Abstract
Mouthwash is a medicated liquid that is swished in the mouth to eliminate the oral pathogens. There are a variety of mouthwashes which are accessible to us nowadays including the chemical and herbal mouthwashes Herbal medicines have least side effects and free of alcohol and sugar content. A mouthwash is recommended to control Halitosis, plaque, toothaches and other oral problems. The aim of our study is to formulate herbal mouthwash formulation and to carry out the evaluation studies such as viscosity, pH, foam height, microscopic test, centrifugation test, etc. for the developed mouthwash. Determination of antibacterial activity of mouthwash wash carried out. Clove, Cinnamon, Rose contain Anti-oxidant and Anti-bacterial activity additionally Fennel is used as flavoring agent, Licorice as sweetening agent and Rosemary as preservative. The herbal mouthwash shows Anti-bacterial activity by using agar well diffusion technique. Due to the Anti-bacterial activity, it minimizes the production of bacteria in the mouth which helps to prevent bad odor in mouth resulting in treatment of halitosis. This in-vitro study determines the antimicrobial activity of herbal mouthwash against streptococcus mutans. Herbal mouthwash offers a promising natural alternative to conventional mouthwashes, exhibiting potential antimicrobial activities. Hence, herbal mouth freshners are the major requirement at present to combat the bad odor and to get instant effect which prevents the risk of hesitation, embarrassment, and anxiety with increased oral quality life of the people.
Keywords
Anti-bacterial, Halitosis, Herbal mouthwash, swished, Oral pathogens.
Introduction
The microbial load in the oral cavity is decreased by using mouthwashes. Therapeutic on the tooth surface and the mouth is provided by the mouthwashes. Mouthwashes are liquids that show Anti-inflammatory, Antioxidant, Antimicrobial, and Analgesic action. There are two types of Mouthwashes available – chemical and herbal. The main Purposes of using mouthwash are good oral hygiene can be obtained by using the mouthwashes routinely at home, they provide anti-inflammatory, anti-Microbial activity. Mouthwash is defined as a liquid that is passively held in the mouth by contraction of the oral muscles and movement of the head, and it may be gargled, in which the head is tilted back and the liquid bubbles in the mouth. Herbal mouthwashes are free of alcohol, artificial preservatives, flavours, and colours. Hence Herbal mouthwashes can be used in place of chemical mouthwashes to Maintain oral hygiene, owing to the additional Benefits provided by herbal Preparations.[1]
Types of Herbal mouthwash: [1]
- Fluoride mouthwash: The cavities in the teeth can be prevented by using the salts of fluoride present in the mouthwashes.
- Antiseptic mouthwash: It is the common type of mouthwash. It contains alcohol and is typically utilized to stop bacterial growth especially for the people with mouth infections and halitosis or bad breath.
- Cosmetic mouthwash: It is a mouthwash that are not having any therapeutic effect to the oral care but it masks the bad breath and freshens the bad breath.
- Natural mouthwash: As the name suggests it contains only natural herbal ingredients obtained from the nature. It is an alcohol-free and chemical lacking mouthwash. The herbal ingredients are safer to use as compared to other types of mouthwashes.
Halitosis is a term derived from the Latin word halitus (breath) and the Greek suffix osis (condition or action); in English it is known as Bad breath. Bad breath, halitosis, is an unpleasant problem that people experience often and dislike it. Halitosis can be a symptom of a serious disease. though it is not a serious Disorder, it affects the overall health of the individual in the society. [3] Halitosis refers to the offensive or unpleasant odor which may come from the mouth or cavities of the nose, sinuses and pharynx. Fetor oris, oral malodor, fetor ex oris, foul or bad breath are the various names given to this objectionable condition. Halitosis is classified as genuine halitosis, pseudo-halitosis or halitophobia. Halitosis whose origin is either physiological or pathological can be termed as Genuine Halitosis whereas pseudo-halitosis may occur where no reasonable cause is known. Almost 80-90% of cases in genuine halitosis, bacterial activities occur in the oral cavity. In the oral microbiota, over 600 species of microorganisms have been detected by researchers. These microorganisms act on sulfur amino acids such as cystine, cysteine and methionine which results in volatile sulfur compounds that include hydrogen sulfide (H2S), methyl mercaptan (CH3SH) and dimethyl sulfide (CH3SCH3).[4]
Causes of Halitosis- Physiologic causes of halitosis are Lack of flow of saliva during sleep, Food where the metabolism of certain foods and beverages produces volatile fatty acids or other malodorous substances which can be excreted through the lungs, Smoking, Menstruation where Women have bad breath during periods, which is secondary to hormonal changes.
Advantages of herbal mouthwashes over chemical mouthwashes:
- Herbal mouthwashes are non-irritant and they have non-staining properties.
- They are less harmful and have very few or no side effects.
- Herbal mouthwashes are gentle for even the most sensitive mouth.[5]
Disadvantages:
- Herbal drugs cannot be found everywhere, they are least availability.
- Pungent in smell.
- Stability problems during the formulation process. [6]
Uses: ;
- To enhance oral hygiene.
- To control dental plaque.
- For eradicating bacteria present in oral cavity.[7]
MATERIALS AND METHODS:
Collection of plant materials:
The herbal ingredients which are used to formulate the herbal mouthwash such as, Cinnamon, Clove, Fennel, Licorice, rose and rosemary were purchased from the local General stores and were subjected for authentication by “Central Ayurveda Research Institute, Bengaluru”. The herbal materials were identified and authenticated as Cinnamomum verum J. Presl belonging to the family Lauraceae (RRCBI-20516), Syzygium aromaticum (L.) Merr. & L.M. Perry belonging to the family Myrtaceae (RRCBI-mus151), Foeniculum vulgare Mill. Belonging to the family Apiaceae (RRCBI-mus145), Glycyrrhiza glabra L. belonging to the family Fabaceae (RRCBI-mus356), Rosa centifolia L. belonging to the family Rosaceae (RRCBl-mus518), Salvia Rosmarinus Schleid. Belonging to the family Lamiaceae (RRCBI-mus519) respectively. After Authentication the herbal materials were chopped into small pieces and they were pulverized into a sterile mixer. The powder was sieved using a fine mesh of 44mm. The powder was stored in an airtight container. Chemicals used in the formulation such as Alcohol or food grade ethanol, Glycerol, Sodium lauryl sulphate, Peppermint oil and Distilled water etc. were used from “The Oxford College of pharmacy, Bangalore” [4]
Extraction process:
About 5g of all the herbal powders of clove, cinnamon, fennel, licorice and rosemary were transferred into a conical flask which contains 100ml of distilled water. It was kept for maceration at 37°C for about 24 hours in a stoppered flask. After maceration the obtained extract was filtered using the Muslin cloth, then again it was Filtered using a filter paper. After filtration a clear liquid extract of the cinnamon which is brown in color, clove which is reddish brown in color, fennel which is light green in color, licorice which is yellow in color and Rosemary which is dark green in color was obtained. The extracts were boiled, cooled and stored in a closed container.[5] The rose petals were boiled in a beaker containing 100ml of distilled water for about 30 Minutes. The petals were stirred occasionally by using a spatula or a stirrer. After 30 minutes it was made to cool down and the extract has been filtered in a Muslin Cloth. The red liquid extract with good aroma was obtained and was transferred to an air tight container.[6]
Pre-Formulation studies:
Compatibility studies: Prior to formulation of our product, a Compatibility study of the herbal drug extractions was carried at “Pooranayu Research Labs, Bengaluru”. To check the interaction of the herbal drugs with each other. Compatibility is the key characteristics to proceed with the formulation of herbal drugs. So, all the herbal extractions of cinnamon, clove, Fennel, liquorice, rose and rosemary 1ml of each extract were collected separately in Eppendorf tubes. Along with these a mixture of glycerol, alcohol and sodium lauryl sulphate was poured in 1ml Eppendorf tube. The compatibility of the herbal ingredients along with the chemicals was tested and based on the result further formulation of the mouthwash was done.
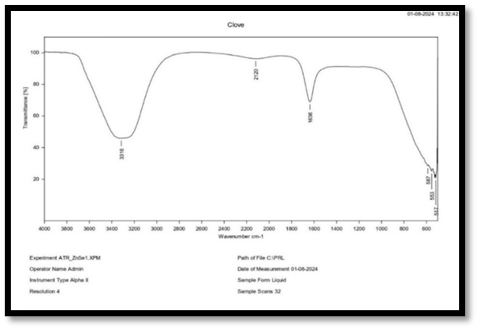
FTIR of Clove
FTIR of Fennel
FTIR of Licorice
FTIR of Rosemary
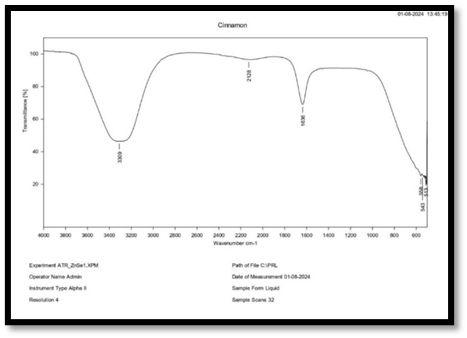
FTIR of Cinnamon
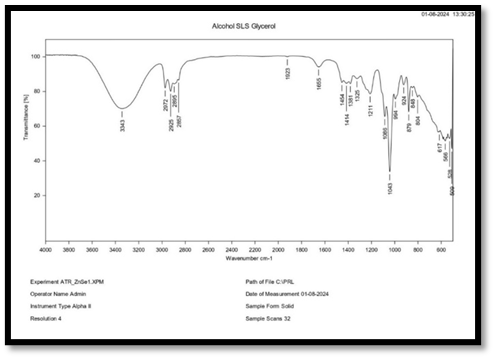
FTIR of Alcohol, SLS, Glycerol
FTIR of Comparision of herbs
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Group identified
|
|
3400- 3200
|
3309
|
O-H stretching
|
|
2920
|
2128
|
C-H stretching
|
|
1720- 1710
|
1720
|
C=O stretching
|
|
1600
|
1636
|
C=C stretching
|
|
1500
|
1500
|
C-H bending
|
|
Below 1200
|
1200
|
C-O stretching
|
Interpretation of FT-IR spectra of cinnamon
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Group identified
|
|
3400- 3200
|
3316
|
O-H stretching
|
|
2924-2854
|
2120
|
C-H stretching
|
|
1637
|
1636
|
C=C stretching
|
|
1514-1433
|
1514
|
C-H bending
|
|
1265
|
597
|
C-O bonding
|
Interpretation of FT-IR spectra of clove
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Group identified
|
|
3400-3200
|
3341
|
O-H stretching
|
|
2924 and 2854
|
2973
|
C-H stretching
|
|
1637
|
1696
|
C=C stretching
|
|
1514-1433
|
1418
|
C-H bonding
|
|
1265
|
1274
|
C-O stretching
|
Interpretation of FT-IR spectra of Fennel
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Group identified
|
|
3400-3200
|
3317
|
O-H stretching
|
|
2950-2850
|
2924
|
C-H stretching
|
|
1730
|
1636
|
C=O stretching
|
|
1610-1580
|
1542
|
C=C stretching
|
|
1500
|
1435
|
C-H bending
|
|
1200-1000
|
1156
|
C=O stretching
|
|
600
|
595
|
C-X bonds
|
Interpretation of FT-IR spectra of Licorice
|
Wavenumber standard in cm-1
|
Wavenumber observed in cm-1
|
Groups identified
|
|
3400-3200
|
3271
|
O-H stretching
|
|
2920
|
2120
|
C-H stretching
|
|
1710-1720
|
1636
|
C=O stretching
|
|
1620
|
1620
|
C=C stretching
|
|
1440
|
1440
|
C-H bending
|
|
1200
|
626
|
C-O stretching
|
Interpretation of FT-IR spectra of Rose
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Groups identified
|
|
3400-3200
|
3354
|
O-H stretching
|
|
2950-2850
|
2971
|
C-H stretching
|
|
1740
|
1686
|
C=O stretching
|
|
1650
|
1648
|
C=C stretching
|
|
1450
|
1425
|
C-H bending
|
|
1200
|
1189
|
C-O stretching
|
|
1000
|
879
|
C-X bonds
|
Interpretation of FT-IR spectra of Rosemary
|
Wave number standard in cm-1
|
Wave number observed in cm-1
|
Groups identified
|
|
3341
|
3343
|
O-H stretching
|
|
2972, 2895
|
2895
|
C-H stretching
|
|
1211,1080,1043
|
1211
|
C-O stretching
|
|
1211
|
1043
|
S=O stretching
|
Interpretation of FT-IR spectra of Alcohol, SLS and glycerol
Conclusion of FTIR: FTIR is a technique that is used to analyze the chemical composition of materials by measuring how they absorb infrared rays. Many functional groups are found in the herbs which shows the chemical elements of the herb. These chemical elements are found to be compatible with each other, which means the herbal drugs when used together in the formulation do not show any interactions. From this compatibility study we conclude that Clove, Cinnamon, Licorice, Rosemary, Rose and the chemical ingredients Alcohol, SLS, Glycerol are having good compatibility which are used for the further formulation of the herbal mouth.
Formulation of herbal mouthwash: Mouthwashes were prepared by adding varying proportions of Clove, Cinnamon, Rose, Fennel, Licorice, Rosemary, Peppermint oil, Glycerol, Sodium lauryl sulphate, Alcohol and Distilled water. A total six formulations of mouthwashes F1, F2, F3, F4, F5 and F6 were prepared. [7]

Fig.1.1. Formulations
Formulation Table:
Table .1.1 Formulation table
|
Ingredients
|
Functions
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
|
Clove (30g/100ml)
|
Antioxidant
|
5ml
|
5ml
|
8ml
|
10ml
|
20ml
|
30ml
|
|
Cinnamon (20g/100ml)
|
Antibacterial, antifungal
|
5ml
|
5ml
|
8ml
|
10ml
|
10ml
|
20ml
|
|
Rose
(10g/100ml)
|
Antibacterial, Anti-inflammatory
|
5ml
|
5ml
|
8ml
|
8ml
|
10ml
|
10ml
|
|
Fennel
(7g/100ml)
|
Flavouring agent
|
5ml
|
6ml
|
6ml
|
6ml
|
7ml
|
7ml
|
|
Liquorice
(10g/100ml)
|
Sweetening agent
|
8ml
|
8ml
|
8ml
|
10ml
|
10ml
|
10ml
|
|
Rosemary
(20g/100ml)
|
Preservative
|
10ml
|
10ml
|
20ml
|
20ml
|
8ml
|
10ml
|
|
Peppermint oil (1%)
|
Flavouring agent
|
0.01ml
|
0.01ml
|
0.01ml
|
0.01ml
|
0.01ml
|
0.01ml
|
|
Glycerol (4%)
|
Co-surfactant
|
2ml
|
2ml
|
2ml
|
2ml
|
2ml
|
2ml
|
|
Sodium lauryl sulphate(1g/100ml)
|
Foaming agent
|
1ml
|
1ml
|
1ml
|
1ml
|
1ml
|
1ml
|
|
Alcohol (10%)
|
Preservative
|
10ml
|
10ml
|
10ml
|
10ml
|
10ml
|
10ml
|
|
Distilled water
|
Solvent
|
Q.s
|
Q.s
|
Q.s
|
Q.s
|
Q.s
|
Q.s
|
|
Total volume
|
|
100ml
|
100ml
|
100ml
|
100ml
|
100ml
|
100ml
|

Fig.1.2. Herbal Formulations F1, F2, F3, F4, F5 and F6
Evaluation of herbal mouthwash:
- Visual Examination: Through visible examination, physical characteristics including odor and color have been checked for the formulated herbal mouthwash. Physical parameters such as color, odor and Consistency are examined by visual inspection. [8]
- Determination of pH: The digital meter was used to measure the pH of the prepared herbal mouthwash. Standard buffer was used to calibrate the pH meter. About 1ml of mouthwash was taken and its pH was determined by pH meter. To check pH of the formulations, the pH meter was connected to the formulations. Readings were taken three times to get an average value.[9]
- Centrifugation test: Using 6 different centrifugation tubes, the herbal mouthwash preparation was centrifuged in the Centrifugation machine at 2800 rpm for 5minutes to examine the phase separation of the Formulated preparation. First ensure that the Centrifugation machine is well calibrated and ready to use. Transfer 10ml of herbal mouthwash preparation to 6 different Centrifugation tubes. Place the tubes in centrifuge machine and set the following parameters; Speed-2800 rpm (revolutions per minute), Time-5 minutes, Temperature-Room temperature (20-25°C) Start the centrifuge machine and run the sample for the set time. After Centrifugation observe the Sample for any visible sediment or phase separation.[10]
- Microscopic test: The transparency of the mouthwash formulation was assessed by using a compound microscope with magnification powers of 10X and 40X.The result is concluded as transparent or non-transparent. [11]
- Viscosity: Viscosity of the formulation is determined using Ostwald viscometer. Viscometer is mounted on a suitable burette stand. Mouthwash was filled in a viscometer in wider opening side. The formulation was sucked until the mark A. The time was noted in a stopwatch to determine the time take by the mouthwash to flow from mark A to mark B. All the 6 formulation’s viscosity was determined in triplicate to get an average value in seconds. [12]
- Foam height: 1ml of mouthwash is mixed in 50ml of distilled water. The mixture is poured in a measuring cylinder. Distilled water was added in the measuring cylinder to make the volume up to 100ml. The mixture received 25 strokes, after which it was kept aside. The height of the foam above the aqueous volume is observed and noted in all 6 formulations. [13]
- Homogeneity: The mouthwash preparations were packed in the container and tested for homogeneity by visual inspection. It shows whether there is a presence of aggregate in the formulation. [14]
- In-vitro Antibacterial activity: In-Vitro Antibacterial activity test is performed on isolated colonies of streptococcus mutans. The agar well diffusion method was used for determining the Zone of Inhibition (ZOI) and Minimum Inhibitory Concentration (MIC).
Table 1.2. Equipment’s for antibacterial activity
|
Sl. No
|
Particulars
|
Source
|
Catalogue No
|
|
1
|
Agar Base
|
Himedia
|
90935
|
|
2
|
Petri plates
|
Genaxy
|
GEN-PTD-90
|
|
3
|
96 well plate
|
Thermo Scientific
|
167425
|
|
4
|
L-spreader
|
Himedia
|
PW1085-1x20N0
|
|
5
|
1000µl tips
|
Genaxy
|
GENUT 1000C
|
|
6
|
200µl tips
|
Genaxy
|
GENUT 200C
|
|
7
|
Micro centrifuge tubes
|
X pet
|
MCT-1.7-B
|
Test organisms: Streptococcus mutans
Test compound as standard: Tetracycline Inoculum: Streptococcus mutans cell suspension was prepared and grown on media and cultures were incubated for 24hrs at 37°C. The cell suspensions of all the cultures were adjusted to 1-2x 106cells/ml.
Test compound: Sample – 250 microgram, Std-Standard: Tetracycline 25 µg/ml
Procedure:
Determination of Antimicrobial activity: Streptococcus mutans were inoculated on media (90 mm), Test compounds: Sample (25µl), Standard Tetracycline (25µl) Streptococcus mutans were added to the 5mm well on agar plates. The treated plates with Streptococcus mutans were incubated in aerobic chamber at 37°C for 24hrs. The treated plates were observed for zone of inhibition around the wells. [15]
RESULTS AND DISCUSSION:
1. Visual Examination:
Table 1.3. Visual recordings
|
Formulation
|
Colour
|
Odour
|
Appearance
|
|
F1
|
Yellow
|
Fragrant
|
Clear
|
|
F2
|
Pale yellow
|
Fragrant
|
Turbid
|
|
F3
|
Brown
|
Fragrant
|
Slightly turbid
|
|
F4
|
Golden yellow to brown
|
Fragrant
|
Clear /transparent
|
|
F5
|
Pale brown
|
Fragrant
|
Clear
|
F4 mouthwash looks clear and transparent when compared to other formulations among the all 6 formulated herbal preparations.
2. Determination of pH:
Table 1.4. pH readings
|
Formulation
|
Trial 1
|
Trail 2
|
Trail 3
|
Average
|
|
F1
|
5.88
|
5.97
|
5.97
|
5.94
|
|
F2
|
5.24
|
5.41
|
5.61
|
5.42
|
|
F3
|
5.06
|
5.41
|
5.31
|
5.26
|
|
F4
|
5.87
|
5.92
|
5.99
|
5.92
|
|
F5
|
5.79
|
5.88
|
5.93
|
5.86
|
|
F6
|
5.88
|
5.97
|
5.96
|
5.93
|
As the skin is having the acidic pH around 5.5, this pH range of the formulated herbal mouthwash is suitable for the oral disorders such as halitosis. Oral problems are appropriate for this pH range of formulation.

Fig.1.3. pH meter
2.Centrifugation test:
Table 1.5. Phase separation
|
Formulation
|
Phase Separation
|
|
F1
|
No
|
|
F2
|
No
|
|
F3
|
No
|
|
F4
|
No
|
|
F5
|
No
|
|
F6
|
No
|

Fig.1.4 observation of phase separation
3.Microscopic test:
Table. 1.6. Microscopic test
|
Formulation
|
Microscope
|
Magnification
Power
|
Observations
|
|
F1
|
Compound microscope
|
10X and 40X
|
Transparent with small aggregates
|
|
F2
|
Compound microscope
|
10X and 40X
|
Transparent with small aggregates
|
|
F3
|
Compound microscope
|
10X and 40X
|
Transparent with small aggregates
|
|
F4
|
Compound microscope
|
10X and 40X
|
Transparent and clear
|
|
F5
|
Compound microscope
|
10X and 40X
|
Transparent with bigger aggregates
|
|
F6
|
Compound microscope
|
10X and 40X
|
Transparent with small aggregates
|
|
Formulation
|
Trial 1
|
Trial 2
|
Trial 3
|
Average
|
|
F1 Viscosity
|
254 seconds
|
295 seconds
|
293 seconds
|
280 seconds
|
|
F2 Viscosity
|
294 seconds
|
293 seconds
|
289 seconds
|
292 seconds
|
|
F3 Viscosity
|
341 seconds
|
342 seconds
|
338 seconds
|
340 seconds
|
|
F4 Viscosity
|
307 seconds
|
306 seconds
|
301 seconds
|
304 seconds
|
|
F5 Viscosity
|
313 seconds
|
311 seconds
|
308 seconds
|
310 seconds
|
|
F6 Viscosity
|
281 seconds
|
271 seconds
|
264 seconds
|
272 seconds
|

Fig.1.5. viscosity observation
As the Viscosity of all the formulation is more than 120 seconds, it concludes that all the formulations have thick consistency. F6 preparation is less viscous whereas formulation F3 is more viscous.
Foam height:
Table.1.7. Height of foam
|
Formulation
|
Foam height in centimetre
|
|
F1
|
1cm
|
|
F2
|
2cm
|
|
F3
|
2.5 cm
|
|
F4
|
1cm
|
|
F5
|
2cm
|
|
F6
|
3cm
|

Fig.1.6. Foam height
In general, a foam height of about 2-3cm is considered as optimum for the herbal mouthwash as it provides a gentle non-irritating foam and helps to distribute the active ingredients effectively. An optimum foam height for the herbal mouthwash is between 1 to 3cm.Thus the formulated mouthwash has a better foaming ability.
Homogeneity:
Table.1.8. Homogeneity
|
Formulation
|
Homogeneity
|
|
F1
|
Good
|
|
F2
|
Good
|
|
F3
|
Good
|
|
F4
|
Good
|
|
F5
|
Good
|
|
F6
|
Good
|
In-vitro Antibacterial activity: Inhibitory activity of test compounds against test organisms:
Conclusion: The zone of inhibition observed against test compound and standard are summarized in Table. Sample shows inhibitory activity against Streptococcus mutans in well diffusion. The zone of inhibition is the circular spot of the sample in which the bacterial colonies do not grow. ZOI between 5-10 mm indicates moderate to high Antibacterial activity. ZOI of 7 mm indicates that the antimicrobial agent has inhibited the growth of streptococcus mutans in a circular area with a diameter of 7 mm around the agent.
Table.1.9 Zone of inhibition measurement
|
Test Organisms
|
Test Compounds
|
Conc. per well
|
Zone of inhibition (mm)
|
Figure reference number
|
|
|
| |
|
|
Tetracycline (Standard)
|
25 µg/ml
|
16
|
|
|
|
Streptococcus mutans
|
Sample
|
-
|
7
|
1
|
|
|
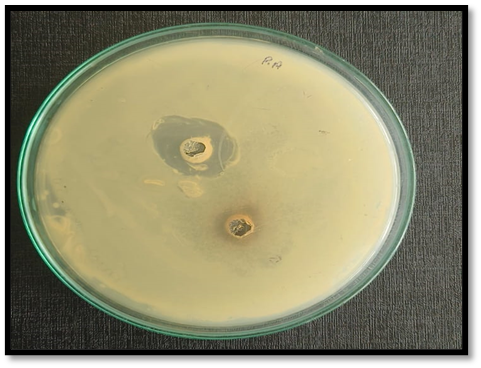
Fig.1.7. Zone of Inhibition
CONCLUSION:
The aim of our study was to formulate herbal mouthwash and carry out the evaluation studies such as viscosity, pH, foam height, microscopic test, centrifugation test, etc. for the developed mouthwash and to carry out anti-bacterial activity of the mouthwash. After performing the compatibility studies, we formulated the herbal mouthwash in different concentrations. The formulated mouthwash was subjected to evaluation tests. The color, odor and appearance of the mouthwash was good. The pH of the mouthwash was suitable to treat the oral disorders. There was no phase separation and homogeneity were also good. The prepared mouthwash was viscous with thick consistency. The mouthwash had a good foam forming ability. Herbal mouthwash exhibited significant Anti-bacterial property against the streptococcus mutans. Thereby we conclude that the formulated mouthwash is helpful in managing the oral pathogens hence, it can reduce the chances of halitosis. Herbal mouthwash is having least toxic effects and convenient to use and because of this the demand for natural herbal products is growing widely. The sample of the mouthwash has shown inhibitory activity against the bacterial colony indicating no growth of bacteria. This infers that the mouthwash is suitable for use in the management of Halitosis, where bad breath is a major problem of the oral health. It avoids the first pass metabolism as it can be used by gargling and spitting out of the mouth. Due, to the Anti-bacterial activity, it minimizes the production of bacteria in the mouth which helps to prevent bad odor in mouth resulting in treatment of halitosis.
Conflict Of Interest:
The authors Declare no conflict of interest.
ACKNOWLEDGEMENT:
We take this opportunity to express our sincere and respectful thanks to our beloved teachers, family, friends and other members who helped in completing this article.
REFERENCES
- Bokhare SH, Maske AS, Mohite RS, Magar AP, Mane VV. Herbal Mouthwash For The Management Of Oral Diseases.
- Winslow LC, Kroll DJ. Herbs as medicines. Archives of internal medicine. 1998 Nov 9;158(20):2192-9.
- Attia EL, Marshall KG. Halitosis. Canadian Medical Association Journal. 1982 Jun 6;126(11):1281.
- Kukreja BJ, Dodwad V. Herbal mouthwashes-A gift of nature. Int J Pharma Bio Sci. 2012 Apr;3(2):46-52.
- Vinod KS, Sunil KS, Sethi P, Bandla RC, Singh S, Patel D. A novel herbal formulation versus chlorhexidine mouthwash in efficacy against oral microflora. Journal of International Society of Preventive and Community Dentistry. 2018 Mar 1;8(2):184-90.
- Tidke S, Chhabra GK, Madhu PP, Reche A, Wazurkar S, Singi SR. The effectiveness of herbal versus non-herbal mouthwash for periodontal health: a literature review. Cureus. 2022 Aug;14(8).
- Bollen CM, Beikler T. Halitosis: the multidisciplinary approach. International journal of oral science. 2012 Jun;4(2):55-63.
- Ayl?kc? BU, Çolak H. Halitosis: From diagnosis to management. Journal of natural science, biology, and medicine. 2013 Jan;4(1):14.
- Richter JL. Diagnosis and treatment of halitosis. Compend Contin Educ Dent. 1996 Apr;17(4):370-2.
- Nigam D, Verma P, Chhajed M. Formulation and evaluation of herbal mouthwash against oral infections disease. Int J Pharm Life Sci. 2020 Jul 1;11(7):6746-50.
- Ojha S. Formulation and evaluation of antibacterial herbal mouthwash against oral disorders. Indo Glob. J. Pharm. Sci. 2018 Feb 11;8(2):37-40.
- Shohayeb M, Abdel-Hameed ES, Bazaid SA, Maghrabi I. Antibacterial and antifungal activity of Rosa damascena MILL. essential oil, different extracts of rose petals. Global Journal of Pharmacology. 2014;8(1):1-7.
- Deshmukh SA, Gholse YN, Kasliwal RH, Chaple DR. Formulation, development, evaluation and optimization of Herbal antibacterial mouthwash. Deshmukh et al. 2019 Feb 24;8(6):828-41.
- Shambharkar SB, Thakare VM. Formulation and evaluation of herba l mouthwash. World J Pharm Res. 2021 May 28;10(9):775-91.
- Venâncio GN, Rodrigues IC, Souza TP, Marreiro RD, Bandeira MF, Conde NC. Herbal mouthwash based on Libidibia ferrea: microbiological control, sensory characteristics, sedimentation, pH and density. Revista de Odontologia da UNESP. 2015 Mar;44:118-24.
- Orasmo EA, Miyakawa W, Otani C, Khouri S. In vitro AFM evaluation of Streptococcus mutans membrane exposed to two mouthwashes. Journal of Applied Pharmaceutical Science. 2013 Sep 30;3(9):024-8.
- Kim YR, Nam SH. Comparison of the preventive effects of slightly acidic HOCl mouthwash and CHX mouthwash for oral diseases. Biomedical Research. 2018;29(8):1718-23.
- Iskandar B, Lukman A, Syaputra S, Al-Abrori UN, Surboyo MD, Lee CK. Formulation, characteristics and anti-bacterial effects of Euphorbia hirta L. mouthwash. Journal of Taibah University Medical Sciences. 2022 Apr 1;17(2):271-82.
- Chaudhary P, Sharma R, Rupanar S, Jadhav S, Bongade A, Shinde P, Gavit S. Preparation and Evaluation of Herbal Mouthwash Containing Hydroalcoholic Extract of Pongamia pinnata. Asian Journal of Biological and Life Sciences. 2023 Jan;12(1):173.
- Mensitieri F, Caggiano M, Gaudino G, Charlier B, Coglianese A, Amato A, Di Spirito F, Amato M, Dal Piaz F, Izzo V. In Vitro Evaluation of Antibacterial and Antibiofilm Activity of Different Chlorhexidine-Containing Mouthwash Formulations against Streptococcus mutans. Applied Sciences. 2023 Jun 26;13(13):7531.
- Aneja KR, Joshi R, Sharma CH. The antimicrobial potential of ten often used mouthwashes against four dental caries pathogens.


 Sanjana R.*
Sanjana R.*
 Adithi P.
Adithi P.















 10.5281/zenodo.14696848
10.5281/zenodo.14696848