Abstract
The study focused on developing a fast-disintegrating oral tablet of Betaxolol Hydrochloride aimed at treating hypertension through a direct compression method. Various formulations were tested using different concentrations of super disintegrants, with Microcrystalline cellulose serving as the diluent. Mannitol was utilized as a directly compressible diluent, while aspartame was chosen for its intense sweetness. Additionally, magnesium stearate and talc acted as lubricants, and aerosil was included to enhance flow properties. Pineapple flavor was added to improve the tablet's palatability. The evaluation of the tablets included assessments of thickness, weight variation, friability, hardness, content uniformity, wetting time, water absorption ratio, disintegration time, in vitro dissolution time, and dispersion time. The formulation labeled MF9, which contained 5.3% Cross Carmellose Sodium, emerged as the optimized batch due to its rapid disintegration time of just 26 seconds and an impressive drug release of 99.59% within 3 minutes. Fourier Transform Infrared (FT-IR) analysis confirmed no incompatibility between the drug and the excipients used. Differential Scanning Calorimetry (DSC) results indicated no significant alterations in peak characteristics, suggesting a lack of chemical interaction among the components. This indicates that the fast-disintegrating oral tablet of Betaxolol Hydrochloride could be a valuable formulation for hypertension treatment where a quick onset of action is essential.
Keywords
Betaxolol Hydrochloride, fast dissolving tablets, cross carmellose sodium, direct compression method.
Introduction
Oral medication administration is widely recognized as a convenient, safe, and cost-effective method for drug delivery. This approach simplifies the intake process, ensuring precise dosing and facilitating self-medication while minimizing discomfort. Fast-dissolving drug delivery systems, such as orodispersible tablets (ODTs), represent an innovative strategy that allows medications to dissolve quickly in the mouth without water, enhancing patient compliance through rapid release and improved bioavailability. The advantages of oral administration include its non-invasive nature, which reduces complications associated with injections, and its accessibility for a diverse range of patients. Fast-dissolving formulations are particularly beneficial for individuals with dysphagia (difficulty swallowing), such as children and the elderly. These systems provide a quick onset of action while bypassing first-pass metabolism, thereby improving therapeutic efficacy and catering to patient preferences for ease of use.Dysphagia poses significant challenges, often leading to noncompliance with medication regimens, especially in pediatric and geriatric populations. Traditional solid dosage forms like tablets can be difficult for these groups, resulting in treatment failures. To address this, ODTs of Betaxolol Hydrochloride are being developed. These tablets disintegrate rapidly in the oral cavity, making them suitable for those with swallowing difficulties and active individuals seeking convenience.[1,2]
The primary objectives of this research include enhancing patient compliance, developing a cost-effective product, and improving the onset of action and overall safety and efficacy of Betaxolol HCl. As a selective beta-1 adrenergic antagonist used for conditions like hypertension and glaucoma, Betaxolol's formulation into ODTs aims to improve its dissolution rate and bioavailability through direct compression techniques using various super disintegrants. Key research activities will involve pre-formulation studies on the drug and polymers, formulation of fast-disintegrating tablets, and evaluation of their physical properties—such as weight variation, friability, hardness, content uniformity, thickness, wetting time, water absorption ratio, and disintegration time. In vitro dissolution studies will also be conducted to assess the release characteristics of the ODTs. Overall, this research seeks to provide effective solutions for patients facing swallowing difficulties while ensuring a rapid therapeutic response from Betaxolol Hydrochloride
METHODOLOGY
Pre-formulation Studies [3,4,5]
Pre-formulation testing is the initial step in developing drug dosage forms, focusing on the physical and chemical properties of the drug substance alone and in combination with excipients. The main objectives are to generate information that aids formulators in creating stable and bioavailable dosage forms for large-scale production.
Identification of Drug
- Melting Point: Determined using the open capillary method for pure Betaxolol HCl.
- UV Spectrum Analysis: A solution of Betaxolol HCl in phosphate buffer (pH 6.8) was scanned from 200-400 nm to identify the maximum wavelength.
- Solubility: Assessed in water and alcohol.
Preparation of Standard Calibration Curve for Betaxolol HCl
Stock Solution Preparation
To prepare a stock solution, dissolve 10 mg of Betaxolol HCl in 100 ml of pH 6.8 buffer, yielding a concentration of 100 ?g/ml. Similar steps were followed using pH 1.2 buffer for another stock solution.
Calibration Curve in pH 1.2 Buffer
- Dissolve 10 mg of Betaxolol HCl in 100 ml of pH 1.2 buffer to create a working standard.
- Prepare aliquots (0.2 ml to 1 ml) corresponding to concentrations of 2 to 10 ?g/ml.
- Measure absorbance at 273 nm using a UV-visible spectrophotometer, plotting absorbance against concentration to confirm linearity per Beer’s law.
Calibration Curve in pH 6.8 Buffer
- Similar steps as above, but measure absorbance at 224 nm.
- Plot absorbance versus concentration, confirming linearity over the range of 2 to 10 ?g/ml.
Preparation of Buffer Solutions and ReagentsAll reagents were prepared according to Indian Pharmacopoeia (I.P.) guidelines, ensuring consistency and reliability in experimental procedures.
Sodium Hydroxide (0.2 M) Solution:
Dissolve 8 grams of sodium hydroxide (NaOH) in approximately 700 ml of distilled water in a 1000 ml volumetric flask, then bring to the mark with additional distilled water.
Potassium Dihydrogen Phosphate (0.2 M) Solution:
Dissolve 27.218 grams of potassium dihydrogen phosphate in about 700 ml of distilled water in a 1000 ml volumetric flask, and adjust the volume to the mark with distilled water.
Phosphate Buffer (pH 6.8):
Transfer 50 ml of the 0.2 M potassium dihydrogen phosphate solution to a 200 ml volumetric flask, add 22.4 ml of the 0.2 M sodium hydroxide solution, and fill to 200 ml with distilled water. Adjust the pH to 6.8 using dilute sodium hydroxide.
Hydrochloric Acid (0.2 M) Solution:
Prepare by diluting concentrated hydrochloric acid to achieve a final concentration of 7.292 grams in 1000 ml.
Potassium Chloride (0.2 M) Solution:
Dissolve approximately 14.911 grams of potassium chloride in distilled water and adjust the total volume to 1000 ml.
pH 1.2 Hydrochloric Acid Buffer:
Combine 250 ml of the 0.2 M potassium chloride solution with about 425 ml of the 0.2 M hydrochloric acid in a 1000 ml volumetric flask, then adjust the volume with distilled water and fine-tune the pH to 1.2 using hydrochloric acid.
- Differential Scanning Calorimetry (DSC): DSC measures heat flow differences between a sample and reference against temperature, helping identify impurities from drug-polymer interactions by comparing DSC curves of pure drug samples with formulations.
- Fourier Transform Infrared (FT-IR) Studies: FT-IR spectroscopy is used to assess compatibility between the drug and excipients, preventing degradation from interactions. FT-IR spectra are obtained for the drug and physical mixtures by preparing KBr pellets from sample powder and KBr.
- Formulation Development [6,7,8]
Table No 1: The formulation design of orodispersible tablets of Betaxolol HCL
|
Sl.no
|
Ingredients (mg)
|
MF1
|
MF2
|
MF3
|
MF4
|
MF5
|
MF6
|
MF7
|
MF8
|
MF9
|
|
1
|
Betaxolol hcl
|
10
|
10
|
10
|
10
|
10
|
10
|
10
|
10
|
10
|
|
2
|
Sodium starch glycolate
|
4
|
6
|
8
|
-
|
-
|
-
|
-
|
-
|
-
|
|
3
|
Crospovidone
|
-
|
-
|
-
|
4
|
6
|
8
|
-
|
-
|
-
|
|
4
|
Cross carmellose sodium
|
-
|
-
|
-
|
-
|
-
|
-
|
4
|
6
|
8
|
|
5
|
Microcrystalline cellulose
|
50
|
50
|
50
|
50
|
50
|
50
|
50
|
50
|
50
|
|
6
|
Aspartame
|
6
|
6
|
6
|
6
|
6
|
6
|
6
|
6
|
6
|
|
7
|
Magnesium stearate
|
1.5
|
1.5
|
1.5
|
1.5
|
1.5
|
1.5
|
1.5
|
1.5
|
1.5
|
|
8
|
Talc
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
|
9
|
Aerosil
|
0.5
|
0.5
|
0.5
|
0.5
|
0.5
|
0.5
|
0.5
|
0.5
|
0.5
|
|
10
|
Pineapple flavour
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
1
|
|
11
|
Mannitol
|
76
|
74
|
72
|
76
|
74
|
72
|
76
|
74
|
72
|
|
|
Total
|
150
|
150
|
150
|
150
|
150
|
150
|
150
|
150
|
150
|
Tablets were prepared in batch of 100
Betaxolol HCl orodispersible tablets were formulated using the direct compression method. The formulation included various superdisintegrants at different concentrations, Microcrystalline cellulose as a diluent, mannitol as a directly compressible diluent, and aspartame for sweetness. Additionally, magnesium stearate and talc served as lubricants, while aerosil acted as a flow promoter. Pineapple flavor was added to enhance the tablets' palatability.
Preparation of Betaxolol HCl Orodispersible Tablets by Direct Compression
Nine formulations (MF1 to MF9) of Betaxolol HCl orodispersible tablets were prepared, maintaining a constant total tablet weight of 150 mg. The process mirrored the powder blend preparation:
- Ingredient Mixing: Microcrystalline cellulose, mannitol, and superdisintegrants were mixed first.
- Drug Addition: The drug and aspartame were incorporated next.
- Lubrication: The blend was lubricated with magnesium stearate, talc, and aerosil.
- Flavoring: Finally, the flavor was added.
The powder blend was compressed into tablets using an 8 mm round flat-faced punch on a rotary tablet compression machine (Cad mach Machineries Ltd.), with constant compression force applied across all formulations.
Post-compression evaluations included hardness, friability, thickness, weight variation, and in vitro dispersion time wetting time, water absorption ratio, drug content, In-vitro disintegration time and In-vitro dissolution
Pre-compression assessment of powder blend[9,10,11]:
Different parameters were evaluated for prepared powder blend using following methods.
- Angle of repose: The angle of repose of the powder was determined using the funnel method. A weighed amount of powder was placed in the funnel, adjusted to touch the apex, and allowed to flow freely onto a surface.
- Bulk Density: Bulk density is the ratio of a powder's total mass to its bulk volume. To measure it, weigh 2g of powder (sieved through a 20 mesh), pour it into a 10ml graduated cylinder, and record the initial volume, known as bulk volume.
- Tapped Density: Tapped density is the ratio of a powder's total mass to its tapped volume. To measure it, an accurately weighed amount of powder is placed in a measuring cylinder, and the volume is measured after tapping the cylinder 500 times.
- Compressibility Index: Compressibility index is indicates the powder flow properties. It is ex pressed in percentage. Compressibility index is based on the bulk density and tapped density, the percentage compressibility of the powder blend was determined by using the following formula.
I=Tapped density-Bulk density Tapped density ×100
- Hausner Ratio: Hausner ratio is an indirect index of ease of powder flow.It was calculated by the following formula.
Hausner ratio = Dt / Db
post-compression assessment of powder blend[12,13,14,15]:
- Hardness: Tablet hardness was measured using a Monsanto tablet hardness tester to determine the crushing strength (fc). Hardness is expressed in kg/cm?2;.
- Friability: The friability test for tablets was conducted using a Roche friabilator to evaluate their resistance to abrasion and shock.The percentage of friability is calculated using the formula:
%F=W initial – W finalW initial ×100
×100
- Thickness: The thickness of tablets was measured by using vernier caliper. Thickness is expressed in mm.
- Weight Variation:The weight variation test was performed following I.P. standards by weighing twenty randomly selected tablets from each batch. The average weight was calculated, and individual weights were compared to it. Tablets passed the test if no more than two tablets were outside the percentage limit and no tablet differed by more than twice that limit.
- In-vitro Dispersion Time: In-vitro dispersion time was measured by dropping a tablet into a petridish containing 10ml of phosphate buffer pH 6.8 solution at 37± 0.50 c. Three tablets from each batch were randomly selected and tested the time required for complete dispersion of a tablet was measured. The in-vitro dispersion time is expressed in seconds.
- Wetting Time: A piece of tissue paper folded double was placed in a Petri dish (6.5cm) containing 6 ml of water. The tablet was placed on the paper, and the time for complete wetting of the tablet was measured in seconds. The method was slightly modified by maintaining water at 370 c. Wetting time corresponding to the time taken for the tablet to disintegrate when kept motionless on the Petri dish.
- Water Absorption Ratio:A piece of tissue paper folded twice was placed in a petri dish (6.5cm) containing 6 ml of water. A tablet was put on the tissue paper and the time required for the complete wetting was measured. The wetted tablet was then weighed. Water absorption ratio, R, was determined using following equation.
R = 100 (Wa-Wb) / Wb
- Disintegration Time: The in-vitro disintegration time of a tablet was assessed using a disintegration test apparatus. One tablet was placed in each of the six tubes, along with a disc, and the apparatus was operated with distilled water at 37°C ± 2°C. The complete disintegration time, measured in seconds, was recorded when no palpable mass remained in the apparatus.
- In-vitro Dissolution Studies: In-vitro dissolution studies for Oro dispersible tablets of Betaxolol HCL were carried out using USP apparatus type II at 50 rpm. The dissolution medium used was phosphate buffer pH 6.8 (300ml) maintained at 37 ± 0.5oC.
RESULTS AND DISCUSSION
A) Melting Point: The melting point of the Betaxolol Hcl was found to be 114 °C, which complies with given in the official reference.
B) UV Spectroscopy: The ?max of pure Betaxolol HCL was found to be 224 nm after scanning on the spectrophotometer, which complies with the reference spectra of Betaxolol HCL.
C) Solubility: The Betaxolol Hydrochloride was found to be freely soluble in water and alcohol.
D) I.R. Spectroscopy:
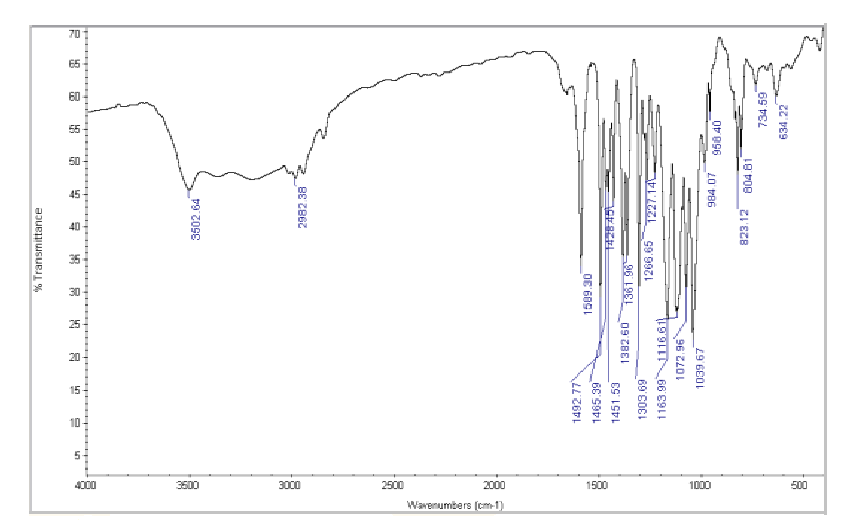
Figure No.1: FTIR Spectrum of Betaxolol HCL
Drug and Excipients Compatibility Study:FTIR spectra of Betaxolol HCl and its physical mixture with excipients were obtained using an IR spectrophotometer, scanning from 4000 to 400 cm??1; at a resolution of 1 cm??1;. The spectra revealed characteristic absorption peaks for both the pure drug and the mixture, indicating compatibility between Betaxolol HCl and the excipients.
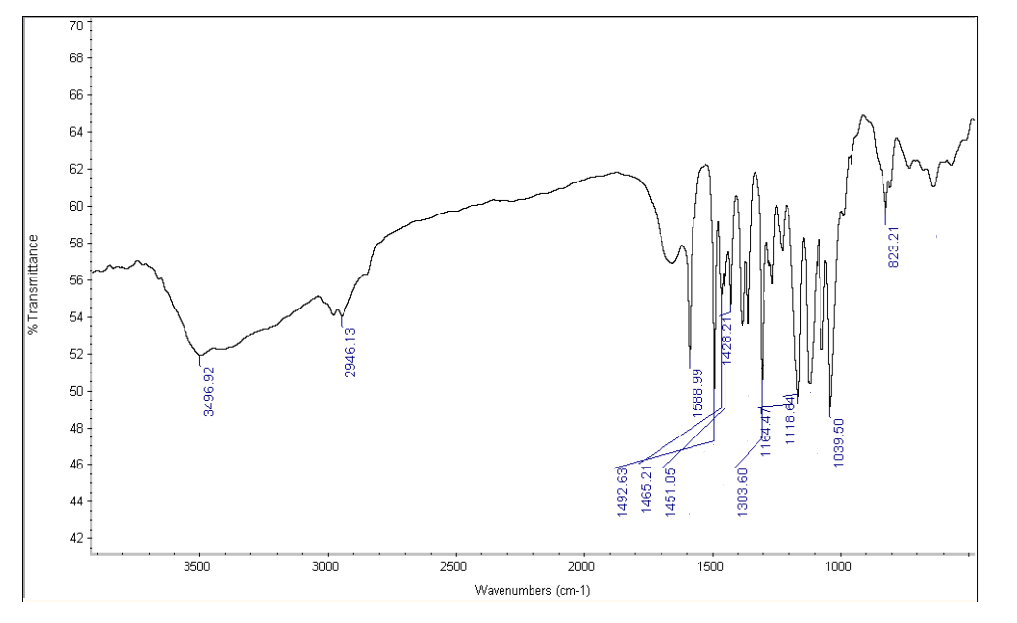
Figure No.2: FTIR Spectrum of Drug with Crospovidone
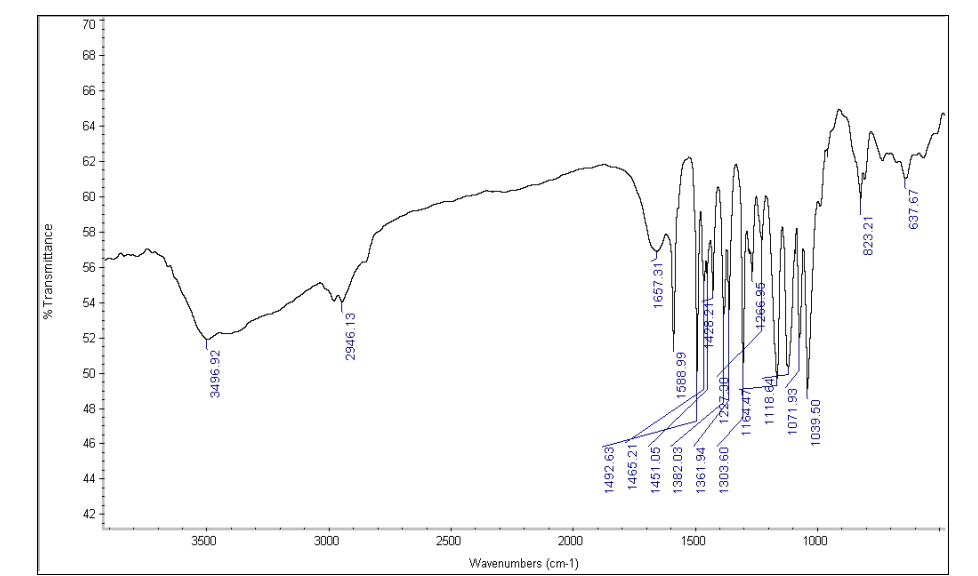
Figure No. 3: FTIR Spectrum of Drug with sodium starch glycolate
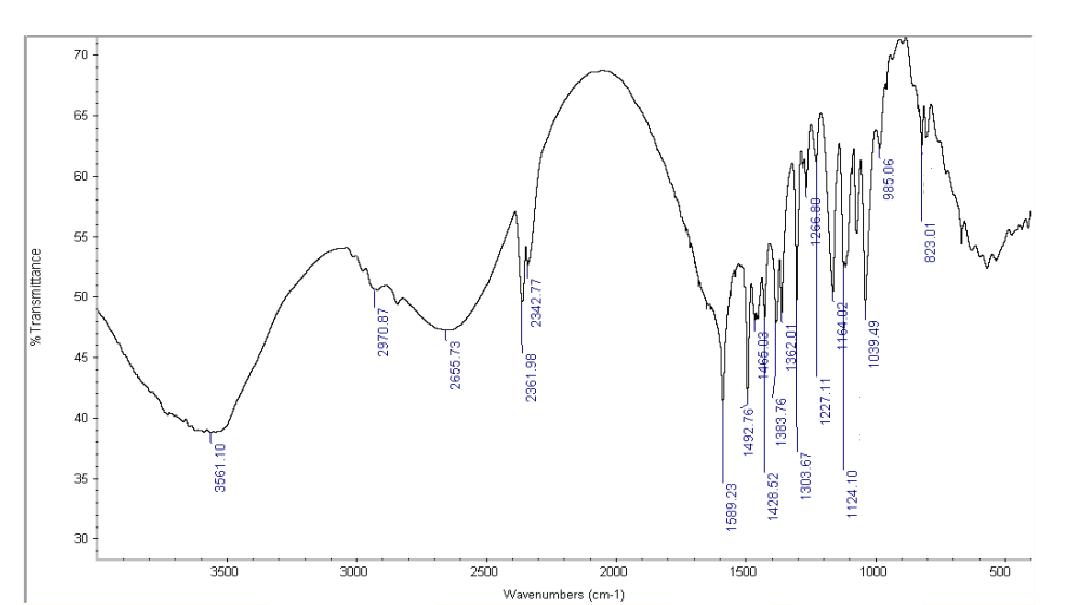
Figure No. 4: FTIR Spectrum of Drug with Cross Carmellose Sodium
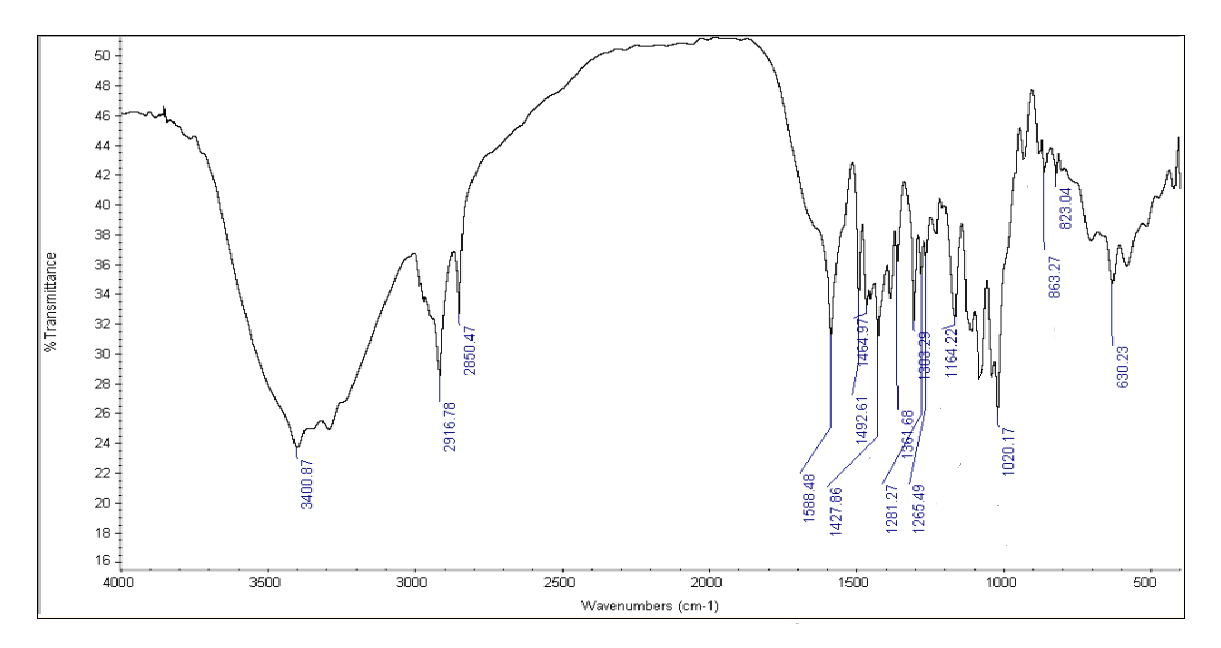
Figure No. 5: FTIR Spectrum of Betaxolol HCL with all excipients
Table No 2: FTIR spectra for the mixture of Betaxolol HCL with various super disintegrants and with all excipients.
|
Transition
|
IR Range
|
Drug +
Crospovidone
|
Drug + sodium
starch glycolate
|
Drug + Cross
Carmellose
Sodium
|
Drug +
all
excipients
|
|
C=C
|
1500-1600
|
1588.99
|
1588.99
|
1589.23
|
1427.66
|
|
C=O
|
1820-1660
|
|
1657.31
|
-
|
1588.48
|
|
C-C
|
800-1300
|
823.21
|
823.21
|
829.01
|
823.27
|
|
N-H
|
3300-3500
|
3496.92
|
3496.92
|
3561.10
|
3400.87
|
|
C-O
|
1300-1000
|
1118.64
|
1118.64
|
1124.10
|
1164.22
|
|
C-H
|
3000-2850
|
2946.13
|
2946.13
|
2970.87
|
2916.78
|
The IR spectra of pure Betaxolol HCl and its 1:1 mixtures with Crospovidone, Cross Carmellose Sodium, and sodium starch glycolate showed no significant differences, indicating no interaction between Betaxolol HCl and the excipients. This suggests that these super disintegrants are suitable for formulating orodispersible tablets. A standard calibration curve for Betaxolol HCl was also established for quantification in formulations.
Preparation of standard calibration curve in hydrochloric acid buffer pH 1.2:
The standard calibration curve for Betaxolol HCl was developed using a hydrochloric acid buffer at pH 1.2. Absorbance values plotted against concentrations (2-10 ?g/ml) at 224.0 nm showed a linear relationship with a correlation coefficient of 0.9993, confirming adherence to Beer-Lambert's Law, as detailed in Table 11 and illustrated in Figure 12.
Table No 3: Absorbance values for standard calibration curve of Betaxolol HCL in hydrochloric acid buffer pH 1.2
|
Sl.no
|
Concentration (?g/ml)
|
Absorbance
|
|
1
|
0
|
0
|
|
2
|
2
|
0.193
|
|
3
|
4
|
0.392
|
|
4
|
6
|
0.584
|
|
5
|
8
|
0.772
|
|
6
|
10
|
0.940
|
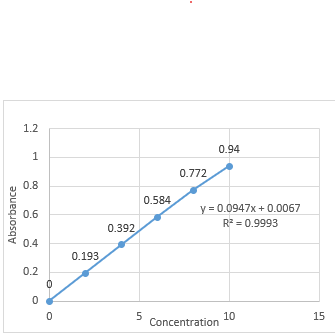
Figure No. 6: standard calibration curve for Betaxolol HCL at 224.0 nm. In Hydrochloric acid buffer pH 1.2
Calibration curve of Betaxolol HCL in phosphate buffer pH 6.8:
1) UV Spectra of drug in phosphate buffer pH 6.8:
A 50 ?g/ml solution of Metoclopramide HCl was prepared in a phosphate buffer with a pH of 6.8 and analysed using a UV-visible spectrophotometer over a wavelength range of 200 to 400 nm. The maximum absorbance (?max) was observed at 224 nm
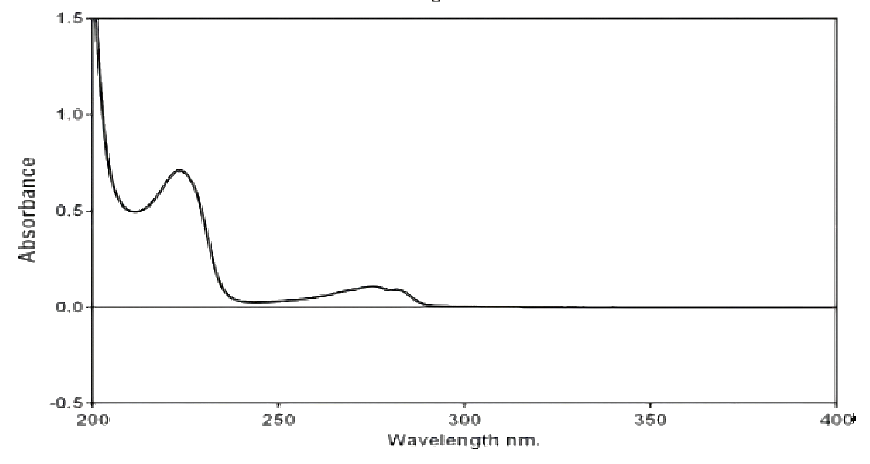
Figure No. 7: UV Spectrum of Betaxolol HCL in phosphate buffer pH 6.8
2) Preparation of standard calibration curve in phosphate buffer pH 6.8:
A standard calibration curve for Betaxolol HCl was created using phosphate buffer at pH 6.8 as the solvent, plotting absorbance against concentration. The absorbance values are in Table 12, and the curve, shown in Figure 14, had a correlation coefficient of 0.9965. It demonstrated linearity in the concentration range of 2-10 ?g/ml at 224.0 nm, confirming compliance with Beer-Lambert's Law.
Table No. 4: Absorbance values for standard calibration curve of Betaxolol HCL in phosphate buffer pH 6.8
|
Sl.no
|
Concentration (?g/ml)
|
Absorbance
|
|
1
|
0
|
0
|
|
2
|
2
|
0.165
|
|
3
|
4
|
0.331
|
|
4
|
6
|
0.512
|
|
5
|
8
|
0.723
|
|
6
|
10
|
0.920
|
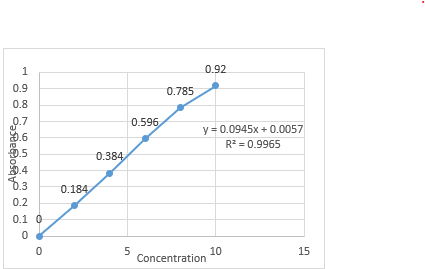
Figure No. 8: Standard calibration curve of Betaxolol HCL in pH 6.8 buffer.
Differential Scanning Calorimetry studies of Betaxolol HCL: The DSC analysis of Betaxolol hydrochloride shows an endothermic peak at 114°C, aligning with its melting point of 113.01°C, indicating energy absorption during melting. No significant changes in peak positions or appearances were observed in mixtures with excipients, suggesting no chemical interactions. This compatibility was further confirmed by a DSC pattern matching approach, indicating the absence of incompatibility between the drugs and excipients. The findings are illustrated in Figures 15-18.
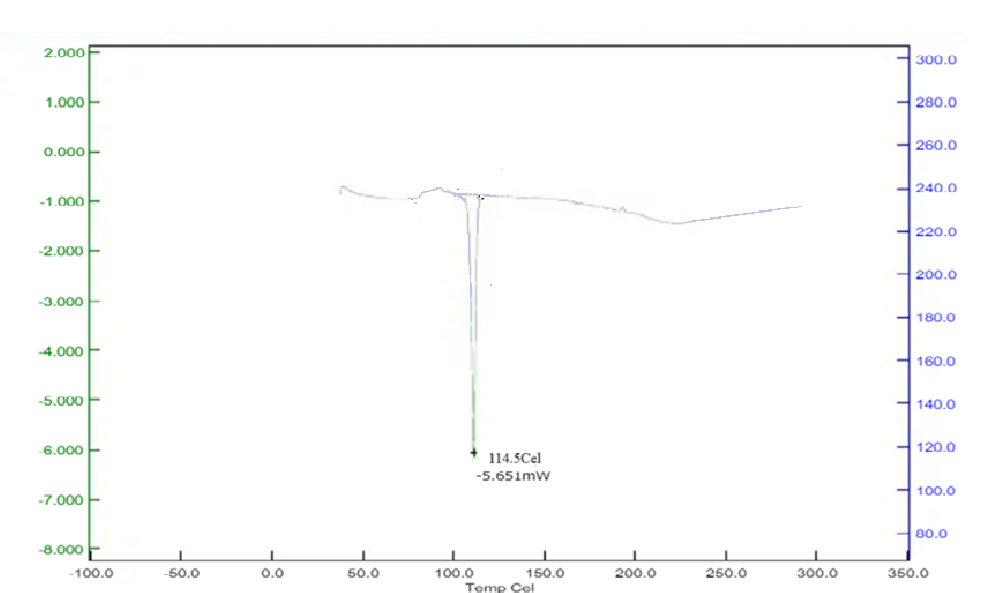
Figure No. 9: DSC of Drug
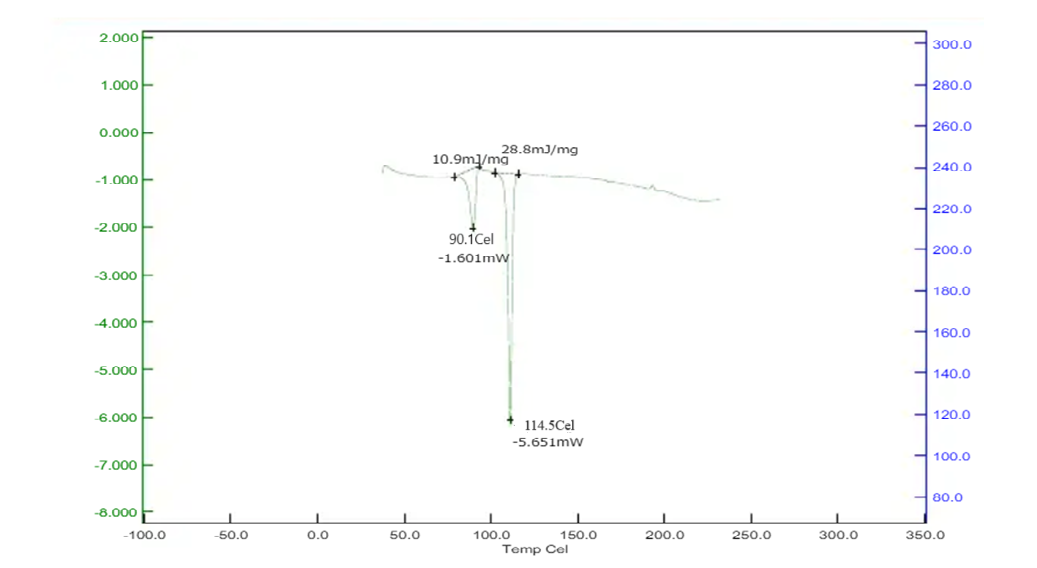
Figure No. 10: DSC of Drug with Sodium Starch Glycolate
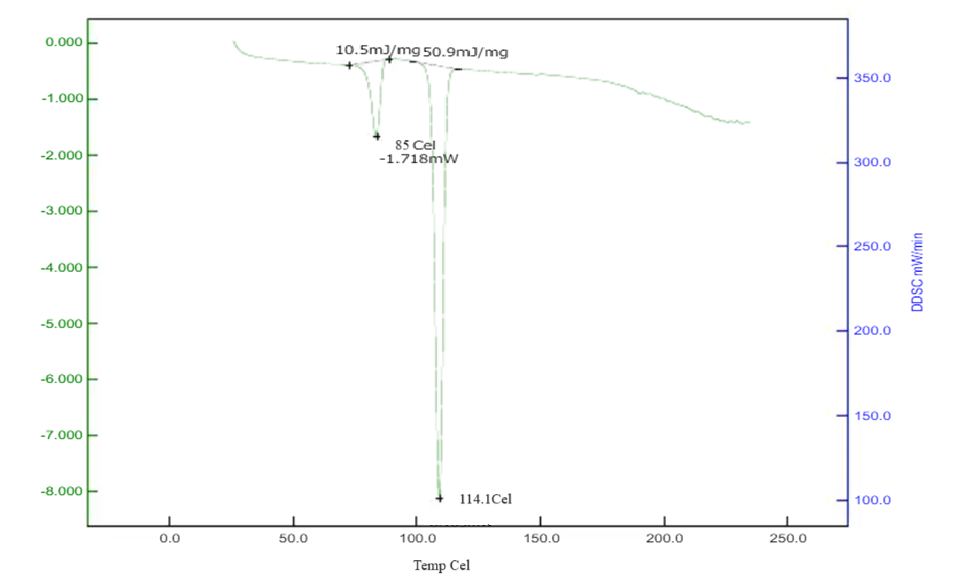
Figure No. 11: DSC of Drug with Cross Carmellose Sodium
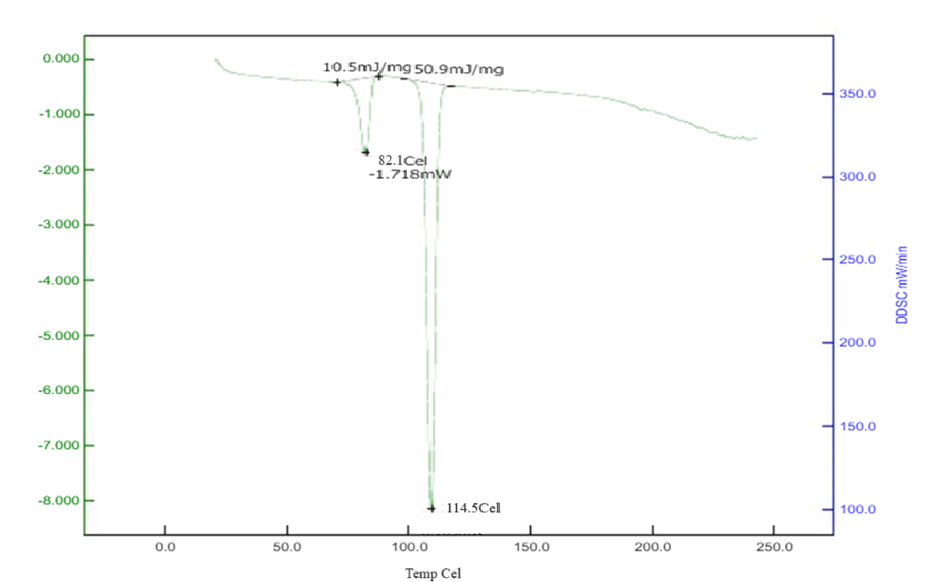
Figure No. 12: DSC of Drug with Crospovidone
Pre-compression study of tablet blend:
Table No. 5: Pre-compression Evaluation of Blended Powder
|
Batch Code
|
Angle of Repose(?)
|
Bulk Density (g/cm3)
|
Tapped density (gm/cm2)
|
Compressibility Index (%)
|
Hausner ratio
|
|
1
|
30.71 ± 0.27
|
0.520 ±0.04
|
0.602 ±0.06
|
17.32 ±0.2
|
1.213±0.05
|
|
2
|
33.11±0.17
|
0.525 ±0.03
|
0.623 ±0.07
|
17.28± 0.3
|
1.211±0.04
|
|
3
|
31.6 ±0.21
|
0.518 ±0.02
|
0.625 ±0.05
|
20.01±0.7
|
1.250±0.08
|
|
4
|
29.52±0.14
|
0.530 ±0.05
|
0.601 ±0.08
|
16.49±0.5
|
1.213±0.06
|
|
5
|
31.2 ±0.19
|
0.506 ±0.07
|
0.619 ±0.06
|
16.66±0.6
|
1.219±0.04
|
|
6
|
32.38 ±0.28
|
0.514 ±0.05
|
0.601 ±0.05
|
14.83±0.8
|
1.114±0.05
|
|
7
|
30.18 ±0.16
|
0.513 ±0.03
|
0.622 ±0.04
|
16.59±0.4
|
1.171±0.07
|
|
8
|
25.5 ±0.23
|
0.512 ±0.04
|
0.625 ±0.02
|
17.08±0.2
|
1.121±0.09
|
|
9
|
28.85 ±0.19
|
0.52 ±0.01
|
0.627 ±0.03
|
16.03±0.1
|
1.211±0.05
|
- Angle of Repose (?): The angle of repose of various powders mixed blend, prepared with different super disintegrants, was measured by cylinder method. Angle of repose was found in the range from 25.5 to 33.11 the good flowability of powder blend was also evidenced with angle of repose which is indicated a good flowability. The result are given in Table no.5
- Bulk density: The bulk density was measured by graduated cylinder. The bulk density was found in the range from 0.506 ±0.07 to 0.520±0.04. The result are given in Table no. 5
- Tapped density: The tapped densities of several powder mixed blends made with distinct super disintegrants were determined using a measuring cylinder. The range of the tapping density was discovered to be 0.601±0.05 to 0.627±0.03. The result are given in Table no. 5
- Compressibility Index: the compressibility index of several powder mixed blends made with various super disintegrants was computed using bulk density and tapped density data. It was discovered between 14.83±0.8 and 20.01±0.7. The result are given in Table no.5
- Hausner ratio: The Hausner ratio of different powder mixed mixes made with distinct super disintegrants was computed using data on bulk density and tapped density as per the Indian Pharmacopoeia (I.P) guidelines. It was discovered to be between 1.14±0.05 and 1.250±0.08. The result are given in Table no.5
Evaluation of orodispersible tablets of Betaxolol HCl:
Table No.6: Post-compression Evaluation of orodispersible tablets of Betaxolol HCl
|
Batch Code
|
Hardness
(kg/cm2)
|
Friability (%)
|
Thickness
(mm)
|
Weight (mg) ± S. D
|
Dispersion Time(sec)
|
Water absorption ratio ± S. D
|
Disintegrating Time(sec)
|
|
1
|
3.96±0.36
|
0.64±0.01
|
2.561±0.02
|
151.85 ±0.6
|
34±5
|
62.45±5.90
|
32±1.17
|
|
2
|
3.95±0.32
|
0.77±0.02
|
2.553±0.03
|
149.25±0.4
|
37±2
|
90.03±2.42
|
34±1.54
|
|
3
|
3.01±0.25
|
0.598±0.08
|
2.585±0.08
|
151.75±0.2
|
41±3
|
65.76±6.04
|
33±1.46
|
|
4
|
3.96±0.14
|
0.781±0.02
|
2.586±0.01
|
149.20±0.3
|
35±7
|
61.60±2.50
|
32±2.20
|
|
5
|
3.91±0.35
|
0.820±0.01
|
2.517±0.05
|
151.30 ±0.8
|
42±6
|
66.56±5.40
|
33±1.36
|
|
6
|
3.02±0.22
|
0.724±0.02
|
2.547±0.08
|
150.48 ±0.4
|
44±9
|
95.10±1.94
|
37±1.23
|
|
7
|
3.94±0.10
|
0.498±0.01
|
2.525±0.01
|
151.30 ±0.4
|
32±1
|
82.81±5.77
|
29±1.06
|
|
8
|
3.20±0.20
|
0.543±0.05
|
2.537±0.05
|
150.60 ±0.2
|
31±3
|
81.10±2.45
|
28±1.23
|
|
9
|
3.95±0.37
|
0.799±0.03
|
2.580±0.08
|
151.11±0.3
|
28±2
|
61.65±5.80
|
25±1.77
|
- Hardness: Tablets were evaluated by using hardness tester. Hardness of the tablets was found in the range 3.91±0.35 to 3.20±0.20. The result are given in Table no.6
- Friability: Friability of tablets was observed in acceptable range 0.49±0.01 to 0.82±0.01 (Less than 1%). The result are given in Table no.6
- Thickness uniformity: The thickness of tablets was found to be exact 2.5 uniform thickness was obtained due to uniform die fill
- Weight variation: Direct compression was used to manufacture the tablets. Since the material had good flowability, the tablets had uniform weight due to consistent die fill. The tablets were within the acceptable weight variation range, with less than 7.5% variation, as per pharmacopeia specifications. The results are presented in Table no. 6.
- In-vitro dispersion time: The in-vitro dispersion time was determined by placing a tablet in a petri dish containing 10 ml of phosphate buffer solution at pH 6.8 and 37 ± 0.5°C as per the Indian Pharmacopoeia (I.P) guidelines. The dispersion time for all batches fell within the range of 28±2 to 44±9 seconds. Among the batches, MF9 exhibited the fastest dispersion time. The results are presented in above Table.
- Water absorption ratio: A piece of tissue paper was folded twice and placed in a small Petri dish with a diameter of 6.5 cm, which contained 6 ml of water. A tablet was positioned on top of the paper, and the time taken for the tablet to become fully saturated was recorded. After the tablet was completely wetted, it was weighed, and the water absorption ratio was determined for each batch. The results of these ratios are presented in above table.
In-vitro release studies: A comparative analysis of Oro dispersible tablets containing Betaxolol HCl was performed using in vitro kinetic parameters, revealing insights into their drug release profiles. Detailed data for all formulations is provided below.
In vitro drug release studies details:
Apparatus used : USP II dissolution test apparatus
Dissolution medium : 6.8Buffer
Dissolution medium volume: 300 ml
Temperature : 37±0.5ºC
Speed of basket paddle : 50 rpm
Sampling intervals : 1 min
Sample withdrawn : 10 ml
Absorbance measured : 224 nm
Table No. 26: Comparative in-vitro drug release profile of MF1, MF2, MF3.
|
Sl.no
|
Time (min)
|
MF1
|
MF2
|
MF3
|
|
1
|
0
|
0
|
0
|
0
|
|
2
|
1
|
16.35±0.92
|
22.31±0.85
|
22.93±0.25
|
|
3
|
2
|
32.6±0.89
|
38.65±0.16
|
48.26±0.85
|
|
4
|
3
|
56.34±1.16
|
52.39±1.21
|
78.93±1.25
|
|
5
|
4
|
65.21±1.08
|
76.55±0.87
|
91.49±1.16
|
|
6
|
5
|
79.32±1.16
|
93.63±0.56
|
99.97±1.79
|
|
7
|
6
|
94.53±1.12
|
-
|
-
|
Mean ± SD, where n=3
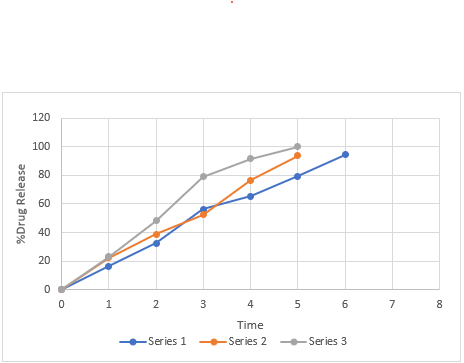
Figure No. 13: Comparative in vitro drug release profile of batches MF1 to MF3
Table No. 27: Comparative in-vitro drug release profile of MF4, MF5, MF6.
|
Sl.no
|
Time(min)
|
MF4
|
MF5
|
MF6
|
|
1
|
0
|
0
|
0
|
0
|
|
2
|
1
|
21.69±0.15
|
21.96±0.21
|
22.37±1.85
|
|
3
|
2
|
31.15±0.21
|
31.15±0.26
|
39.26±0.26
|
|
4
|
3
|
54.09±0.14
|
55.09±1.14
|
68.93±0.65
|
|
5
|
4
|
76.59±0.85
|
76.95±0.13
|
79.5±0.46
|
|
6
|
5
|
85.35±1.03
|
87.62±0.85
|
92.36±0.52
|
|
7
|
6
|
91.34±1.14
|
98.96±1.16
|
-
|
|
8
|
7
|
99.25±0.16
|
-
|
-
|
Mean ± SD, where n=3
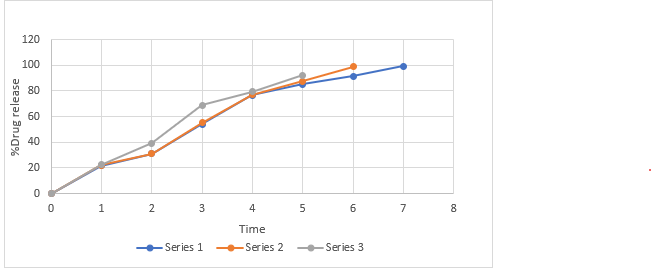
Figure No. 14: Comparative in vitro drug release profile of batches MF4 to MF6
Table No. 28: Comparative in-vitro drug release profile of MF7, MF8, MF9.
|
sl.no
|
Time(min)
|
MF7
|
MF8
|
MF9
|
|
1
|
0
|
|
0
|
0
|
|
2
|
1
|
21.54±0.12
|
31.39±0.36
|
52.63±1.78
|
|
3
|
2
|
41.7±0.56
|
53.85±1.12
|
74.63±1.32
|
|
4
|
3
|
63.35±1.03
|
75.68±1.18
|
99.59±1.23
|
|
5
|
4
|
97.15±1.16
|
98.79±0.78
|
-
|
Mean ± SD, where n=3
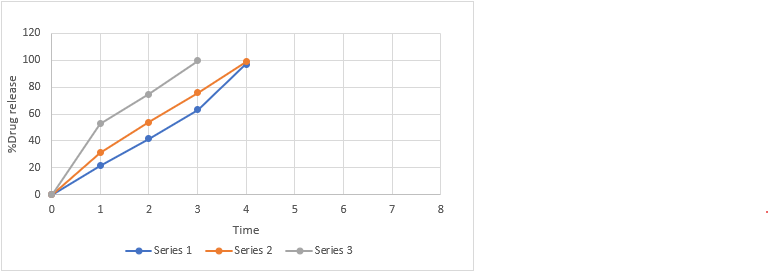
Figure No. 15: Comparative in vitro drug release profile of batches MF7 to MF9
CONCLUSION
The study focused on developing an orodispersible tablet of betaxolol hydrochloride using superdisintegrants such as sodium starch glycolate, crospovidone, and cross carmellose sodium in varying concentrations. The objective was to create a formulation that allows for once-daily dosing, thereby enhancing patient compliance. Compatibility studies using FTIR and DSC confirmed that betaxolol hydrochloride was compatible with the selected excipients. Precompression studies indicated good flow properties of the powder blends. The optimized formulation, MF9, containing 8 mg of cross carmellose sodium, achieved a minimum disintegration time of 26 seconds and a drug release of 99.59% within 3 minutes, meeting all post-compression evaluation parameters.
REFERENCES
- Nautiyal U, Singh S, Singh R, Gopal, Kakar S. Fast dissolving tablets as a novel boon: a review. J Pharm Chem Biol Sci. 2014; 2:5-26.
- Masih A, Kumar A, Singh S, Tiwari AK. Fast dissolving tablets: A review. Int J Curr Pharm Res. 2017;9(2):8-18.
- Geethalakshmi A, Gyaltsen K. Formulation and Comparative In-vitro Evaluation of Fast Disintegrating Mouth Films of Betaxolol Hydrochloride for Hypertension. International Journal of Research and Scientific Innovation. 2018;5(10):34-9.
- Naman Doshi; Avani Sheth; Tejas Patel,J B Dave,C.N.Patel. Spectrophotometric absorption factor method development and Validation for estimation of Rovastatin calcium and Telmisartan in solid dosage form. Journal of Chemical and Pharmaceutical Research 2010,2(3);15-24.
- Jagirapu b, Harini U, Divya M, Sushma P. Simultaneous estimation of telmisartan and atorvastatin calcium in API and tablet dosage form. Journal of Drug Delivery and Therapeutics. 2019 Jan 15;9(1):175-9.
- Patil NC, Raja K. Formulation and Evaluation of Orodispersible Tablets of Metoclopramide Hydrochloride. Tamilnadu Dr. MGR Medical University. 2012:638-52.
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Philadelphia: Lea & Febiger; 1976.
- Khan S, Kataria P, Nakhat P, Yeole P, Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid?disintegrating tablets. AAPS Pharm.Sci.Tech.; 8(2), 2007, 46.
- Patel SS, Patel MS, Patel NM. Flowability testing of directly compressible excipients according to British pharmacopoeia. J Pharm Res 2009;8: 66 -9.
- Deshmukh VN. Mouth Dissolving Drug Delivery System: A Review. Int J Pharm Tech Res. 2012;4(1):412- 44 21. 49. Patel ST, Sengupta M. Fast Dissolving Tablet Technology – A Review. World J Pharm Pharm Sci. 2013;2(2):485-508.
- Shah V, Patel SS, Jatav RK, Jain A, Sheorey RV. Formulation and evaluation of mouth dissolving tablets of metoclopramide hydrochloride by direct compression technique. Int. J. Drug Disc. Herbal Res. 2011;1(2):100-3.
- Chiou AH, Yeh MK, Chen CY, Wang DP. Micronization of meloxicam using a supercritical fluids process. The Journal of supercritical fluids. 2007 Aug 1;42(1):120-8.
- Rangole US, Kawtikwar PS, Sakarkar DM. Formulation and in-vitro evaluation of rapidly disintegrating tablets using hydrochlorothiazide as a model drug. Res. J. Pharm. Technol.2008;1(4):349-52.
- Viswanath V, Tulasi P. Formulation, optimization and characterization of Betaxolol hydrochloride proniosomes using 3-2 factorial design. Int. J. Res. Pharm. Sci & Tech., 2020 Feb 18;1(3):89-97.
- Sawarikar PP, Sridhar BK, Shivkumar S. Formulation and evaluation of fast dissolving/disintegrating tablets of isoxsuprine hydrochloride. J Curr Pharma Res. 2010 Apr; 3:41-6.


 Nagendra R.*
Nagendra R.*

















 10.5281/zenodo.14050550
10.5281/zenodo.14050550