Background: Diabetes has primarily become a lifestyle condition that is alarmingly increasing across all age group in India, even among the younger population the prevalence is above 10%. The situation in urban areas is worse compared to rural areas, where the prevalence of the disease is almost double across all socia-ecomic groups. Objective: To prepare aqueous extract of carica papaya leaf. To evaluate the antihyperglycemic activity of aqueous extract of leaves of carica papaya (AECPL) in alloxan induced diabetic albino rat. To formulate and in vitro evaluation of tablet of extract of carica papaya leaf. Material and Methods: Rats fasted overnight for 12h were randomly (simple random sampling technique) divided into 6 groups of 4 rats per group. Group 1 served as normal control or nondiabetic group was treated with 10 mL/kg/day of distilled water orally. Group 2 served as untreated diabetic control received 0.5 mg/100 g of vehicle (2% gum acacia).Group 3 served as standard group and was treated with 0.1 mg/kg/day of glibenclamide. Groups 4, 5, and 6 were treated orally with 100, 200, and 400 mg/kg/day of AECPL, respectively, based on their acute oral toxicity study. Fasting blood glucose estimation was done at 0, 3, 6, and 9 h after treatment. Treatment was continued for 15 consecutive days. Fasting blood glucose levels were estimated at 0, 1, 5, 10 and 15 days. Result: The present study showed the antihyperglycemic effect of AECPL in alloxan-induced diabetic rats. The diabetic rats when treated with the Aq. Extract of carica papaya leaf in the dose 400 mg/kg b.w., showed 6.44%, 12.68% and 22.43?cline in the blood glucose level in the initial 5, 10, and 15 h, respectively. Then they showed 38.25%, 40.50%, 42.58%, 46.98% and 55.02?cline in the blood glucose level on day 1, 5, 10, 15 and 20 respectively. Formulation and Evaluation of dosage form were satisfactory. Conclusion: From detailed study of Aq. Extract of carica papaya leaf, I have concluded that Aq. Extract of carica papaya leaf show antihyperglycemic activity. Tablet formulated and evaluated in vitro. Also, these formulations fulfil all requirements of pharmacopoeial and non pharmacopoeial standards and method can be routinely used for an industrial process.
Carica Papaya Leaf, Formulation and Evaluation, Antihyperglycemic Activity.
Ayurveda, the treatment done from ancient period, always over takes other types and treatment. Oldest form of healthcare known to mankind is Ayurveda. It is all about natural herbs, also known to be herbal medicine. Primitively man observed great diversity of plants available. Then as it provide food, clothes, shelter and medicines. At that time medicinal use of plants have been developed through trial and error basis, as the time move each tribe added the medicinal power of herbs, to its knowledge and later developed well define herbal pharmacopoeias. Herbs, being and integral part of development of modern civilization. Today at present, drug commonly used are of herbal origin. Taking an example of US about 25% prescribed drugs contain at least one active ingredient which is derived from plant, some from plant extracts (1, 2). Talking about herbal medicinal products, it contain one or more active substance. Even WHO reports shows world population relies on drug from natural origin. Considering a major metabolic disorder that is Diabetes with major complications like hyperglycemia, inflammation, foot ulcer, nerve disorder and sexual depression. Taking into this many complications if treatment means to cure the disease, there is no such drug, which can cure diabetes completely. Then yoga and Ayurveda is the only evidence I found practically and theoretically treating the diabetes (3). Keeping in view the impotence of disease and also considering the fact that green medicine are safe. So, I have selected and focus on herbal origin drug for the project.
- AIM:
India is the capital of Diabetes in the world. Diabetes has primarily become a lifestyle condition that is alarmingly increasing across all age group in India, even among the younger population the prevalence is above 10%. The situation in urban areas is worse compared to rural areas, where the prevalence of the disease is almost double across all socia-ecomic groups. The current rise especially in younger population is a cause of great concern for public health. In 2020, according to international diabetes federation, the number of people with diabetes in India is currently around 7.7 million (4, 5). The prevalence of diabetes in the population is 8.9%. India is the second highest number of children with type 1 diabetes, after United States. Main reasons of diabetes are trend of urbanization and change in lifestyle. Increase in sedentary lifestyle. Less physical demanding work. Due to global nutrition transition. Intake of food than are high in energy but poor in nutrition. (Often high in sugar and saturated fats, sometimes referred to as western pattern diet) Treatment on diabetes are controlling blood sugar through proper diet. Intake of oral medication, or insulin is the main treatment. Exercise, weight loss, nutrition counselling are other ways too. Herbal medicines have been used throughout the beginning of human history and played a pivotal role in the prevention and treatment. Plants have always been a very good source of drug since long time (6, 7). Most of the drugs currently available have been derived directly or indirectly from them. The Indian traditional system of medicine uses plants for management of diabetic condition. According to WHO up to 90% population in developing countries use plants and its product as medicine for primarily health care (8, 9). There are about 800 plants which have been reported to show antidiabetic potential.
- Objective:
- To prepare aqueous extract of carica papaya leaf.
- To evaluate the antihyperglycemic activity of aqueous extract of leaves of carica papaya (AECPL) in alloxan induced diabetic albino rat.
- To formulate and in vitro evaluation of tablet of extract of carica papaya leaf.
- METHODS:
4.1 Preformulation:
4.1.1 Physicochemical Evaluation:
Properties:
Extract of carica papaya leaf was studied for organoleptic characters such as colour, odour and appearance etc.
4.1.2 Melting Point:
The Melting point of aq. Extract of carica papaya leaf was determined by melting point apparatus. The melting point was determined by introducing small amount of substance in the capillary attached to graduated thermometer and constant heat was applied with the assembly suspended in the paraffin bath. The temperature at which Aq. Extract of carica papaya leaf melted was recorded.
Melting point was also measured by using DSC spectra.
4.1.3 Determination of Flow properties:
Angle of repose:
Angle of repose was determined by using funnel method. The blend was poured through funnel that can be raised vertically until a maximum cone height (h) was obtained. Radius of the heap was measured and angle of repose was calculated using the formula,
? =tan?1 h/r
Bulk density:
Bulk density of a powder is defined as the ratio of the mass of the powder and its bulk volume. It is used to describe packing of particles. For bulk determination, a weighed quantity of the powder material was introduced into a graduated measuring cylinder and volume of powder was determined.
Bulk Density (g/cm3) = Mass of the powder/ bulk volume
Tapped density:
For determination of the tapped density, a weighed quantity of the powder was introduced into a graduated measuring cylinder and was tapped mechanically either manually or using a taping device till a constant volume was obtained.
Tapped Density (g/mL) = Mass of the powder/ tapped volume
Compressibility index:
The simplest way for measuring of free flow of powder was compressibility, an indication of the ease with which a material can be induced to flow was given by compressibility index.
Compressibility index = Tapped density-Bulk density × 100/Tapped Density
Hausner’s ratio:
Hausner’s ratio is an index of ease of powder flow. It is calculated by the following formula,
Hausner’s ratio = Tapped density/Bulk density
Lower Hausner?s ratio (< 1>1.25)
4.2 Plant material:
For the present study fresh leaves of Carica papaya were collected from village Sakharpa, Ratnagiri, Maharashtra, India. Identity of the plant was authenticated by botanist Prof. A .D. Rangwala (HOD Horticulture Dr. B. S. Konkan Krishi Vidyapeeth, Dapoli and Principal of College of Agril. Biotechnology Kharawate-Dahiwali, Tal-Chiplun.
4.3 Phytochemical Screening:
The collected fresh leaves of carica papaya were washed with running tap water, then with distilled water to make it free from any kind of contamination it was washed with 95% ethanol. All the plant materials were shade dried until all the water molecules were evaporated and leaves became well dried for grinding, with the help of dry grinder all the leaves ground well in to fine powder form and passed through sieve and the stored in the polyethene bag with proper labelling.
4.3.1 Preparation of the Aq. Extract of carica papaya leaf:
The collected fresh leaves of carica papaya were washed with running tap water, then with distilled water to make it free from any kind of contamination then the leaves of the plant were subjected to surface sterilization using 30% alcohol, and then dried in shade. The dried leaves were subjected to size reduction to a coarse powder by using dry grinder and passed through sieve. The powder sample stored in the polyethene bag with proper labelling. The powder sample (50 g) was boiled in hot water for 30 min after which it was filtered using piece of white cotton gauze. The filtrate was lyophilized to producing brown color solid residue. The residue was weighted and stored in air and water proof containers, kept in refrigerator at 4oC. From this stock, fresh preparation was made whenever required. Each extract was used for screening the following bioactive compounds: alkaloids, terpenoids, phenol and tannins, sugar, saponins, flavonoids, quinones, and proteins, according to the standard procedure described by (Ayoola et al., 2020).
4.4 Test for phytochemical analysis:
Test for Alkaloids:
About 0.5g of powdered sample of plant material was stirred with 5ml of 1% aqueous hydrochloric acid on a steam bath. A few drops of Dragendorff’s reagent were used to treat 1ml of the filtrate. Turbidity or precipitation with this reagent indicates presence of alkaloids.
Test for Terpenoids:
1ml of extract was mixed with 1ml of Conc.H2SO4 and incubated in water bath for 2-4 minutes, the formation of greyish colour indicates the presence of terpenoids.
Test for Tannins:
Approximately 0.5g of powdered sample of plant material was boiled in 20ml of distilled water in a test tube and then filtered. 0.1?Cl2 was added to the filtered sample and observed for brownish green colouration, which indicated the presence of tannins.
Test for Saponins:
Two grams (2g) of powdered sample of the plant material was boiled together with 10ml of distilled water in a water bath and filtered, 10ml of the filtered sample was mixed with 5ml of distilled water in a test tube and shaken vigorously to obtain a stable persistent frothing which was then mixed with 3 drops of olive oil and observed for the formation of emulsion which indicates the presence of saponins.
Test for Cardiac Glycosides:
One millilitre (1ml) of concentrated H2S04 was prepared in a test tube. 5ml of aqueous extract from the plant material samples was mixed with 2ml of glacial CH3C02H containing 1 drop of FeCl2. The above mixture was carefully added to the 1ml of conc. H2S04, so that the conc. H2S04 is underneath the mixture. If glycoside is present in the sample, a brown ring will appear indicating the presence of the cardiac glycoside constituent.
Test for Anthraquinones:
Five grams (5g) of powdered sample of plant material was added to 10ml benzene, filtered and ammonia solution was added. A pink, red or violet colouration in the ammoniacal phase indicated the presence of anthraquinones.
Test for Flavonoids:
Add few drops of dilute NaOH, in 1ml of extract, neutralize the solution by adding few drops of HCl. The sample will discolor indicating the presence of flavonoids.
Tests for proteins:
Few drop of Conc. nitric acid was added to 1ml of extract, the formation of yellow colour indicates the presence of proteins.
4.5 Evaluation of aq. extract of carica papaya leaf:
4.5.1 Animals
Healthy Wistar albino rats of either sex weighing about 160-210 g were used. The animals were housed in polypropylene cages, maintained under standard conditions (12:12 h light: dark cycle; 25+ 2oC, 35%-60% humidity). They were fed with standard rat pellet diet and water. Animal study approved by the IAEC with approval no. BVCPK/CPCSEA/IAEC/01/23/2020.
4.5.2 Sample Collection:
Blood samples were collected by the retro orbital plexus puncture method from overnight fasted rats under light ether anesthesia and blood glucose levels were estimated using Dr. Morepen Gluco one Bg 03 Glucometer test strips and test meter device (Erba Glucose kit, TRANSASIA BIO-MEDICALS LDT.), which measures the blood glucose level by GOD–POD method (Glucose oxidase-peroxidase method).
4.5.3 Determination of LD50 of the Extract:
For acute oral toxicity study and LD50 determination, the Organization for Economic Co-operation and Development (OECD) guideline 425[24] was followed.
4.5.4 Induction of Diabetes:
A single dose (120 mg/kg, b.w., i.p.) of alloxan monohydrate (Explicit Chemicals Private Limited) dissolved in normal saline was used for induction of diabetes in rats after overnight fasting. After 1 h of alloxan administration, the animals were fed standard pellets and water. The animals were stabilized for a week and animals showing blood glucose level more than 250 mg/dL were selected for the study.
4.5.5 Experimental design:
Rats fasted overnight for 12h were randomly (simple random sampling technique) divided into 6 groups of 4 rats per group. Group 1 served as normal control or nondiabetic group was treated with 10 mL/kg/day of distilled water orally. Group 2 served as untreated diabetic control received 0.5 mg/100 g of vehicle (2% gum acacia).Group 3 served as standard group and was treated with 0.1 mg/kg/day of glibenclamide. Groups 4, 5, and 6 were treated orally with 100, 200, and 400 mg/kg/day of AECPL, respectively, based on their acute oral toxicity study. Fasting blood glucose estimation was done at 0, 3, 6, and 9 h after treatment. Treatment was continued for 15 consecutive days. Fasting blood glucose levels were estimated at 0, 1, 5, 10 and 15 days.
4.5.6 Determination of Solubility:
Solubility of Aq. Extract of carica papaya leaf, polymer Carbopol and Ethyl cellulose was determined in water
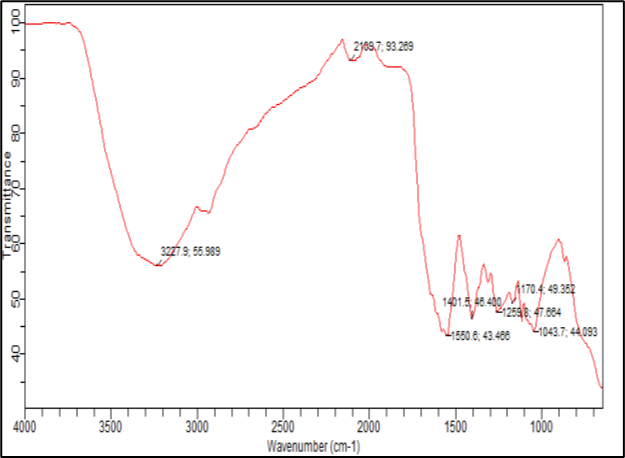
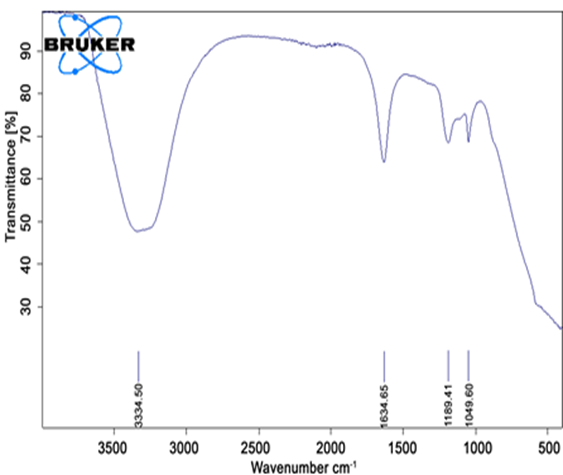
4.5.7 FTIR spectroscopy:
The FTIR spectrum of Aq. extract of carica papaya leaf, mixture of Aq. extract of carica papaya leaf and carbopol, mixture of Aq. extract of carica papaya leaf and ethyl cellulose was recorded using FTIR spectrophotometer.
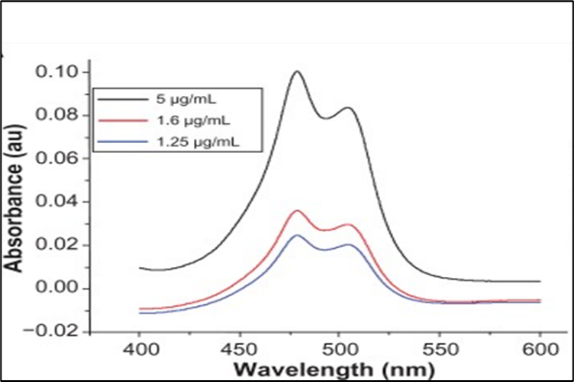
4.5.8 Determination of ? max of Aq. Extract of carica papaya leaf:
The absorption maximum Aq. Extract of carica papaya leaf was found to be 479 nm in phosphate buffer pH 6.8.
4.6 Formulation of Tablet:
In this study, all tests was a performed within product expiration dates. The ingredients such as carbopol, microcrystalline cellulose, dibasic calcium phosphate, PEG 4000, methyl paraben may be used for formulation Aq. Extract of carica papaya leaf tablet.
Table No.1- Formulation of tablet
|
Sr. No.
|
Ingredient
|
Quantity per Tablet (mg)
|
|
1
|
Plant Extract
|
400
|
|
2
|
Carbopol
|
20
|
|
3
|
Microcrystalline cellulose
|
40
|
|
4
|
Dibasic calcium phosphate
|
30
|
|
5
|
PEG 4000
|
10
|
|
6
|
Methyl paraben
|
0.1%
|
|
|
Total
|
500
|
4.7 Evaluation of Tablet:
4.7.1 Determination of drug content:
The tablets will be finely powdered and a quantity of powder equivalent to 100 mg of Aq. Extract of carica papaya leaf will be accurately weighed and transferred to 100 ml of buffer solution (pH 6.8) and mixed thoroughly. The solutions will be filtered, diluted with buffer solution (pH 6.8), and analyzed for the content of Aq. Extract of carica papaya leaf using UV-visible spectrophotometer at 479 nm. The drug content of each sample will be estimated from their previously prepared standard curve.
4.7.2 Uniformity of weight:
The variation of the weight of individual tablets is a valid indication of the corresponding variation in the drug content . The average tablet weight will be determined by weighing 20 tablets individually using a digital analytical balance.
4.7.3 Thickness and diameter measurement:
20 tablets will be taken and their thickness and diameter will be determined individually by Vernier calliper. Mean and standard deviation will be calculated.
4.7.4 Friability Test:
Twenty tablets will be weighed and subjected to friability test using a Roche Friabilator. After the given number of rotations (100 rotations/4 min) loose dust will be removed from the tablets. Finally, tablets will be weighed. The loss in weight indicates the ability of the tablets to withstand this type of wear. It is expressed in percentage (%) and calculated by the following formula:
Friability (%) = Initial weight-Final weight × 100/Initial weight
4.7.5 Hardness testing:
The crushing strength of the tablets will be determined using a Monsanto tablet hardness tester.
4.7.6 Disintegration time:
Disintegration time of six tablets of Aq. Extract of carica papaya leaf will be determined in distilled water maintained at 37±0.5 C using tablet disintegration apparatus. The disintegration time will be taken to be the no particle remained on the basket of the system.
4.7.7 Dissolution Test:
The dissolution studies were performed using USP Apparatus II dissolution tester. Aq. Extract of carica papaya leaf tablets were placed in dissolution vessel containing 900 ml phosphate buffer pH 6.8 maintained at 37 ± 0.5 °C and stirred with paddle at 100 rpm. Samples were collected periodically and replaced with dissolution medium. After filtration through Whatmann filter paper, concentration of Aq. Extract of carica papaya leaf was determined spectrophotometrically at 479 nm. All studies were done in triplicate.
- RESULT AND DISCUSSION:
5.1 Description:
Visual inspection of drug is done.
Table No.2 - Description of Aq. Extract of carica papaya leaf
|
Drug
|
Description
|
|
Aq. Extract of carica papaya leaf
|
Slightly yellowish and greenish crystalline powder, having bitter taste and characteristic odour.
|
5.2 Melting point
Melting point of Aq. Extract of carica papaya leaf, Aq. Extract of carica papaya leaf and carbopol, Aq. Extract of carica papaya leaf and ethyl cellulose was determined by capillary method and was also measured by using DSC spectra.
Table No.3 - Determination of melting point
|
Drug
|
By DSC
|
By manual
|
|
Aq. Extract of carica papaya leaf
|
131.58o C
|
132.20 o C
|
|
Aq. Extract of carica papaya leaf+ carbopol
|
119.95o C
|
120.10 o C
|
|
Aq. Extract of carica papaya leaf+ Ethyl cellulose
|
368.47 o C
|
370.12 o C
|
5.3 Flow properties of powder:
Table no.4 - Flow properties of powder
|
Sr. No.
|
Parameter
|
Result
|
|
1
|
Bulk Density* (gm/ml)
|
0.43±0.003
|
|
2
|
Tapped Density* (gm/ml)
|
0.62±0.12
|
|
3
|
Carr’s Index (%)
|
11.67
|
|
4
|
Hausner’s ratio
|
1.15
|
|
5
|
Angle of repose (0)
|
26.32± 1.22
|
* indicates Mean ± S.D. (n = 3)
5.4 Result of Phytochemical screening:
The phytochemical screening of aqueous extract of leaves sample of Carica papaya revealed the presence or absence of some secondary metabolites (phytochemical constituents) such as alkaloids, terpenoids, flavonoids, sugar, protein, phenols, saponins, glycosides, Anthraquinones as shown in Table no.-16.
Table No.5- phytochemical constituents in aqueous extracts of carica papaya leaf
|
Phytochemical Test
|
Papaya Leaves
|
|
Alkaloids
|
-
|
|
Terpenoids
|
-
|
|
Phenol & Tannins
|
+
|
|
Sugar
|
+
|
|
Saponins
|
-
|
|
Flavonoids
|
+
|
|
Anthraquinones
|
-
|
|
Glycosides
|
-
|
|
Proteins
|
+
|
+ Present, - Absent
5.5 Evaluation of aq. Extract of carica papaya leaf
5.6 Result of Animal study


 Dr. Lalit Nemade*
Dr. Lalit Nemade*
 Mitali Gandhi
Mitali Gandhi


 10.5281/zenodo.14587840
10.5281/zenodo.14587840