Abstract
Cadmium contributes to nephrotoxicity in humans and animals by inducing oxidative stress. The dataset demonstrates the in vivo renal histology and biochemical activity of Glucosamine (Chitosamine) in cadmium-induced nephrotoxic rat model. A total of 42 female albino Wistar rats weighing between 180 to 220 g were categorized into seven groups (n=6). Except for the first group (control), all of the groups received CdCl2 5mg/kg body weight per oral (p.o). Second-group is the negative control group (toxic, CdCl2 treated), the third and fourth received EDTA 495mg/kg, and Vitamins 50mg/kg (p.o) respectively. Glucosamine 0.2g/kg (p.o) was given to the fifth and sixth groups, whereas Vitamins 50mg/kg (p.o) were given to the sixth and seventh groups, respectively. Seventh group additionally received EDTA 495mg/kg (p.o). Kidney function parameters were estimated in serum and oxidative damage parameters and histopathology were estimated in kidney tissues. Cadmium chloride raised, blood urea nitrogen (BUN), creatinine (Cr), uric acid, urea and tissue malondialdehyde (MDA) levels while decreasing tissue superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) levels. Glucosamine administration significantly reduced lipid peroxidation, enhanced glutathione and antioxidant enzyme activities, and considerably lowered BUN and creatinine levels. Data were supported by histological examination indicated improved changes in kidney, indicating protective potential of Glucosamine in cadmium chloride-induced nephrotoxicity.
Keywords
Cadmium chloride, Glucosamine, Kidney injury, Oxidative stress, Proximal tubule, Renal toxicity.
Introduction
One of the prime threats to the ecosystem is the contamination caused by heavy metals.[1] Cd is commonly utilized in various industrial processes but is recognized as one of the toxic metals to the environment.[2] Occupational and environmental exposures are associated with industrial exposure. Smoking is a major source of exposure to Cd.[3] Cd mainly accumulates in the liver, bones, and other organs, causing irreversible damage. The kidneys are particularly more vulnerable to Cd poisoning, with reports indicating that almost half of the total body load of Cd accumulates in the epithelial cells of the proximal tubule, producing proteinuria and reabsorption problems.[4] The elimination half-life of cadmium is around 10-30 years. [5] The Occupational Safety and Health Administration considers a blood cadmium level of 5-10 µg/L of whole blood as hazardous, necessitating medical evacuation from workplace exposure.[6] The kidneys store approximately 60% of the total amount of Cd in the body. [7] Previous studies indicated that the absorption of Cd could potentially lead to oxidative stress. [8] In the biological system Cd produces both morphological and biochemical alterations. [3] The free ionic form of cadmium (Cd2+) in the proximal tubular region of the nephron can bind to renal metallothionein protein and induce further renal expression of metallothionein.[9] Proximal tubule dysfunction is an indicator of renal impairment triggered by chronic dietary exposure to Cd. [10] The specific mechanisms behind cadmium poisoning are unclear. Multiple studies have found that the generation of reactive oxygen species (ROS) and oxidative stress are important factors in the toxicity and carcinogenicity of cadmium. [11] Cd predominantly leads to oxidative phosphorylation and cellular respiration by interacting with mitochondria. [12] It produces ROS, which breaks single-strand DNA and interferes with protein and nucleic acid formation, resulting in cancer in numerous organs. Furthermore, it speeds up cell division, prevents DNA damage, and suppresses apoptosis. [12,13] In addition it binds with the sulfhydryl groups (SH) to proteins, depletes glutathione (GSH), and increases the production of reactive oxygen species (ROS) which includes hydroxyl radicals (OH.-), hydrogen peroxide (H2O2), and superoxide ions.[12] Cd affects cellular thiol redox balance which results in decreased intracellular glutathione content and reduced activities of cellular antioxidant enzymes (i.e. superoxide dismutase, peroxidase and catalase).[14] When cadmium is present in the human diet, it puts the kidneys at a higher risk of danger. [15] Cadmium is typically absorbed in its free ionic state (Cd?2;?) by passive diffusion or carrier-mediated transport. MTP1 and DMT1 are metal transporter proteins that help in absorption, along with calcium channels. When Cd?2;? penetrates the cells, it disturbs calcium homeostasis, increasing oxidative stress, suppressing autophagy, and causing cell death. It interacts with cytoplasmic components and metallothionein, though the latter doesn’t sequester most Cd?2;? due to its high affinity for calcium sites. Exposure to heavy metals causes calcium release from the endoplasmic cells reticulum, resulting in disrupted calcium distribution. [16,17,18] Chronic exposure to cadmium chloride (CdCl2) in rats can lead to morphological and metabolic alterations, resulting in elevated blood urea nitrogen (BUN) and creatinine levels in a dose-dependent manner. [19] Cd exposure results in increased malondialdehyde (MDA) peroxidation and decreased antioxidant markers such as catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx), and superoxide dismutase (SOD). [20,21] Glucosamine (Chitosamine) a derivative of chitosan with the chemical formula: C6H13NO5 and molecular weight: of 179.17, has been shown to possess anti-inflammatory [22], antioxidant [23], anticancer [24], antibacterial [25], and antitumor effects [26] in experimental study. Glucosamine (GlcN) is widely used orally both in Europe and in the US in an attempt to palliate the pain and disability of osteoarthritis (OA) and it is also a component of a large number of dietary supplements in the US. It has also gain great interest in the fitness and athletics communities because of claims that it has cartilage building and lubricant properties for the joints [27] Overall, the widespread emission of Cd has raised the risk of exposure in the working and general population and demanded further research to prevent health-related hazards. Although several experiments have indicated the antioxidant and anti-inflammatory potential of glucosamine, but few experimental studies have investigated and reported the protective effect of glucosamine on toxic metals in an animal model with renal failure. [28] As per our understanding, the protective role of glucosamine on Cadmium-induced nephrotoxicity in rats has not been demonstrated. Hence, the main goal of the current study was to investigate in vivo biochemical and histological changes induced by CdCl2 on the kidney of an adult rat model. We evaluated the possible role of glucosamine in protecting CdCl2-induced renal toxicity in rats and in ameliorating the renal oxidative stress biomarkers and histological changes in rats. Confirming the hypothesis that oxidative stress contributes to Cd intoxication and alteration in kidney tissue, glucosamine may shed light on the prevention of Cd poisoning.
MATERIALS AND METHODS:
Ethical consideration:
Prior to the experiment, we reviewed and ensured that the protocols were in accordance with the guidelines of animal acts proposed by the Committee for the Control and Supervision of Experimental Animals (CCSEA) guidelines, New Delhi, India and Institutional Animal Ethical Committee (IAEC) of Y.B. Chavan College of Pharmacy, Aurangabad (CCSEA/IAEC/P’cology/78/2023-24/190).
Experimental Animals:
We used adult Wistar female rats (n?=?42) weighing between 180 and 220?g. The animals were kept in metal cages under hygienic conditions and maintained at 22?±?2°C and 25 ± 5% R.H. with 12-h light-dark cycles with free access to food and water. Rats were acclimatized for two weeks as an adaptation period before treatment commenced.
Drugs and Chemicals:
Glucosamine was purchased from Research-lab fine chem industries, Mumbai Cadmium chloride was obtained from Research-lab fine chem industries, Mumbai. Biochemical markers were assessed using SOD, GSH, MDA at the institute. BUN, Serum Uric acid, Serum Urea, and Serum Creatinine were outsourced to diagnostic center. Chemicals purchased were of standard grade and purity for experimental testing.
Animal groupings and treatments:
Cadmium chloride (5mg/kg) was dissolved in distilled water and administered orally at a dosage of 2ml/kg. The amount of cadmium chloride used was based on animal toxicity data, which showed acute poisoning at 1-3 mg/kg body weight after intraperitoneal injection. [19] Glucosamine (dose of 0.2g/kg) and EDTA (dose of 495 mg/kg) dissolved in distilled water and administered by gastric tube (gastric gavages) at a volume of 2ml/kg. In the previous study at the dose of 0.2g/kg revealed a potential therapeutic effect against liver and kidney tissues. [28] A combination of vitamin C and E (dose of 50 mg/kg) was given in emulsion form using Tween 80 as surfactant. [29, 30] Cadmium chloride or its vehicle (normal saline) was administered 30 minutes before gavage delivery of therapy or its vehicle. Treatments occurred during the following timeline: Rats were treated with intra-gastric gavage of saline or Cadmium chloride for 10 weeks along with treatment at the interval of 30 minutes via oral route. On the last day of study the blood and tissue samples were collected for biochemical and histological analysis. The Wistar rats were divided randomly into seven groups of six animals each. Except for the control group, all groups were treated with CdCl2 for ten weeks. The first group of animals received saline solution. The second group received CdCl2 at a dosage of 5mg/kg/day by oral gavage. The third, fourth and fifth groups were co-treated with EDTA (495mg/kg/day), Vitamin C & E (50mg/kg/day) each and Glucosamine (0.2g/kg/day) respectively for the duration of 10 weeks via oral route. Group six had received a combination of glucosamine (0.2g/kg/day) with Vitamins C & E (50mg/kg/day) and EDTA (495mg/kg/day), and seventh group was treated with vitamins (50mg/kg/day) each. The co-treatments were given after 30 minutes of CdCl2 treatment. 24-hours after the last treatment, all rats were anaesthetized with diethyl ether. Blood samples were taken from the retro-orbital-plexus, and immediately thereafter the animals were sacrificed under ether anaesthesia, the kidneys were isolated, dissected and washed using cold saline solution. The kidney tissue were fixed in 10% formalin in saline solution and processed for histopathological sectioning and staining with haematoxylin and eosin (H&E). Other one kidney was used for the biochemical estimation of MDA, SOD, and GSH. Tissue homogenate was prepared immediately after isolation of kidneys to prevent the degradation of enzymes.

Figure 1: The time schedule for experimental treatments
Biochemical analysis
Data collected on cadmium chloride-induced nephrotoxicity by measuring Blood Urea Nitrogen (BUN), Urea, Uric acid and Creatinine (CR) levels in serum. The levels of MDA, SOD, CAT and GSH in kidney tissue were used to assess oxidative stress. Kidney tissue samples were washed with cold saline. The right kidneys were homogenized in phosphate buffered solution to measure tissue lipid peroxidation and antioxidative markers. To test oxidative stress indicators (SOD and CAT), kidney tissues were homogenized in phosphate buffered saline, centrifuged at 10,000 rpm for 20 minutes at 4°C, and stored at -20°C.[31] Blood samples were collected from the retro- orbital plexus of animals and the serum was obtained. Concentrations of MDA, GSH, CAT, and SOD in renal tissues were measured as per protocol. When homogenate is combined with trichloroacetic acid, it forms a reactive red complex called Thiobarbituric acid. The response of the produced red colour of the compound was determined at 540 nm using UV Visible Spectroscopy. This method determines the MDA level [32]. SOD activity in kidney tissue is determined based on the ability of SOD to inhibit spontaneous oxidation of epinephrine. [33] The test to determine GSH level in serum is based on the chemical interaction of GSH with DTNB, as Ellman's reagent, which produces the oxidized glutathione-TNB adduct (GS-TNB) and the TNB chromophore, with a peak absorbance of 412 nm. The amount of GSH in the sample correlates directly with the rate of TNB formation, which may be detected at 412 nm.[34] The protein level in supernatants was assayed by the Lowry’s method using standard bovine serum albumin at 680 nm.[35]
Histological examinations:
Following fixing removed kidney tissues in formalin, a 10 ?m section was taken from each tissue block. After sectioning and drying the slides, they were stained with haematoxylin and eosin (H&E) method. The criteria for cell injury included abnormal architecture renal cortex, medulla, renal papilla, renal tubules and glomerular tufts (nuclear dilation, loss of staining capacity) and obvious cellular swelling. Five histological fields were randomly picked on each slide, shot at 4×100 magnification, and reviewed by a pathologist and histologist acquainted with the method.
Statistical data analyses:
The data were analyzed using GraphPad Instat Prism Version 10.2 computer programs. At first, all of data were analyzed by one-way analysis of variance (ANOVA), followed by, Dunnett’s tests were performed to compare the means of all groups to the mean of every other group. Results were presented as mean ± SE and Probability value of P< 0>
RESULTS:
EFFECTS OF CdCl2 AND TREATMENTS ON ANTIOXIDANT ENZYMES
The levels of lipid peroxidation marker (MDA) and non-enzymatic antioxidants (GSH), enzymatic antioxidant enzymes (SOD and CAT) in the rat kidney are shown in Table 1.
Effects of CdCl2 and Treatments on kidney MDA level
The data is indicated as Mean ± SEM. No. of samples (n) =6. MDA level in the rat kidney treated with CdCl2 group increased significantly as compared to the control group A, Group B: CdCl2, 5 mg/ kg/day, ****P<0 P=0.0128, P=0.9560). P=0.0023. P=0.0016, P=0.0116,>
Effect on kidney Glutathione (GSH)
The data is indicated as Mean ± SEM. No. of samples (n) =6. The administration of CdCl2 significantly decreased GSH antioxidant compared to control values in kidney (Group B: CdCl2, 5 mg/kg/day, ****P<0>?P = 0.0437 and Group E: Glucosamine, 0.2 g/kg/day, **P = 0.0090). Co-treatment with combination with vitamins has shown more significant results than treatment alone (Group F: Glucosamine + Vitamins, 0.2 g/kg/day +50 mg/kg/day, **P=0.0016, Group G: EDTA + Vitamins, EDTA, 495 mg/kg/day + 50 mg/kg/day, *P=0.0110), While Vitamins alone had displayed non-significant result on GSH level (ns P=0.9951). (Figure 2b)
Effects on kidney Superoxide dismutase (SOD)
The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, SOD activity significantly lowered compared to the control group (****P< 0 xss=removed xss=removed P=0.0001, P=0.0155), P=0.8739).>
Effects on kidney Catalase (CAT)
The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, CAT activity significantly was decreased as compared to the control group (****P< 0 xss=removed xss=removed P=0.0014), P=0.9995).>
Table 1: The effect of Glucosamine, Vitamins, and EDTA on Oxidative stress parameters in Cadmium-induced Nephrotoxicity in rats
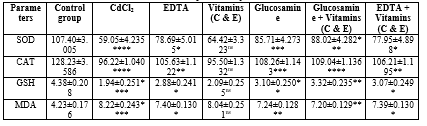
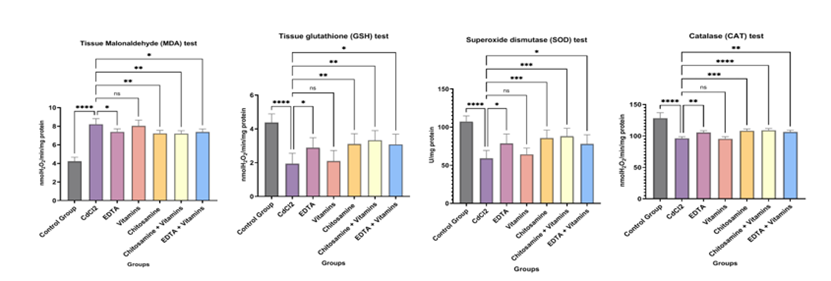
Figure 2: The effect of Glucosamine, Vitamins, and EDTA on Oxidative stress parameters in Cadmium-induced Nephrotoxicity in rats.
KIDNEY FUNCTION TEST:
The activities of treatments on kidney parameters, BUN, Serum Creatinine, Serum Urea and Serum Uric acid in the kidney of rats are shown in Table 2.
Table 2: The effect of Glucosamine, Vitamins, and EDTA on Biochemical Parameters (Kidney Function Test) in Cadmium-induced Nephrotoxicity in rats.

Effects on Serum level of BUN
In CdCl2 treated group a significant increase in BUN level was observed as compared to control depicted in Figure 3(c). The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, BUN activity significantly increased compared to the control group (****P< 0 P=0.0026 P=0.0001). P=0.0005), P=0.8788).>
Effects on Serum Creatinine
In CdCl2 treated group a significant increase in creatinine level was observed as compared to control is depicted Figure 3(c). The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, creatinine activity significantly increased compared to the control group (****P< 0 P=0.0378 P=0.0005). P=0.0014, P=0.0201), P=0.9540).>
Effects on Serum Uric Acid:
In CdCl2 treated group a significant increase in uric acid level was observed as compared to control depicted in Figure 3(c). The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, uric acid activity significantly increased compared to the control group (****P< 0 P=0.0001). P=0.0001), P=0.9660).>
Effects on Serum Urea:
In CdCl2 treated group a significant increase in serum urea level was observed as compared to control depicted in Figure 3(c). The data is indicated as Mean ± SEM. No. of samples (n) =6. In rats given CdCl2 at a dose of 5 mg/kg, serum urea activity significantly increased compared to the control group (****P<0 P=0.0350 P=0.0002). P=0.0007, P=0.0068), P=0.1958).>

Figure 3: The effect of Glucosamine, Vitamins, and EDA on Biochemical Parameters (Kidney Function Test) in Cadmium-induced Nephrotoxicity in rats.
Effects of CdCl2 and drug treatments on organ body weight ratio:
The data is indicated as Mean ± SEM. No. of samples (n) =6, as shown in Table 3. Organ/ body weight ratio in the rat kidney increased significantly in the group treated with CdCl2 compared to the control group A (Group B: CdCl2, 5 mg/ kg/day, ****P<0 P=0.0350, P=0.0108. P=0.0045, P=0.0323, nsP=0.9887.>
Table 3: The effect of Glucosamine, Vitamins, and EDTA on Kidney body weight ratio in Cadmium-induced Nephrotoxicity in rats.
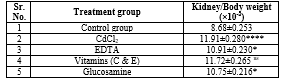
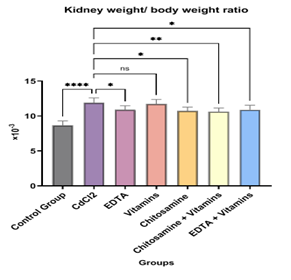
Figure 4: The effect of Glucosamine, Vitamins, and EDTA on Kidney body weight ratio in Cadmium-induced Nephrotoxicity in rats.
HISTOPATHOLOGICAL FINDINGS:
The histopathological findings of the experimental animals were highlighted in Figure 5, where the results reflect the normal parenchymal architecture of the kidney observed in the control group with its respective changes owing to heavy metal (Cadmium) intoxication as perceived in the following slides can be studied. ?
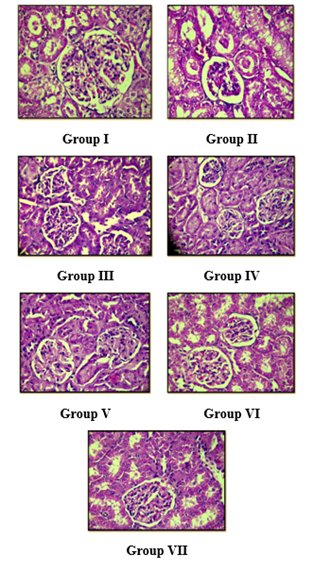
Figure 5: Photomicrographs of histopathological examination (H&E, 400x) of kidney tissue.
Group I:
Showed normal histology of kidney of the control group,
Group II:
Showed hypocellularity of glomeruli, oedematous cell, hydropic degeneration in tubular epithelial cell of kidney of the toxic group receiving merely Cadmium chloride (5 mg/kg).
Group III:
Showed glomerular cellularity is slightly higher though not normal, hydropic degeneration in tubular epithelial cell are less prominent as compared to CdCl2 treated group receiving EDTA (495 mg/kg) comparable to the parenchyma of control group.
Group IV:
Showed glomerular cellularity is higher, hydropic degeneration are less prominent as compared to CdCl2 treated group receiving treatment with (Vit E & Vit C 50mg/kg).
Group V:
Showed glomerular cellularity higher, hydropic degenerative changes in tubular epithelial lesser as compared to CdCl2 treated group receiving treatment with
(Glucosamine 0.2g/kg).
Group VI:
Showed normal glomerular cellularity, tubules are well demarked and no degenerative changes Group receiving co-treatment with (Glucosamine + Vit E & Vit C).
Group VII:
Showed normal glomerular cellularity with no degenerative changes and normal
demarked tubules as normal receiving co-treatment with (EDTA + Vit E & Vit C).
DISCUSSION
This in vivo experimental study examined the potential effect of glucosamine alone and in combination with vitamin C and E on CdCl2 induced nephrotoxicity in rats. Our results show that administration of CdCl2 significantly increased MDA levels and decreased the enzymatic and non-enzymatic antioxidants in the kidney of rats. From previous studies it was suggested that oxidative stress is the main mechanism for Cd toxicity. Previous studies have reported significantly increased MDA levels and decreased in GSH levels in the kidney due to CdCl2 administration in rats. [36,37] In addition, it was reported that CdCl2 (5 mg/kg/day) orally for 30 days significantly increased MDA, lowered activity of GSH, SOD, CAT and histopathological changes in the rat. [38] Our study suggest that CdCl2 administration resulted in the reduction of renal GSH and SOD levels as compared to the control group. These findings were in parallel with the study was done in people working in smelter factory indicated decreased plasma antioxidants enzymes and increased MDA. [39] This study also demonstrated that concurrent workplace exposure to cadmium might result in a remarkable increase in lipid peroxidation (MDA) and alter antioxidants enzymes (CAT, SOD) and oxidative stress. [39] In our study, the functional nephrotoxicity was indexed through BUN, urea and uric acid and creatinine levels, which were increased in CdCl2-treated rats as compared to control rats. Our results confirm the study revealed a link between Cd levels in urine and blood with exposure to Cd and chronic kidney disease. [40,41,42] Our findings also correlated with the results that suggested Cd (2 mg/kg BW) significantly increased BUN and creatinine compared to a control group CdCl2- induced oxidative stress in rat kidneys. [42] Previous research has found substantial histopathological alterations in the kidneys of CdCl2-treated rats. Our investigation found that CdCl2 exposure caused toxic histopathological damage, decreased glomerular function, and progressive renal failure in the kidney, which is consistent with previous findings. [38] In the present study, the treatment with glucosamine, standard drug EDTA and combinations of these drugs with vitamin C & E has improved kidney function, biochemical and histopathological parameters. In this study, glucosamine (0.2 g/kg/day) treatment has significantly improved the studied parameters. A study suggested treatment of Vitamin C (200 mg/kg) has protective effect on oxidative damage of testis. [43] Another study has reported treatment with Vitamin E (100mg/kg) orally for 30 days prior to cadmium chloride administration showed decreased lipid peroxidation. [44] In our study cotreatment with Vitamin E and C (50 mg/kg) orally has not shown any significant results on antioxidant level and kidney parameters. Whereas co-treatment of glucosamine (0.2g/kg) with Vitamins (50 mg/kg) has showed improved results on kidney as compared to alone. In this study it was found that administering CdCl2 in rats may cause nephrotoxicity. Histological investigation revealed significant changes in renal tissue structure after exposure to CdCl2 (5 mg/kg) (Figure 5(b)). Previous investigations have shown that CdCl2 (5 mg/kg b.w., orally for 4 weeks) causes glomerular damage, immediate dilatation of the Baumans capsule, congestion of renal blood vessels, and harm to glomerular epithelial cells in rats.[38] Our results indicated that cadmium-induced nephrotoxicity after treatment with glucosamine (0.2 gm/kg/day) significantly improved the levels of SOD, GSH, CAT in rat kidneys and significantly decreased tissue MDA, serum BUN, creatinine and Uric acid levels. Additionally, our findings suggest that the administration of glucosamine (0.2 gm/kg/day) might reduce cadmium-induced tissue damage. CONCLUSION
The chelation properties of natural chelator Glucosamine (Chitosamine) along with combined effect of antioxidants (Vitamin C and E) are mainly due to their ability to claw and complex with Cadmium ions and chain breaking ability to eradicate them out of the body harmlessly. This process of detoxification not only cleanses the body but also boosts the overall immune system to fight various ailments alongside rectifying renal impairment due to Cadmium toxicity.
ACKNOWLEDGEMENT:
The authors are thankful to the Management Maulana Azad Educational Trust, Dr. Rafiq Zakaria Campus, Aurangabad 431001(MS), India for providing the laboratory facility. REFERENCE
- Gasmi, A., Noor, S., Piscopo, S., & Menzel, A. (2022). Toxic Metal -Mediated Neurodegradation: A Focus on Glutathione and GST Gene Variants. Archives of Razi Institute, 77(2), 525–536. https://doi.org/10.22092/ARI.2021.356279.1816
- Njoga, E. O., Ezenduka, E. V., Ogbodo, C. G., Ogbonna, C. U., Jaja, I. F., Ofomatah, A. C., & Okpala, C. O. R. (2021). Detection, Distribution and Health Risk Assessment of Toxic Heavy Metals/Metalloids, Arsenic, Cadmium, and Lead in Goat Carcasses Processed for Human Consumption in South-Eastern Nigeria. Foods (Basel, Switzerland), 10(4), 798. https://doi.org/10.3390/foods1004079
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. Lyon (FR): International Agency for Research on Cancer; 1993. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 58).https://www.ncbi.nlm.nih.gov/books/NBK499756/
- Hernández-Cruz, E. Y., Amador-Martínez, I., Aranda-Rivera, A. K., Cruz-Gregorio, A., & Pedraza Chaverri, J. (2022). Renal damage induced by cadmium and its possible therapy by mitochondrial transplantation. Chemico-biological interactions, 361, 109961. https://doi.org/10.1016/j.cbi.2022.109961
- Johns, C. E., Gattu, M., Camilli, S., Desaraju, A., Kolliputi, N., & Galam, L. (2023). The Cd/Zn Axis: Emerging Concepts in Cellular Fate and Cytotoxicity. Biomolecules, 13(2), 316. https://doi.org/10.3390/biom13020316
- Johns, C. E., Gattu, M., Camilli, S., Desaraju, A., Kolliputi, N., & Galam, L. (2023). The Cd/Zn Axis: Emerging Concepts in Cellular Fate and Cytotoxicity. Biomolecules, 13(2), 316. https://doi.org/10.3390/biom13020316
- Guo, A. H., Kumar, S., & Lombard, D. B. (2022). Epigenetic mechanisms of cadmium induced nephrotoxicity. Current opinion in toxicology, 32, 100372. https://doi.org/10.1016/j.cotox.2022.100372Gatto N. M. (2021). Environmental Carcinogens and Cancer Risk. Cancers, 13(4), 622. https://doi.org/10.3390/cancers13040622
- Beyersmann, D., & Hartwig, A. (2008). Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Archives of toxicology, 82(8), 493–512. https://doi.org/10.1007/s00204-008-0313-
- Yan, L. J., & Allen, D. C. (2021). Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules, 11(11), 1575. https://doi.org/10.3390/biom11111575
- Chunhabundit R. (2016). Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicological research, 32(1), 65–72. https://doi.org/10.5487/TR.2016.32.1.065
- Wang, Y., Fang, J., Leonard, S. S., & Rao, K. M. (2004). Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free radical biology & medicine, 36(11), 1434–1443. https://doi.org/10.1016/j.freeradbiomed.2004.03.010
- Rafati Rahimzadeh, M., Rafati Rahimzadeh, M., Kazemi, S., & Moghadamnia, A. A. (2017). Cadmium toxicity and treatment: An update. Caspian journal of internal medicine, 8(3), 135–145. https://doi.org/10.22088/cjim.8.3.135
- Sarkar, Angshuman & Ravindran, Geethanjali & Krishnamurthy, Vishnuvardhan. (2013). A brief review on the effect of cadmium toxicity: from cellular to organ level. International Journal of Bio-Technology and Research (IJBTR). 3. 17-36.
- Filipi? M. (2012). Mechanisms of cadmium induced genomic instability. Mutation research, 733(1-2), 69–77. https://doi.org/10.1016/j.mrfmmm.2011.09.002
- Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. Experientia supplementum (2012), 101, 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
- Liu, Y., Chen, Q., Li, Y., Bi, L., Jin, L., & Peng, R. (2022). Toxic Effects of Cadmium on Fish. Toxics, 10(10), 622. https://doi.org/10.3390/toxics10100622
- Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., & Catalano, A. (2020). The Effects of Cadmium Toxicity. International journal of environmental research and public health, 17(11), 3782. https://doi.org/10.3390/ijerph17113782
- Benoff, S., Hauser, R., Marmar, J. L., Hurley, I. R., Napolitano, B., & Centola, G. M. (2009). Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Molecular medicine (Cambridge, Mass.), 15(7-8), 248–262. https://doi.org/10.2119/molmed.2008.00104
- Karami, E., Goodarzi, Z., Ghanbari, A., Bandegi, A. R., Yosefi, S., & Dehdashti, A. (2022). In vivo antioxidant and kidney protective potential of Atorvastatin against cadmium chloride-induced kidney injury in male Wistar rat. All Life, 15(1), 1025–1036. https://doi.org/10.1080/26895293.2022.2126900
- Jawad Hassan, M., Ali Raza, M., Ur Rehman, S., Ansar, M., Gitari, H., Khan, I., Wajid, M., Ahmed, M., Abbas Shah, G., Peng, Y., & Li, Z. (2020). Effect of Cadmium Toxicity on Growth, Oxidative Damage, Antioxidant Defense System and Cadmium Accumulation in Two Sorghum Cultivars. Plants (Basel, Switzerland), 9(11), 1575. https://doi.org/10.3390/plants9111575
- Charkiewicz, A. E., Omeljaniuk, W. J., Nowak, K., Garley, M., & Nikli?ski, J. (2023). Cadmium Toxicity and Health Effects-A Brief Summary. Molecules (Basel, Switzerland), 28(18), 6620. https://doi.org/10.3390/molecules28186620
- Sy, M., Newton, B. L., Pawling, J., Hayama, K. L., Cordon, A., Yu, Z., Kuhle, J., Dennis, J. W., Brandt, A. U., & Demetriou, M. (2023). N-acetylglucosamine inhibits inflammation and neurodegeneration markers in multiple sclerosis: a mechanistic trial. Journal of neuroinflammation, 20(1), 209. https://doi.org/10.1186/s12974-023-02893-9
- Li, F., Zhang, Z., Bai, Y., Che, Q., Cao, H., Guo, J., & Su, Z. (2023). Glucosamine Improves Non-Alcoholic Fatty Liver Disease Induced by High-Fat and High-Sugar Diet through Regulating Intestinal Barrier Function, Liver Inflammation, and Lipid Metabolism. Molecules (Basel, Switzerland), 28(19), 6918. https://doi.org/10.3390/molecules28196918
- Chesnokov, V., Gong, B., Sun, C., & Itakura, K. (2014). Anti-cancer activity of glucosamine through inhibition of N-linked glycosylation. Cancer cell international, 14, 45. https://doi.org/10.1186/1475-2867-14-45
- Le, A. Q., Dang, V. P., Nguyen, N. D., Nguyen, C. T., & Nguyen, Q. H. (2023). Antibacterial Activity against Escherichia coli and Cytotoxicity of Maillard Reaction Product of Chitosan and Glucosamine Prepared by Gamma Co-60 Ray Irradiation. Polymers, 15(22), 4397. https://doi.org/10.3390/polym15224397
- Hosea, R., Hardiany, N. S., Ohneda, O., & Wanandi, S. I. (2018). Glucosamine decreases the stemness of human ALDH+ breast cancer stem cells by inactivating STAT3. Oncology letters, 16(4), 4737–4744. https://doi.org/10.3892/ol.2018.9222
- Block, J. A., Oegema, T. R., Sandy, J. D., & Plaas, A. (2010). The effects of oral glucosamine on joint health: is a change in research approach needed?. Osteoarthritis and cartilage, 18(1), 5–11. https://doi.org/10.1016/j.joca.2009.07.005
- Neha Nausheen, Quadri & Ali, Syed Ayaz & Syed, Shoaeb Mohammad & Patave, Tarannum. (2022). Amelioration of Hepato-renal Impairment by Natural Chelators in Lead-induced Poisoning in Rats. Indian Journal of Pharmaceutical Education and Research. 56. 1123-1133. 10.5530/ijper.56.4.194.
- Blanusa, M., Varnai, V. M., Piasek, M., & Kostial, K. (2005). Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Current medicinal chemistry, 12(23), 2771–2794. https://doi.org/10.2174/092986705774462987
- Becker, A., & Soliman, K. F. (2009). The role of intracellular glutathione in inorganic mercury-induced toxicity in neuroblastoma cells. Neurochemical research, 34(9), 1677– 1684. https://doi.org/10.1007/s11064-009-9962-3
- Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian journal of biochemistry & biophysics, 21(2), 130–132.
- Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry, 95(2), 351–358. https://doi.org/10.1016/0003-2697(79)90738-3
- Sun, M., & Zigman, S. (1978). An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Analytical biochemistry, 90(1), 81–89. https://doi.org/10.1016/0003-2697(78)90010-6
- Sedlak, J., & Lindsay, R. H. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical biochemistry, 25(1), 192– 205. https://doi.org/10.1016/0003-2697(68)90092-4
- Lowry, O. H., Rosenbrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of biological chemistry, 193(1), 265–275.
- Nna, V. U., Ujah, G. A., Mohamed, M., Etim, K. B., Igba, B. O., Augustine, E. R., & Osim, E. E. (2017). Cadmium chloride-induced testicular toxicity in male wistar rats; prophylactic effect of quercetin, and assessment of testicular recovery following cadmium chloride withdrawal. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 94, 109–123. https://doi.org/10.1016/j.biopha.2017.07.087
- Zhang, K., Long, M., Dong, W., Li, J., Wang, X., Liu, W., Huang, Q., Ping, Y., Zou, H., Song, R., Liu, G., Ran, D., & Liu, Z. (2024). Cadmium Induces Kidney Iron Deficiency and Chronic Kidney Injury by Interfering with the Iron Metabolism in Rats. International journal of molecular sciences, 25(2), 763. https://doi.org/10.3390/ijms25020763
- Eman T. Mohammed and Khalid S. Hashem, 2019. Ameliorative Effect of Lipoic Acid on Cadmium Induced Hepatotoxicity and Nephrotoxicity in Rats. Journal of Applied Sciences, 19: 637-646. https://scialert.net/abstract/?doi=jas.2019.637.646
- Moitra, S., Brashier, B. B., & Sahu, S. (2014). Occupational cadmium exposure-associated oxidative stress and erythrocyte fragility among jewelry workers in India. American journal of industrial medicine, 57(9), 1064–1072. https://doi.org/10.1002/ajim.22336
- Sanders, A. P., Mazzella, M. J., Malin, A. J., Hair, G. M., Busgang, S. A., Saland, J. M., & Curtin, P. (2019). Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12-19 in NHANES 2009-2014. Environment international, 131, 104993. https://doi.org/10.1016/j.envint.2019.104993
- Sun, H., Wang, N., Chen, C., Nie, X., Han, B., Li, Q., Zhu, C., Chen, Y., Xia, F., Chen, Y., Zhai, H., Jiang, B., Hu, B., & Lu, Y. (2017). Cadmium exposure and its association with serum uric acid and hyperuricemia. Scientific reports, 7(1), 550. https://doi.org/10.1038/s41598-017-00661-3
- Cirmi, S., Maugeri, A., Micali, A., Marini, H. R., Puzzolo, D., Santoro, G., Freni, J., Squadrito, F., Irrera, N., Pallio, G., Navarra, M., & Minutoli, L. (2021). Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines, 9(12), 1797. https://doi.org/10.3390/biomedicines9121797
- Zhou, J., Qin, H., Li, X. H., & Wang, Y. H. (2022). Zhongguo ying yong sheng li xue za zhi = Zhongguo yingyong shenglixue zazhi = Chinese journal of applied physiology, 38(3), 233–237. https://doi.org/10.12047/j.cjap.6258.2022.043
- Olaniyan, O. T., Ojewale, A. O., Eweoya, O. O., Adedoyin, A. A., Adesanya, O. A., Adeoye, A. O., & Okeniran, O. S. (2021). Modulatory Role of Vitamin E on Proton Pump (ATPase) Activity of Cadmium Chloride-Induced Testicular Damage in Wistar Rats. BioMed research international, 2021, 4615384. https://doi.org/10.1155/2021/4615384


 Zeenat Jahan Bashir Ansari*
Zeenat Jahan Bashir Ansari*
 Syed Ayaz Ali
Syed Ayaz Ali






 10.5281/zenodo.13996127
10.5281/zenodo.13996127