antacid phosphatase can be put away at 4?C with its conjugate sodium azide. Antacid phosphatase and P-nitro-phenyl phosphate are utilized as substrates, are accessible in secure tablet shapes, and deliver a yellow color in positive responses. For the peroxidase conjugate, 5 amino salicylic corrosive and orthophenylenediamine are utilized as the substrates and the generation of a brown color is considered a positive response. In the event that beta galactosidase is utilized, the test must be studied in a fluorometer. The catabolic impacts of proteins decide both the speeding up and the specificity of the immunological response amid the enzyme-substrate response [6]. The enzyme-substrate response is usually completed inside 30–60 min. The response can be halted utilizing sodium hydrox ide (NaOH), hydrochloric corrosive (HCl) or sulfuric corrosive (H2SO4) [7]. The comes about are read on a spectrophotometer and at 400–600 nm depending on the characteristics of the conjugate utilized
1. Coordinate ELISA (antigen screening)
2. Circuitous ELISA
3. Sandwich ELISA (counter acting agent screening)
4. Competitive ELISA (antigen/antibody screening)
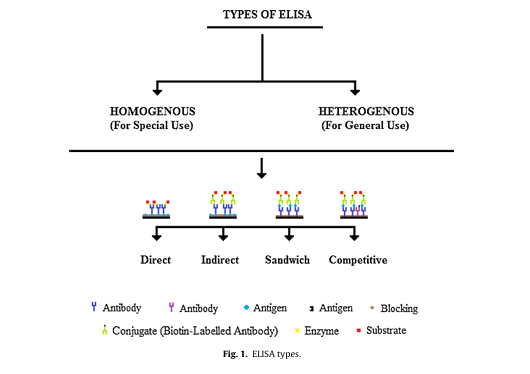
- Focal points and drawbacks of ELISA strategy :
In later a long time, ELISA test has been utilized exceptionally commonly in get up and go tide and protein examinations [8]. Usually a delicate and specific test that quickly produces comes about. It incorporates a wide region of application due to its ease of utilize and speed [8]. Other than, it is more commonsense as there's no got to think about two serum tests. The ELISA test is nearly as sen sitive as RIA and does not require extraordinary gear or radioactive names. Be that as it may, its unwavering quality is moo, in comparison to RIA [9]. The points of interest and contrasts of ELISA tests are presented in Table 1.

Protein immunoassays (EIAs) utilize the catalytic properties of proteins to distinguish and measure immunologic responses. Enzyme-linked immunosorbent test (ELISA) could be a heterogeneous EIA strategy utilized in clinical analyses.[10] In this sort of test, one of the response components is nonspecifically adsorbed or covalently bound to the surface of a strong stage, such as a microtiter well, a attractive molecule, or a plastic globule. This connection encourages the partition of bound and free-labeled reactants.[11] Within the most common approach to utilizing the ELISA procedure, an aliquot of test or calibrator containing the antigen (Ag) to be evaluated is included to and permitted to tie with a solid-phase counter acting agent (Ab). After washing, an enzyme-labeled antibody is included and shapes a “sandwich complex” of solid-phase Ab-Ag-Ab enzyme. Unbound counter acting agent is at that point washed absent, and protein substrate is included. The sum of item produced is corresponding to the quantity of antigen in the sample.[12] Particular antibodies in a test can moreover be measured utilizing an ELISA method in which antigen rather than counter acting agent is bound to a strong stage. The moment reagent is an enzyme-labeled counter acting agent particular to the analyte counter acting agent. In expansion, ELISA tests have been utilized broadly to distinguish antibodies to infections and autoantigens in serum or entire blood. In expansion, protein conjugates coupled with substrates that deliver obvious items have been utilized to create ELISA-type tests with comes about that can be translated outwardly. Such measures are exceptionally valuable in screening, point-of-care, and domestic testing applications.[13]
Etiology and The study of disease transmission
Two distinctive inquire about groups at the same time concocted the coordinate ELISA:
researchers Engvall and Perlman and researchers Van Weemen and Schuurs. The ELISA was created by the adjustment of the radioimmunoassay (RIA). This was done by conjugating labeled antigens and antibodies with chemicals instead of radioactive iodine 125. The unused strategy was to begin with utilized in deciding the levels of IgG in rabbit serum. Inside the same year, researchers evaluated human chorionic gonadotropin in pee utilizing horseradish peroxidase. Since at that point, the ELISA strategy has been utilized in numerous distinctive applications and has ended up a schedule research facility investigate and diagnostic method worldwide.[10]
The primary ELISA strategy included chromogenic columnist particles and substrates in creating perceptible color alter that monitors the nearness of antigen. Advance progression within the ELISA procedure driven to the advancement of fluorogenic, quantitative PCR, and electrochemiluminescent columnists to create signals.[14] In any case, a few of these procedures don't depend on utilizing enzyme-linked substrates but non-enzymatic columnists that utilize the rule of ELISA.[15] The most recent improvement, in 2012, was an ultrasensitive enzyme-based ELISA that controls nanoparticles as chromogenic correspondents. This strategy can produce a color flag obvious to the bare eye, with a blue color showing positive comes about and ruddy color showing negative comes about. Be that as it may, this strategy is subjective and can decide as it were the nearness or nonattendance of an analyte and not its concentration.[16]
Example Prerequisites and Strategy
ELISAs are performed in polystyrene plates, regularly 96-well plates coated to bind protein strongly.[11] Depending on the ELISA sort, testing requires a essential and/or auxiliary location counter acting agent, analyte/antigen, coating antibody/antigen, buffer, wash, and substrate/chromogen.[12] The essential location counter acting agent could be a particular counter acting agent that as it were ties to the protein of interest. In contrast, a auxiliary location counter acting agent may be a moment enzyme-conjugated counter acting agent that ties to a essential counter acting agent that is not enzyme-conjugated.[13]
There are four fundamental general steps to completing an ELISA immunoassay. These steps are:
1. Coating (with either antigen or counter acting agent)
2. Blocking (regularly with the expansion of bovine serum egg whites [BSA])
3. Location
4. Last examined
Location is carried out by including a substrate that can create a color. There are numerous substrates accessible for utilize in ELISA location. Be that as it may, the substrates most commonly utilized are horseradish peroxidase (HRP) and antacid phosphatase (AP).[12] The substrate for HRP is hydrogen peroxide, coming about in a blue color alter. AP measures the yellow color of nitrophenol after room temperature hatching periods of 15 to 30 minutes and ordinarily employments p-nitrophenyl-phosphate (pNPP) as its substrate.[11] Between each of the over four steps could be a “wash” of the plate employing a buffer, such as phosphate-buffered saline (PBS) and a non-ionic cleanser, to expel unbound fabric. The wells are washed two or more times amid each wash step, depending on the particular protocol.[13] In a usual ELISA convention, a serial weakening of concentrations is set within the wells of the plate. After the comes about are measured, a standard curve from the serial weakenings information is plotted with a concentration on the x-axis employing a log scale and absorbance on the y-axis employing a direct scale.[17]
- Coordinate ELISA
Both coordinate and backhanded ELISAs start with the coating of antigens to the ELISA plates.[19] The primary official step includes including antigens to the plates, which are brooded for one hour at 37 °C or can be hatched at 4 °C overnight. Once the brooding step is completed, the following step is to wash the plates of any potential unbound antigens and piece any unbound locales on the ELISA plate utilizing operators like BSA, ovalbumin, aprotinin, or other creature proteins.[11] This moment step is significant since it anticipates the authoritative of any non-specific antibodies to the plate and minimizes false-positive comes about. After including the buffer, the plate is rewashed, and a chosen enzyme-conjugated essential location counter acting agent is included. The plate is advance brooded for one hour.[18] In a coordinate ELISA, the essential discovery counter acting agent ties straightforwardly to the protein of interest.[11] Another, the plate is rewashed to evacuate any unbound antibodies. An protein, such as antacid phosphatase (AP) or horseradish peroxidase (HRP), is included to the plate, which comes about in a color alter. The color alter of the test happens by either the hydrolysis of phosphate bunches from the substrate by AP or by the oxidation of substrates by HRP.[18] The preferences of utilizing coordinate ELISA incorporate dispensing with auxiliary counter acting agent cross-reactivity, and due to less steps, it is fast compared to backhanded ELISA. Its impediments incorporate its moo affectability compared to the other sorts of ELISA and its tall fetched of reaction.[12]
- Backhanded ELISA
The steps of the circuitous ELISA are indistinguishable to the coordinate ELISA, but for an extra wash step and the sorts of antibodies included after the buffer is removed.[20] Roundabout ELISA requires two antibodies: a essential location counter acting agent that sticks to the protein of intrigued and a auxiliary enzyme-linked counter acting agent complementary to the essential antibody.[18] The essential counter acting agent is included to begin with, taken after by a wash step, and after that the enzyme-conjugated auxiliary counter acting agent is included and brooded. After this, the steps are the same as the coordinate ELISA, which incorporates a wash step, the expansion of substrate, and the location of a color change.[21] The roundabout ELISA contains a higher sensitivity when compared to the coordinate ELISA.[21] It is additionally less costly and more adaptable due to the numerous conceivable essential antibodies that can be utilized. The as it were major drawback of this sort of ELISA is the hazard of cross-reactivity between the auxiliary location antibodies.[14]
- Sandwich ELISA
Not at all like coordinate and backhanded ELISA, the sandwich ELISA starts with a capture counter acting agent coated onto the wells of the plate.[18] It is named a “sandwich since the antigens are sandwiched between two layers of antibodies (capture and area antibodies).[13] After counting the capture counter acting specialist to the plates, the plates are at that point secured and brooded overnight at 4 °C. Once the coating step is total, the plates are washed with PBS and after that buffered/blocked with BSA. The buffer washes are carried out at room temperature for at slightest 1 to 2 hours. At long last, the plate is washed with PBS once once more some time recently the antigen is added.[22] The antigen of intrigued is included to the plates to tie to the capture counter acting agent and brooded for 90 minutes at 37 °C. The plate is rewashed, and the essential discovery counter acting agent is included to the plate and hatched for another 1 to 2 hours at room temperature, taken after by a buffer wash. At that point the auxiliary enzyme-conjugated counter acting agent is included and brooded for another 1 to 2 hours. The plate is rewashed, and the substrate is included to convey a color change.[13] The sandwich ELISA has the foremost critical affectability among all the ELISA types.[22] The major impediments of this sort of ELISA are the time and cost and the vital utilize of “matched pair” (divalent/multivalent antigen) and auxiliary antibodies.[14]
- Competitive ELISA
The competitive ELISA tests for the nearness of an counter acting agent particular for antigens within the test serum.[23] This sort of ELISA utilizes two particular antibodies:
an enzyme-conjugated counter acting agent and another counter acting agent show within the test serum (in case the serum is positive). Combining the two antibodies into the wells will permit for competition for official to antigens. The nearness of a color alter implies that the test is negative since the enzyme-conjugated counter acting agent bound the antigens (not the antibodies of the test serum). The nonattendance of color demonstrates a positive test and the nearness of antibodies within the test serum.[14] The competitive ELISA contains a moo specificity and cannot be utilized in weaken tests. In any case, the benefits are that there's less test decontamination required, it can degree a huge run of antigens in a given test, it can be utilized for little antigens, and it has moo variability.[19]
Enzyme-linked immunosorbent tests are connected in numerous symptomatic tests.[12-14] A few of the employments of ELISA can incorporate the taking after:
Identify and Degree the Nearness of Antibodies within the Blood
• Autoantibodies (anti-dsDNA, anti-dsg1, ANA, etc.)
• Antibodies against irresistible infection (antibacterial, antiviral, antifungal)
• Hepatitis A, B, C, HIV, etc.
Identify and Appraise the Levels of Tumor Markers
• Prostate-specific antigen (PSA)
• Carcinoembryonic Antigen (CEA)
Identify and Gauge Hormone Levels
• Luteinizing hormone
• Follicular fortifying hormone
• Prolactin
• Testosterone
• Human chorionic gonadotropin (hCG)
Following Malady Flare-ups
• Cholera
• HIV
• Flu
Identifying Past Exposures
• HIV
• Lyme infection
• Hepatitis
Screening Given Blood for Conceivable Viral Contaminants
• Anti-HIV-1/2
• Anti-HCV
• HBsAg
Identifying Sedate Manhandle
• Amphetamine
• Methamphetamine
• 3,4-methylenedioxymethamphetamine
• Cocaine
• Benzoylecgonine
Interferometer Components
Variables that can meddled with suitable ELISA testing can happen at any stage of the testing prepare, starting with example collection. The quality and judgment of the measure plate, coating buffer, capture counter acting agent, blocking buffer, target antigen, discovery counter acting agent, protein conjugate, washes, substrate, and flag location can all meddled with appropriate ELISA testing.[10] A few of the components that can interfere with testing are the taking after:
• Plate Measure:
the shape and quality of the wells, the fabric of the plate, potential pre-activation, and indeed or uneven coating.[13]
• Buffer:
pH, contamination.[21]
• Capture and location counter acting agent:
hatching time, temperature, specificity, titer, affinity.[19]
• Blocking buffer:
cross-reactivity, concentration, contamination.[22]
• Target antigen:
compliance, soundness, epitopes.[14]
• Protein conjugate:
sort, concentration, work, cross-reactivity.[24]
• Washes:
defilement, recurrence, volume, term, composition.[10]
• Substrate:
quality/manufacturer.[13]
• Discovery:
instrument-dependent factors.[24]
• Reader/human error.[14]
Comes about, Detailing, and Basic Discoveries
Information assembled from ELISA tests can be quantitative, subjective, or semiquantitative.[10] The quantitative concentration comes about are plotted and compared to a standard bend. The subjective comes about affirm or deny the nearness of a specific antigen/antibody in a sample.[13] The semiquantitative comes about compare the concentrated of the signals, which can reach relative antigen levels in a sample.[24]
Once color changes are measured from the test, the comes about are charted either on paper or in software.[10] Regularly, the chart compares optical thickness to log concentration, which gives a sigmoidal bend. Known concentrations grant the graph's standard bend, and estimation of questions can at that point happen when test values are compared to the direct parcel of the charted standard curve.[23]
Clinical Importance
ELISAs can be utilized in numerous settings, counting quick counter acting agent screening tests for Human immunodeficiency infection (HIV), location of other infections, microbes, organisms, immune system maladies, nourishment allergens, blood writing, nearness of the pregnancy hormone hCG, research facility and clinical inquire about, measurable toxicology, and numerous other demonstrative settings.[25,26] In HIV testing, a blood or spit example is collected for testing, regularly utilizing roundabout ELISA-based tests.[15] The ELISA may be a screening instrument for HIV discovery, but not symptomatic. Determination requires advance testing by Western smudge due to potential untrue positives. Another infection, Molluscum contagiosum infection (MCV), which commonly contaminates the skin of children and youthful grown-ups, can be identified by ELISA testing.[27] ELISA testing in this setting is right now being assessed to survey worldwide MCV seroprevalence.[28] ELISA has too been utilized to identify desmogleins 1 and 3 and bullous pemphigoid antigen 180 autoantibodies ensnared in pemphigus and bullous pemphigoid immune system rankling infections, respectively.[29] In nourishment sensitivity, the advancement of the ELISA has played an imperative part in hypersensitivity inquire about and determination. Ultrasensitive ELISA varieties have been created to identify amounts of allergens on the scale of picograms. Usually vital since of the life-threatening part that nourishment sensitivities can have on a open wellbeing scale.[30]
Quality Control and Lab Security
As with quantitative methods, it is vital to confirm that the comes about of subjective and semi-quantitative examinations are adjust some time recently detailing them to the asking healthcare provider.[31] The research facility must build up a quality control program for all of its subjective and semi-quantitative tests.[32] When building up this program, set approaches, prepare staff, dole out duties, and guarantee all essential assets are accessible. Guarantee that the recording of all quality control information is total which the quality director and the research facility chief conduct an fitting audit of the information.[33]
Positive and negative controls are prescribed for numerous subjective and semi-quantitative tests, counting a few strategies that utilize extraordinary stains or reagents and tests with endpoints such as agglutination or color change.[34] These controls ought to for the most part be utilized with each test run.[35] The utilize of controls will too offer assistance approve a unused parcel number of test units or reagents, check on temperatures of capacity and testing areas, and assess the method when unused testing work force is carrying out the testing.[36] Keep the taking after in intellect when utilizing conventional controls for subjective or semi-quantitative tests:
test control materials within the same way as testing quiet tests; utilize a positive and negative control, ideally once each day of testing, or at slightest as regularly as prescribed by the producer; select positive controls that are near to the cut-off esteem of the test, to be beyond any doubt the test can distinguish frail positive responses; for agglutination strategies, incorporate a powerless positive control as well as a negative control and more grounded positive control.[32]
Fundamental security rules for research facility conduct ought to be watched at whatever point working in a laboratory.[37] Consider all examples, control materials, and calibrator materials as possibly irresistible. Work out the normal safety measures required for dealing with all research facility reagents.[38] Transfer of all squander fabric ought to be in understanding with neighborhood rules. Wear gloves, a lab coat, and security glasses when dealing with human blood examples. Put all plastic tips, test glasses, and gloves that come into contact with blood in a biohazard squander holder. Dispose of all expendable crystal into sharps squander containers.[39] Ensure all work surfaces with expendable permeable seat beat paper, disposed of into biohazard squander holders week by week or at whatever point blood defilement happens. Wipe all work surfaces week after week. All gear ought to be routinely assessed for wear or weakening. Gear ought to be kept up concurring to the manufacturer's prerequisites, and records of certification, support, or repairs ought to be kept up for the life of the equipment.[40] Computers and instrumented ought to be labeled to show whether gloves ought to be worn. Conflicting glove utilize around keyboards/keypads could be a source of potential defilement. Avoid wearing adornments within the lab, as this could posture different security hazards. Safety rules for laboratory-specific operations will be given in suitable research facility SOPs.[41]
CONCLUSION
ELISA may be a capable strategy not as it were for common biomedical inquire about but moreover as a symptomatic instrument. permits location of all sorts of organic atoms at exceptionally moo concentrations and amounts. In spite of the fact that it has its confinements, ELISA remains an critical apparatus in both clinical and essential inquire about, as well as in clinical diagnostics.
REFERENCES
- Coons AH, Creech HJ, Jones RN. Immunological properties of an anti body containing of fluorescent group. Pros Soc Exp Biol Med 1941;47: 200–2.
- Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest 1960;39:1157–75.
- Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quanti tative assay of immunoglobulin G. Immunochemistry 1971;8:871–4.
- Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett 1971;15:232–6
- Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest 1960;39:1157–75.
- Engvall E. The ELISA enzyme-linked immunosorbent assay. Clin Chem 2010;56:319–20.
- Hornbeck P, Enzyme-linked immunosorbent assays. Curr Protoc Immunol 2001, http://dx.doi.org/10.1002/0471142735.im0201s01 (Chapter 2: Unit 2.1).
- Engvall E. The ELISA enzyme-linked immunosorbent assay. Clin Chem 2010;56:319–20
- Revoltella RP, Laricchia Robbio L, Liedberg B. Comparison of conventional immunoassays (RIA, ELISA) with surface plasmon resonance for pesticide detection and monitoring. Biotherapy 1998;11:135–45.
- Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015 Oct:72():4-15. doi: 10.1016/j.peptides.2015.04.012. Epub 2015 Apr 20 [PubMed PMID: 25908411]
- Engvall E. The ELISA, enzyme-linked immunosorbent assay. Clinical chemistry. 2010 Feb:56(2):319-20. doi: 10.1373/clinchem.2009.127803. Epub 2009 Oct 22 [PubMed PMID: 19850633]Level 3 (low-level) evidence
- Shah K, Maghsoudlou P. Enzyme-linked immunosorbent assay (ELISA): the basics. British journal of hospital medicine (London, England : 2005). 2016 Jul:77(7):C98-101. doi: 10.12968/hmed.2016.77.7.C98. Epub [PubMed PMID: 27388394]
- Konstantinou GN. Enzyme-Linked Immunosorbent Assay (ELISA). Methods in molecular biology (Clifton, N.J.). 2017:1592():79-94. doi: 10.1007/978-1-4939-6925-8_7. Epub [PubMed PMID: 28315213]
- Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008 Aug:63(8):879-84 [PubMed PMID: 18772478]
- Gelkop S, Sobarzo A, Brangel P, Vincke C, Romão E, Fedida-Metula S, Strom N, Ataliba I, Mwiine FN, Ochwo S, Velazquez-Salinas L, McKendry RA, Muyldermans S, Lutwama JJ, Rieder E, Yavelsky V, Lobel L. The Development and Validation of a Novel Nanobody-Based Competitive ELISA for the Detection of Foot and Mouth Disease 3ABC Antibodies in Cattle. Frontiers in veterinary science. 2018:5():250. doi: 10.3389/fvets.2018.00250. Epub 2018 Oct 12 [PubMed PMID: 30370272]Level 1 (high-level) evidence
- de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nature nanotechnology. 2012 Dec:7(12):821-4. doi: 10.1038/nnano.2012.186. Epub 2012 Oct 28 [PubMed PMID: 23103935]
- Tabatabaei MS, Ahmed M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods in molecular biology (Clifton, N.J.). 2022:2508():115-134. doi: 10.1007/978-1-0716-2376-3_10. Epub [PubMed PMID: 35737237]
- Kohl TO, Ascoli CA. Direct Competitive Enzyme-Linked Immunosorbent Assay (ELISA). Cold Spring Harbor protocols. 2017 Jul 5:2017(7):pdb.prot093740. doi: 10.1101/pdb.prot093740. Epub 2017 Jul 5 [PubMed PMID: 28679705]
- Lin AV. Direct ELISA. Methods in molecular biology (Clifton, N.J.). 2015:1318():61-7. doi: 10.1007/978-1-4939-2742-5_6. Epub [PubMed PMID: 26160564]
- Lin AV. Indirect ELISA. Methods in molecular biology (Clifton, N.J.). 2015:1318():51-9. doi: 10.1007/978-1-4939-2742-5_5. Epub [PubMed PMID: 26160563]
- Kohl TO, Ascoli CA. Indirect Immunometric ELISA. Cold Spring Harbor protocols. 2017 May 1:2017(5):. doi: 10.1101/pdb.prot093708. Epub 2017 May 1 [PubMed PMID: 28461658]
- Kohl TO, Ascoli CA. Immunometric Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay. Cold Spring Harbor protocols. 2017 Jun 1:2017(6):pdb.prot093724. doi: 10.1101/pdb.prot093724. Epub 2017 Jun 1
- Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clinical chemistry. 2005 Dec:51(12):2415-8 [PubMed PMID: 16179424]
- Tighe PJ, Ryder RR, Todd I, Fairclough LC. ELISA in the multiplex era: potentials and pitfalls. Proteomics. Clinical applications. 2015 Apr:9(3-4):406-22. doi: 10.1002/prca.201400130. Epub 2015 Mar 25 [PubMed PMID: 25644123]
- Kuo HT, Yeh JZ, Wu PH, Jiang CM, Wu MC. Application of immunomagnetic particles to enzyme-linked immunosorbent assay (ELISA) for improvement of detection sensitivity of HCG. Journal of immunoassay & immunochemistry. 2012:33(4):377-87. doi: 10.1080/15321819.2012.655820. Epub [PubMed PMID: 22963487]
- Tiscione NB. The Validation of ELISA Screening According to SWGTOX Recommendations. Journal of analytical toxicology. 2018 Apr 1:42(3):e33-e34. doi: 10.1093/jat/bkx095. Epub [PubMed PMID: 29300967]
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2015 Jun 5:64(RR-03):1-137 [PubMed PMID: 26042815]
- Sherwani S, Chowdhury M, Bugert JJ. ELISA for Molluscum Contagiosum Virus. Current protocols in microbiology. 2017 Nov 9:47():14A.6.1-14A.6.9. doi: 10.1002/cpmc.42. Epub 2017 Nov 9 [PubMed PMID: 29120484]
- Atzori L, Deidda S, Aste N. Enzyme-linked immunosorbent assay in autoimmune blistering diseases: preliminary experience of the Dermatology Department of Cagliari. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2008 Feb:143(1):1-8 [PubMed PMID: 18833046]
- Weng X, Gaur G, Neethirajan S. Rapid Detection of Food Allergens by Microfluidics ELISA-Based Optical Sensor. Biosensors. 2016 Jun 7:6(2):24. doi: 10.3390/bios6020024. Epub 2016 Jun7 [PubMed PMID: 27338488]
- Kearney E. Internal quality control. Methods in molecular biology (Clifton, N.J.). 2013:1065():277-89. doi: 10.1007/978-1-62703-616-0_18. Epub [PubMed PMID: 23996371]Level 2 (mid-level) evidence
- Kinns H, Pitkin S, Housley D, Freedman DB. Internal quality control: best practice. Journal of clinical pathology. 2013 Dec:66(12):1027-32. doi: 10.1136/jclinpath-2013-201661. Epub 2013 Sep 26 [PubMed PMID: 24072731]Level 2 (mid-level) evidence
- Westgard JO. Internal quality control: planning and implementation strategies. Annals of clinical biochemistry. 2003 Nov:40(Pt 6):593-611 [PubMed PMID: 14629798]Level 2 (mid-level) evidence
- Dubey A, Sonker A. Implementation of internal quality control program for monitoring of enzyme-linked immunosorbent assay performance at a blood center. Asian journal of transfusion science. 2021 Jan-Jun:15(1):21-29. doi: 10.4103/ajts.AJTS_59_19. Epub 2021 Jun 12 [PubMed PMID: 34349453]Level 2 (mid-level) evidence
- Charting methods for internal quality control for competition ELISA. Methods in molecular biology (Clifton, N.J.). 2009:516():335-80. doi: 10.1007/978-1-60327-254-4_9. Epub [PubMed PMID: 19219591]Level 3 (low-level) evidence
- Internal quality control and external quality management of data in practice. Methods in molecular biology (Clifton, N.J.). 2009:516():517-41. doi: 10.1007/978-1-60327-254-4_13. Epub [PubMed PMID: 19219595]Level 3 (low-level) evidence
- Lo J. Biological safety in the medical laboratory. Hong Kong medical journal = Xianggang yi xue za zhi. 2015 Jun:21(3):200. doi: 10.12809/hkmj154581. Epub [PubMed PMID: 26045068]
- Klein RC, Party E, Gershey EL. Safety in the laboratory. Nature. 1989 Sep 28:341(6240):288 [PubMed PMID: 2797146]Level 3 (low-level) evidence
- Yücesan B, Özkan Ö. Laboratory Safety in Parasitology Laboratory. Turkiye parazitolojii dergisi. 2018 Jun:42(2):144-153. doi: 10.5152/tpd.2018.5598. Epub [PubMed PMID: 30070646]
- Rojo-Molinero E, Alados JC, de la Pedrosa EG, Leiva J, Pérez JL. [Safety in the Microbiology laboratory].
- Enfermedades infecciosas y microbiologia clinica. 2015 Jun-Jul:33(6):404-10. doi: 10.1016/j.eimc.2014.06.014. Epub 2014 Nov 8 [PubMed PMID: 25444041]Level 3 (low-level) evidence
- Vonesch N, Tomao P, Di Renzi S, Vita S, Signorini S. [Biosafety in laboratories concerning exposure to biological agents]. Giornale italiano di medicina del lavoro ed ergonomia. 2006 Oct-Dec:28(4):444-56 [PubMed PMID: 17380946]


 Harshwardhan Yesugade *
Harshwardhan Yesugade *
 Omkar Shelake
Omkar Shelake


 10.5281/zenodo.14404235
10.5281/zenodo.14404235