Abstract
This review composition gives knowledge of about HPTLC- grounded logical system development and confirmation parameter in agreement to practical evaluation. It meets norms and minimizes crimes and disquisition. This review composition helps to choose stylish mobile phase and gives guidelines for the good confirmation practice and understand the way of logical procedure. High- Performance Thin Subcaste Chromatography (HPTLC) is an enhanced and automated system of thin- subcaste chromatography TLC) that offers superior separation performance and discovery limits and is constantly a great cover for GC and HPLC.
Keywords
Method Development, Validation, HPTLC.
Introduction
High Performance Thin layer Chromatography (HPTLC) is a sophisticated and automated form of the thin- layer chromatography (TLC) with better and advanced separation effectiveness and discovery limits. It's also known as High Pressure Thin Layer Chromatography/ Planar chromatography or Flat- bed chromatography. It's a important logical system inversely suitable for qualitative and quantitative logical tasks. (1, 2) Any solid, liquid, or gassy emulsion may make up the sample being examined, and the analysis will produce data of some kind that will be applicable to the original query posed about the sample. Some information about the sample can be picked from the data attained during the study. This information could be qualitative or quantitative. (3) Presently, an logical chemical analysis generally involves some kind of logical device that conducts the factual analysis, with computer software handling data processing and instrument control. In light of this, it's accurate to claim that logical chemistry has been computerized. Also, the format of the results from logical chemical studies has altered. moment, a single sample can yield tremendous quantities of data after only a many ages of examination. (4) This may make it delicate to comprehend the results and validate styles. We cover material ways of colorful parameters in quantitative high- performance thin- subcaste liquid chromatographic styles and confirmation disciplines in pharmaceutical analysis to pro in the planning of confirmation styles. also, this composition provides a full review of HPTLC system development that should be useful as an preface to logical confirmation for practical operations in academic exploration or the artificial sector. (5)
Defination: HPTLC (high-performance thin layer chromatography) is a sophisticated form of TLC, which provides superior separation efficiency. The HPTLC concept includes validated methods for qualitative and quantitative analysis.
Principle: HPTLC uses the same physical principles as TLC (adsorption chromatography), i.e., adsorption is the abecedarian unit of separation. action causes the detergent from the mobile phase to pass through. According to their affections with the adsorbent, the factors resettle. The element that's further attracted to the stationary phase moves more sluggishly. The factor that have a lower affinity for the immobile phase move more snappily. A chromatographic plate is used to separate the factors as a result. (6)
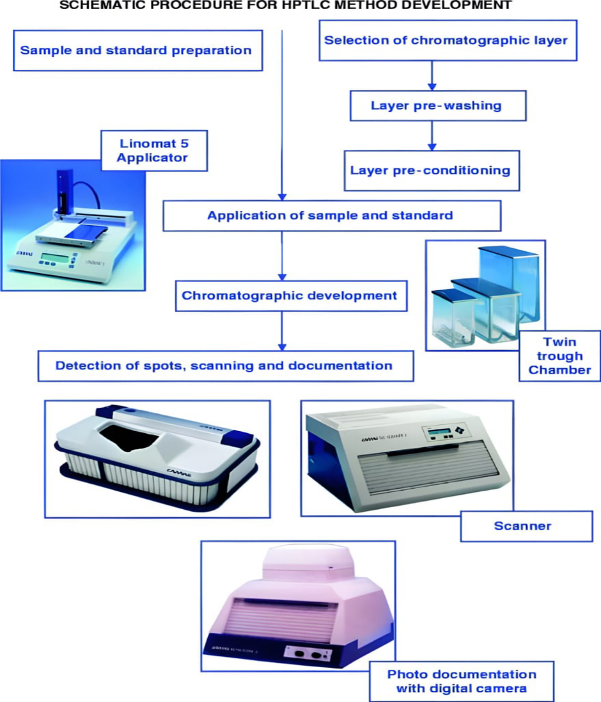
Common Methodology For Hptlc Analysis:
System development in thin- subcaste(planar) chromatography is one of the most significant way for a qualitative and quantitative analysis. During establishing a new logical procedure, always starts with wide literature check (7) i.e. primary information about the physicochemical characteristics of sample and nature of the sample (structure, opposition, volatility, stability and solubility). It involves considerable trial and error procedures.

Selection of the Stationary Phase:
During system development, stationary phase selection should be grounded on the type of composites to be separated. (8) HPTLC uses lower plates (10 * 10 or 10 * 20 cm) with significantly dropped development distance (generally 6 cm) and analysis time (7 – 20 min). HPTLC plates give bettered resolution, advanced discovery perceptivity, and bettered in situ quantification and are used for artificial medicinal densitometric quantitative analysis. (9)
Selection of Mobile Phase:
The choice of mobile phase depends on the adsorbent material used as the stationary phase and the physicochemic parcels of the analyte. Common mobile phases employed according to the colorful selectivity parcels are diethyl ether, methylene chloride, and chloroform mixed collectively or on with hexane as a strength- modifying detergent for normalphaseTLC and methanol, acetonitrile, and tetrahydrofuran amalgamated with water for conforming the strength in reversed- phase TLC. Separations involving ion- pairing on C- 18 layers are carried out with a mobile phase, similar as methanol – 0.1 M aceticacid derivate cradle (pH 3.5), containing 25 mM sodium pentanesulfonate. (10/11/12) Exact volumetric estimations of the factors of the mobile phase must be carried out collectively and absolutely in a satisfactory volumetric outfit and shaken to guarantee applicable blending of the content. Volumes of over to 1 mL are estimated with an applicable micropipette. Volumes of over to 20 mL are estimated with a graduated volumetric pipette of reasonable size. Volumes larger than20 mL are estimated with a graduated cylinder of reasonable size.
HPTLC Validation Procedure:
Validation shouldn't be seen independently from the development of a system. It begins from a plainly characterized logical thing, system selection, optimization, and development, known as pre confirmation considerations before coming to the elaboration of a confirmation protocol. This is the morning stage of the factual confirmation. After performing each analysis portrayed in the confirmation protocol, the data attained are assessed and matched with the acceptance criteria. In the case of all criteria being met, the system can be viewed as valid. In a less formal way, some confirmation data might be taken from trials that were performed preliminarily as part of the system development. The important parameters for system confirmation in HPTLC. This approach is generally conceded for confirmation of qualitative HPTLC ways for identification in routine use. It's possible that the confirmation fashion in some circumstances may bear a many changes in the standard confirmation protocol. similar changes may incorporate limitations with regard to moisture, staying times, perfection, and so on. The confirmation protocol is a crucial instrument for organizing, controlling, and recording the confirmation processes, counting on the quality operation system. The following factors must be incorporated. (13/14)
Main Features Of HPTLC:
Features [15/16/17/18]
* Analysts work simultaneously.
* Lower analysis time and fewer cost per analysis.
•No Cost effective
* Several prior treatments for solvent.
* Accuracy and precision of quantification is high.
* Per sample low mobile-phase consumption.
* Visual Detection possible-open system.
* No interference from previous analysis-fresh stationary and mobile phase-no contimation.
* Low maintenance cost.
Parameters: [19/20]

Selectivity:
Selectivity The capability of the logical fashion developed is to fete an analyte quantitatively in the presence of other com ponents that are anticipated to be present in the sample matrix. The results are communicated as a resolution. In the event that normal impurity is present, it ought to be chromatographed alongside the analyte to check the system felicity, retention factor, trailing factor, and resolution.
Sensitivity:
perceptivity is the capability of the fashion to acquire test results inside a given range in direct proportion to the attention of analyte in the sample – estimation wind for the analyte.
Precision:
Precision gives an suggestion of arbitrary error. Its outgrowth ought to be expressed as a relative standard divagation (RSD) or measure of variation (COV). Precession is seen in terms of replication perfection under the same conditions, same expert, same mechanical assembly, a short time interval, and analogous reagents using the same sample; estimation of peak area RSD ought not be further than 1 grounded on measuring the same sample seven times; peak position RSD ought not be further than 2 grounded on displacing the instrument seven times after every dimension; test operation an equal volume applied as seven spots and RSD ought not be further than 3 under different conditions, similar as different analyte, different laboratory, and different days and reagents from different sources exercising the same sample. RSD ought not be further than 10 within laboratory reproducibility.
Accuracy:
The delicacy of an examination is mandated by the methodical error involved. It's defined as how well the real value and the mean logical value attained by applying the test system at different time intervals agree. The delicacy is satisfactory if the similarity between the true value and the mean estimated does n't surpass the RSD values attained for unremarkable of the system. This parameter is essential for formulated pharmaceutical lozenge forms as it provides data about the recovery of the analyte from the test sample and the impact of the matrix. However, it means that the proposed logical fashion is free from constant and commensurable methodical error, If the recovery rate is observed to be 100. A blank matrix and known contaminations must be present to test the delicacy of the strategy.
Ruggedness:
This is one of the most critical parameters for confirmation of an HPTLC fashion. Tests are generally specified for the ruggedness of an HPTLC system
? Sample medication, which includes composition, volume of detergent, pH, shaking time, temperature, and number of lines
? Sample operation, including volume applied, spot shape and size, band, and spot stability.
? Chromatographic conditions, which include chamber achromatism, eluent composition, eluent volume, temperature, moisture, and development distance.
? Spot visualization, including post chromatographic derivatization, scattering, dipping, response temperature and time, quantitative evaluation, drying of plates, discovery, and wavelength. Once the logical strategy is developed, it ought to be performed autonomously by three experts who are well acquainted with the functional corridor of the strategy, assaying the same sample under the same trial conditions to check the reproducibility of the strategy.
Limit of detection:
The lowest quantum of analyte that can be honored isn't further than 10 of the individual contamination limit. However, also the quantum of analyte to be applied should be increased, If this is n't possible. The limit of discovery (LOD) is expressed as the rate of signal to noise. An normal of the areas of 15 noise peaks and their absolute SD values are measured. The LOD is the quantum of applied sample producing a peak area that's original to the aggregate of the mean blank area and three times the standard divagation.
Stability:
The analyte ought not to decay amid advancement of the chromatogram and ought to be stable in result and on the stationary phase, for no lower than 30 and 15 min, independently. The intensity of the spot on the chromatogram ought to be steady for no lower than 60 min during optimization of the birth/ decontamination system and one must flash back the chemical parcels and chastity of the birth detergent. responses between detergents and their pollutants may produce fresh spots peaks, hence egging false test results. Another important factor is the pH of the waterless phase used for birth/ filtration, which may prompt hydrolysis, oxidation, and isomerization. The complete evacuation of the organic detergent should be avoided.
Advantages of HPTLC include:-(18- 22)
i)samples in nanosecond amounts like in nano- gram range.
ii) minimal running and mortal crimes due to robotization.
iii) better delicacy and perceptivity.
DISADVANTAGES: includes [23-25]
ii) big instrumentation, large space demand, numerous crowds precious, requires strict condition of operation like qualitative and quantitative logical tasks (1, 2) dust free terrain and temperature controlled conditions, and technically professed person with the knowledge to run the system.
Application:
1)Pharmaceutical applications:
- Quality control
- Content Uniformity Test (CUT)
- Identity- and chastity checks
- Stability test.
2)Clinical applications:
- Lipids
- Metabolism studies
- medicine webbing
- Doping control
3)Cosmetics:
- Identity of raw material
- Preservatives, colouring accoutrements, etc.
- webbing for illegal substance etc.
4)Herbal medicines and botanical dietary supplements:
- Identification
- Stability tests
- discovery of contamination
- Assay of marker compounds, etc.
5)Food and feed stuff:
- Quality control
- complements (e.g. vitamins)
- fungicides
- Stability tests (expiration), etc.
6)Industrial applications:
- Process development and optimization
- Process monitoring
- drawing confirmation, etc.
7)Forensics:
- Discovery of document phony
- disquisition of poisoning
- dye analyses, etc.
CONCLUSION:
A simple, accurate, precise system grounded on HPTLC system has been developed for routine analysis of the samples. And this system was validated for linearity, perfection, delicacy, robustness and particularity. HPTLC system has considerable advantage over the other logical fashion like bear small mobile phase, larger sample capacity, multiple or regular times. This system can be used for remedial medicine monitoring in order to optimize medicine lozenge on an individual base and are suitable to measure bioavailability studies and cure
REFERENCES
- Sharma A., Shanker C., Tyagi L. K., Singh M., Rao V. Herbal Medicine for Market Potential in India: An Overview. Academic J. Plant Sci. 2008; 1 (2): 26-36.
- Kadam P. V., Yadav K. N., Shivatare R. S., Pande A. S., Patel A. N., Patil M. J. Standardization of GomutraHaritaki Vati: An Ayurvedic Formulation. Int. J. Pharm. Bio. Sci. 2012; 3 (3): 181–18
- McPolin O. An introduction to HPLC for pharmaceutical analysis. Lulu. com; 2009 Mar 1.
- Kirbiš A, Marinšek J, Flajs VC. Introduction of the HPLC method for the determination of quinolone residues in various muscle tissues. Biomedical Chromatography. 2005 May;19(4):259-65.
- Srivastava M, editor. High-performance thin-layer chromatography (HPTLC). Springer Science & Business Media; 2010 Nov 15.
- Puskuri Divya1, Dr .M Sathishkumar2, Dr. A Malik3, Dr. N Jyothi4. A REVIEW ON HIGH PERFORMANCE THIN LAYER CHROMATOGRAPHY. EPRA International Journal of Research and Development (IJRD) Volume: 6 | Issue: 10 | October 2021https://doi.org/10.36713/epra2016
- Shrivastava, M.M. (2011) An Overview of HPTLC: A Modern Analytical Technique with Excellent Potential for Automation, Optimization, Hyphenation, and Multidimensional Applications. In: Shrivastava, M.M. HPTLC.
- Bele A. A., khale A. Standardisation of herbal drugs: an overview. Int. j. pharmacy 2011; 2 (12): 56-60.
- Indian Pharmacopoeia, Volume-1, published by The Indian Pharmacopoeia Commission, Central Indian pharmacopoeia Laboratory Govt. Of India, Ministry of Health & Family Welfare Sector-23, Raj Nagar, Ghaziabad-201 00, 2007.
- British Pharmacopeia (BP). Vol. II, Her Majesty”s Stationary Office, London. 2001.
- Patra K. C., Pareta S. K., Harwansh R. K., Kumar J. K. Traditional approaches towards standardization of herbal medicines -A review. J. Pharm. Sci. Technol. 2010; 2 (11): 372-379.
- Folashade K. O., Omoregie E. H., Ochogu A. P. Standardization of herbal medicines - A review. Int. J. Biodiversity Conserv. 2012; 4 (3): 101-112.New York: Springer, pp. 3- 24
- Annalakshmi, R., Mahalakshmi, S., Charles, A., Sahayam, C.S., 2013. GC-MS and HPTLC analysis of leaf extract of Madhuca longifolia (Koenig) Linn. Drug Invent. Today 5, 76–80
- Mishra, N., Acharya, R., Gupta, A.P., Singh, B., VK, Kaul, Ahuja, P.S., 2005. A simple microanalytical technique for the determination of Podophyllotoxin in Podophyllum hexandrum roots by quantitative RP- HPLC and RP-HPTLC. Curr.
- Vishal Modi, Sanchita Bhatkar, Parixit Prajapati, TarashankarBasuri. “Various application of HPTLC in Pharmaceutical.” International journal of innovative Pharmaceutical Sciences and Research,2016;4(3):384-407.
- Bidkar J.S., Vidhate G.J., Todkar V.D., Bidkar S.J., Tare H.L., Dama G.Y. “HPTLC A Technology of Industrial utility.” International journal of Institutional Pharmacy and life sciences,2017;7(2):106-119Sci. 88 (9): 1372–1373.
- Seth H. A., Bihani L. G. “High-Performance Thin- Layer Chromatography Instrumentation: An overview of High-Performance Thin-Layer Chromatography (HPTLC).” Pharmatutor-Art-1260.
- Kumar V., Kamle P., Mithal A. “High-Performance Thin- Layer Chromatography (HPTLC): A Review.” International Journal of Analytical and Bioanalytical chemistry;2014,2231-5021.
- Abirami Ganesan and Vetrichelvan Thangarasu. “Validated HPTLC method for simultaneous estimation of sitagliptin phosphate and simvastatin in tablet dosage for]. m.” Scholar Research Library,2016,8(4):6-12.
- Rashmin B. Patel, Mrunali R. Patel, Madhira B. Shankar, Kashyap K. Bhatt. “Simultaneous determination of alprazolam and fluoxetine hydrochloride in tablet formulation by high-performance column liquid chromatography and high-performance thin layer chromatography.” Journal of AOAC International,2009,92(4):1082-1088.
- Koll K., Reich E., Blatter A., Veit M. Validation of standardized high-performance thin-layer chromatographic methods for quality control and stability testing of herbals. J. AOAC Int. 2003; 86: 909-915.
- Sherma J. Review of HPTLC in drug analysis: 1996-2009. J. AOAC Int. 2010; 93: 754-764


 Divya Bhondve *
Divya Bhondve *
 Rasika Kamthe
Rasika Kamthe
 Samruddhi Zagade
Samruddhi Zagade
 Biradar S. M.
Biradar S. M.



 10.5281/zenodo.14264166
10.5281/zenodo.14264166