Abstract
In this study plant extract is use to screen preliminary phytochemical study and analyse the stem of vitex negundo using methanol solvent were use to extract vitex negundo stem, and the result shows the presence of alkaloids, tannins, flavonoids, saponins, terpenoids, proteins, amino acids, carbohydrates, as a result, this study will promote or needs more research into phytochemical component and other related investigations of plants.
Keywords
Phytochemical, Preliminary, Screening
Introduction
Medicinal herbs have been used to prevent illness since ancient times. We understand how important plants are.(1) Medicinal plants, shrubs, and trees are widely used in both developed and developing countries to make traditional medicines used in both residential and commercial settings.(2) Plant-based drugs are affordable, effective, and safe, with little side effects. According to the World Health Organization (WHO), medicinal plants are the most effective source of several drugs. In rich countries, around 80% of people utilize traditional medicines derived from medicinal plants. Additional research is needed to further understand the properties, security, and effectiveness of these plants.(3) Medicinal plants include a variety of chemical components, including tannins, steroids, sugars, terpenoids, alkaloids, and flavonoids, which have specific effects on humans.(4)(5) Plant products have been used for medicinal purposes since ancient times. This recipe can be made with barks, fruits, flowers, roots, leaves, or seeds. Knowing the chemical components of plants will improve the ability to synthesize complicated chemical molecules.(6)(7)
Description of the plant
Vitex negundo L. is popularly known as Huang ping, although locals also name it Bana or Surae. In Sanskrit, it is known as Nirgundi. This plant belongs to the Lamiaceae family. The kingdom is Plantae, the class is Magnoliopsida, the genus is Vitex, and the species is Vitex negundo L.(8)
Large bushes or tiny trees up to 4 metres tall.White-tomentose with quadrangular branches. Leaflets are petiolute, lanceolate, 2.5-12 cm long, acuminate, entire or crenate, subglabrous above and white-tomentose beneath; the petolule of the terminal leaflets is long. The leaves are opposite, with long stalks and digitally 3-5-foliolate. Large, terminal panicles with branched clusters of lavender to blue blooms measuring 8 mm long are arranged. Calyx is 5-toothed, white-woolly, and bell-shaped. Outside, the corolla is hairy, has two unclear lips, and five little, unequal lobes, the largest at the bottom. Stamens emerge in unequal pairs. Style shown.(9)
Classification:
- Kingdom – Plantae
- Phylum – Tracheophyta
- Class – Magnoliopsida
- Order – Lamiales
- Family – Lamiaceae
- Genus – Vitex
- Species – Vitex negundo L

Figure 1 vitex negundo l.
CULTIVATION
Bana is a branching shrub or small tree native to the Western Himalaya that can be found growing in hedges, roadsides, field margins, and wastelands up to 1500 meters in elevation. It is an easy-to-grow plant that prefers a mild, well-drained, sunny location. Bana can be reproduced through seeds and cuttings.
CULTIVATION
Vitex negundo L. stems were collected from the surrounding area of Sundarnagar, Himachal Pradesh, India, and shadow-dried for 25 days before being milled into powder. Amber-colored bottles were used to store powder. A 200-gram sample of plant powder was obtained, and depending on its polarity. We prepare our plant extract utilizing the Soxhlet Extraction process, which entails employing multiple solvents depending on their polarity. Examples of solvents include alcohol, petroleum ether, methanol, ethanol, and chloroform. The stem was extracted using methanol as a solvent. Following four extraction cycles, the solvent was drained off and an evaporator was used to concentrate the extract.
MORPHOLOGY
The bark is thin and grey, and the branchlets are quadrangular, pale, and covered in fine tomentum. The plant has 3-5 foliate leaves with lancoelate, acute leaflets. The terminal leaflet measures 5-10 cm by 1.6-3.2 cm and has a 1-1.3 cm long petiole. The lateral leaflets are smaller and have a very short petiole. The leaves are virtually glaborous above and coated with a fine white tomentum beneath. The base is acute. The common petioles are 2.5-3.8 cm long.(10) Flowers in pedunculate branched tomentose cymes, opposed along the quadrangular tomentose rachis of a big terminal compound pyramidal panicle (axillary peduncles in the top axils may be present). Bracts are 1.5-2.5 mm long and lanceolate caduceus. The calyx is 3 mm long and white tomentose, with triangular teeth measuring 0.8-1 mm in length. The ovary is glaborous, as is the style, and the stigma is forked. Drupes are less than 6 mm in diameter and turn black when ripe.(10)(11) The plant has a pungent, bitter, and acrid taste. It has a heating, astringent, stomachic, and anthelmintic properties. It also encourages hair development and is beneficial for eye diseases, consumption, inflammation, leucoderma, spleen enlargement, bronchitis, asthma, biliousness, and difficult teething in infants. The root is an antidote against snake poison.(12) The root is used as a tonic, febrifuge, expectorant, and treatment for a variety of ailments including otalgia, arthritis, dyspepsia, colic, rheumatism, leprosy, verminosis, flatulence, dysentery, urinary disorders, wounds, ulcers, bronchitis, cough, malarial fever, haemorrhoids, dysmenorrhea, skin diseases, and general debility. The plant's properties include expectorant, carminative, digestive, anodyne, antiseptic, alterant, antipyretic, diuretic, emmenagogue, depurative, rejuvenating, ophthalmic, vulnerary, and tonic.(12) The leaves are fragrant, tonic, and vermifuge. Nirgundi leaves decocted with long pepper are used to treat catarrhal fever symptoms such as heaviness of head and impaired hearing. To relieve headaches, place a cushion packed with Nirgundi leaves beneath the head.(13) The juice of the leaves is believed to remove foetid discharges and worms from ulcers. The flowers can help treat diarrhoea, cholera, fever, haemorrhages, hepatopathy, and heart problems. The leaves and bark can be used to treat scorpion stings, while the seeds can help with eye illnesses like anjan.(13)
MICROSCOPY
Transverse section
The root exhibits cork cells with 10 to 18 tangential rows, varying in size from rectangular to cubic, and reasonably thick walls. There are also a few rows of cork cells that are organized radially. The inner three to five rows of cork cells have thin walls. Cork cambium is made up of squarish to transversely elongated cells arranged in a single row. Secondary cortical cells range from rectangular to elongated, with 4-12 rows and some containing starch grains. Numerous, small clusters of stone cells were discovered scattered.(14)

Figure 2 Transverse Sections Of Vitex Negundo Linn. Root.
The secondary phloem is made up of sieve tubes filled with cells, fibers, and parenchyma, all connected by phloem rays. Each band of phloem is made up of thin-walled phloem tissues that alternate with transverse bands of thick-walled phloem fibres. A few tangential strips of obliterated phloem tissue can also be found in the outer phloem zone. Each fiber group consists of 6-60 thick-walled, long and short fibers. The inner zone of the phloem is made up of thin-walled tissues such as sieve tubes, companion cells, and parenchyma.(14)(15)
Powder characteristics
Powder is brown in colour. The powder contains parenchymatous cells with oval to round and compound starch grains measuring 8 to 12 µ in diameter. Stone cells are elongated, rectangular, and squarish in shape, with wide and narrow lumens, radiating canals, and conspicuous striations. The xylem vessels have pitted thickening, and the xylem and phloem fibers have thick walls.(14)

Figure 3 Fibre, Medullary rays, Stone cell, Starch grains, etc.

Figure 4 Powder form of Vitex negundo linn. root
Phytochemical study: -
The entire herb was then subjected to a preliminary phytochemical examination in accordance with conventional protocols. Several reagents are available for phytochemical screening.
Preliminary Qualitative Analysis:-
Phytochemical analysis is perform to identify different phytochemical present in the stem part of Vitex negundo l. by using different tests (14).
1. Test for Alkaloids
a. Mayer’s test
Take few ml of plant sample extract, two drops of Mayer’s reagent are added along the sides of test tube. Appearance of white creamy precipitate indicates the presence of alkaloids.[15]
b. Wagner’s test
A few drops of Wagner’s reagent are added to few ml of plant extract along the sides of test tube. A reddish- Brown precipitate confirms the test as positive.[16]
2 Test for Carbohydrates
A. Molish’s test
To 2 ml of plant sample extract, two drops of alcoholic solution of ?- naphthol are added. The mixture is shaken well and few drops of concentrated sulphuric acid is added slowly along the sides of test tube. A violet ring indicates the presence of carbohydrates.
b. Benedict’s test
To 0.5 ml of filtrate, 0.5 ml of Benedict?s reagent is added. The mixture is heated on a boiling water bath for 2 minutes. A characteristic coloured precipitate indicates the presence of sugar.
3. Test for Fixed oils and Fats
a. Spot test
A small quantity of extract is pressed between two filter papers. Oil stain on the paper indicates the presence of fixed oils.
b. Saponification test
A few drops of 0.5 N alcoholic potassium hydroxide solution is added to a small quantity of extract along with a drop of phenolphthalein. The mixture is heated on a water bath for 2 hours. Formation of soap or partial neutralization of alkali indicates the presence of fixed oils and fats.[17]
4. Test for Glycosides
For 50 mg of extract is hydrolysed with concentrated hydrochloric acid for 2 hours on a water bath, filtered and the hydrolysate is subjected to the following tests.
a. Borntrager’s test
To 2 ml of filtered hydrolysate, 3 ml of choloroform is added and shaken, choloroform layer is separated and 10% ammomia solution is added to it. Pink colour indicates presence of glycosides.[15]
b. Legal’s test
50 mg of extract is dissolved in pyridine, sodium nitroprusside solution is added and made alkaline using 10% NaOH. Presence of glycoside is indicated by pink colour.
5. Test for Phenolic compounds and Tannins
a. Ferric Chloride test
The extract (50 mg) is dissolved in 5 ml of distilled water. To this few drops of neutral 5?rric chloride solution are added. A dark green colour indicates the presence of phenolic compound.[18]
b. Gelatin test
The extract (50 mg) is dissolved in 5 ml of distilled water and 2 ml of 1% solution of Gelatin containing 10% NaCl is added to it. White precipitate indicates the presence of phenolic compounds.[15]
c. Lead acetate test
The extract (50 mg) is dissolved in of distilled water and to this 3 ml of 10% lead acetate solution is added. A bulky white precipitate indicates the presence of phenolic compounds.
d. Alkaline reagent test
An aqueous solution of the extract is treated with 10% ammonium hydroxide solution. Yellow fluorescence indicates the presence of flavonoids.
e. Magnesium and Hydrochloric acid reduction
The extract (50 mg) is dissolved in 5 ml of alcohol and few fragments of magnesium ribbon and concentrated hydrochloric acid (drop wise) are added. If any pink to crimson colour develops, presence of flavonol glucosides is inferred.[19]
6. Test for Proteins
The extract (100 mg) is dissolved in 10 ml of distilled water and filtered through Whatmann No. 1 filter paper and the filtrate is subjected to test for proteins.
a. Millon’s test
To 2 ml of filtrate few drops of Millon?s reagent are added. A white precipitate indicates the presence of proteins.[20]
b. Biuret test
2 ml of filtrate is treated with 1 drop of 2% copper sulphate solution. To this 1 ml of ethanol (95%) is added, followed by excess of potassium hydroxide pellets. Pink colour ethanolic layer indicates the presence of protein.[note]
7. Test for Saponins
The extract (50 mg) is diluted with distilled water and made up to 20 ml. The suspension is shaken in a graduated cylinder for 15 minutes. A two cm layer of foam indicates the presence of saponins.[17]
RESULT: -
The yield of leaf extracts in methanol was 3.4, 2.12, 3.5, and 1.8%.
Phytochemical Studies :
Preliminary Phytochemical Screening: The color reactions were graded using the following symbols: (-) for no visible changes, (+) for mild intensity, and (++) for extreme intensity. The powdered sample was chemically screened for alkaloids, phenols, tannins, flavonoids, quinones, terpenes, carbohydrates, proteins, gums, and mucilage using conventional techniques.
Preliminary of phytochemical analysis of extracts of V. negundo stem. We perform several tests, such a
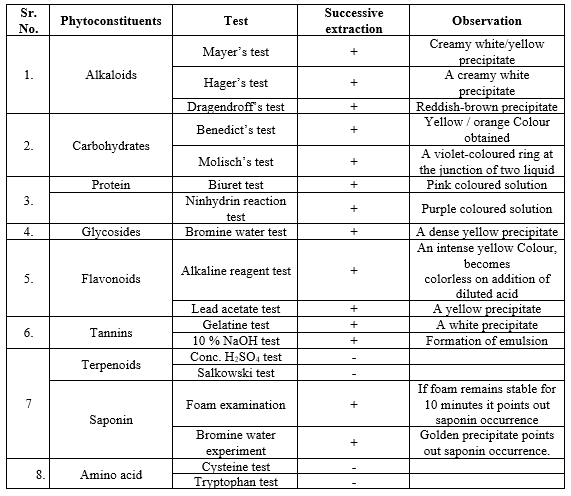
OBSERVATION
Pharmacogostic study of vitex negundo l. shows that the bark is thin and grey, the plant has 3-5 foliate leaves. The terminal leaflet measures 5-10 cm by 1.6-3.2 cm and has a 1-1.3 cm long petiole. The root exhibits cork cells with 10 to 18 tangential rows, there are also a few rows of cork cells that are organized radially. Secondary cortical cells range from rectangular to elongated, with 4-12 rows and some containing starch grains. Numerous, small clusters of stone cells were discovered scattered. In this study of vitex negundo l. preliminary phytochemical analysis observation of (creamy white / yellow precipitate) showed the presence of alkaloids, (dense yellow precipitate) showed the presence of glycosides, (white precipitates) showed the presence of tannins, (pink colour solution) showed the presence of proteins.
CONCLUSION
In vitex negundo l. research pharmacognostic study shows its bark which is thin and grey, and the plant has 3-5 foliate leaves. The root exhibits cork cells with 10 to 18 tangential rows with 4-12 rows and some containing starch grains. Vitex negundo l. preliminary phytochemical analysis observation showed the presence of alkaloids, glycosides, tannins, proteins, etc. The plant must explore more as it has various medicinal properties like it is used in anti-malarial, anti-oxidant, also in anti-inflammatory, and many more.
REFERENCES
- Bijauliya RK, Jain SK, Alok S, Dixit VK, Singh D and Singh M: Dalbergia sissoo Linn. An overview morphology, phytochemistry and pharmacology. Int J Pharm Sci Res 2017; 8(4): 1522-33.doi: 10.13040/IJPSR.0975-8232.8(4).1522-33
- Hassan LG, Mshelia HE, Umar KJ, Kangiwa SM, Ogbiko C, Yusuf AJ. Phytochemical Screening, Isolation and Characterization of Beta-Sitosterol from ethyl acetate Extract of Stem Bark of Entada africana (Fabaceae) Guill. et Perr. J Chem Soc Niger. 2018;43(3).
- Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World journal of agricultural sciences. 2009;5(5):572-6.
- Edoga, H.O., Okwu, D.E., Mbaebie, B.O. 2005. Phytochemicals constituents of some Nigerian medicinal plants. Afr. J. Biotechnol., 4(7): 685-688.
- Mojab, F., Kamalinejad, M., Ghaderi, N., Vanidipour, H.R. 2003. Phytochemicals screening of some species of Iranian plants. Iran. J. Pharm. Res., 3: 77-82.
- Parekh, J., Chanda, S. 2007. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biomed. Res., 10: 175-181.
- Parekh, J., Chanda, S. 2008. Phytochemicals screening of some plants from western region of India. Plant Arch., 8: 657-662.
- Thakur S. Ethnobotany of Rewalsar Himalaya. Deep Publications; 2004.
- Chatterjee A, Pakrashi SC. The Treaties On Indian medicinal plants, volume-3. Natl Inst Sci Commun (CSIR), New Delhi. 1997;16.
- Kirtikar KR, Basu BD. Indian medicinal plants. Text Vol.3, International Book Distributors, Dehradun 2008; 1937- 1940.
- Sharma PC, Yelne MB, Dennis T.J. Database on Medicinal Plants used in Ayurveda. CCRAS Publication, Vol. 3, New Delhi: Reprint Edition 2005; p. 450-471.
- Gupta AK, Tandon N, Madhu S. Quality standards of Indian Medicinal Plants. Indian Council of Medical Research, Vol. 3, New Delhi 2008; p. 130-135.
- Giri Prasad T, Das V. Evaluation of phytochemical and antimicrobial activities of Vitex negundo L. leaf extracts. International journal of research and analytical reviews. 2018;5(3):888-92.
- Arthi, I; S, Neethu Krishnan pharmacognostic and phytochemical evaluation on vitex negundo l. 2021.
- Jain M, Bhandari A, Bhandari Ankansha, Patel P. Isolation, characterization and in-vitro Antiurolithiatic activity of cerpegin alkaloid from Ceropegia bulbosa var.lushii root. Int J Drug Dev & Res, 2012; 4(4): 154-160.
- De Silva GO, Abeysundara AT, Aponso MMW. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. Am J Essent Oils Nat Prod. 2017;5(2):29–32.
- Singh V, Kumar R. Study of phytochemical analysis and antioxidant activity of Allium sativum of Bundelkhand region. Int J Life-Sciences Sci Res. 2017;3(6):1451–8.
- Raaman N. Phytochemical techniques. New India Publishing; 2006.
- Kumar MK, Kaur G, Kaur H. INTERNATIONALE PHARMACEUTICA SCIENCIA. 2011;
- Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). In: Veterinary research forum: an international quarterly journal. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran; 2014. p. 95.


 Dinesh Kumar Thakur*
Dinesh Kumar Thakur*
 Dev Prakash Dahiya
Dev Prakash Dahiya





 10.5281/zenodo.11190750
10.5281/zenodo.11190750