Abstract
The objective of this study is to create and design buoyant microspheres containing gliclazide, and subsequently assess their properties. Gliclazide, a derivative of sulfonyl urea, is used to treat type-II diabetes. In order to address the medicine's limited solubility in water and improve its absorption when taken orally, floating microspheres were developed as a controlled drug delivery system. The development of this formulation was achieved by the utilization of the ionotropic gelation process. A total of six formulations, labeled F1-F6, were created using natural polymers in three distinct ratios. The micromeritic properties, percentage swelling, buoyancy, in-vitro release, and entrapment efficiency of the microspheres were evaluated. The flow characteristics of all the formulated microspheres were determined to be satisfactory. The formulation that demonstrated the most effectiveness was identified as F6. It exhibits zero order drug release with a rate of 82.15%. Additionally, it has a percentage yield of 88.4%, a percentage swelling of 92%, a buoyancy percentage of 96%, and an entrapment efficiency of 89%. Formulation F6 was prepared, utilizing natural polymers for the first time to generate floating microspheres of gliclazide.
Keywords
Floating drug delivery delivery systems, Floating microspheres, Gastro retention, Gliclazide, ionotropic gelation method
Introduction
Gliclazide, a second-generation sulfonylurea, is extensively utilized for the treatment of type 2 diabetes mellitus. It significantly reduces blood glucose levels by boosting insulin secretion from active pancreatic beta cells, hence improving peripheral tissue sensitivity to insulin [1]. Gliclazide (Figure 1) has an elimination half-life of roughly 16 hours and is extensively metabolized by the liver, with merely 4% of its total clearance occurring through renal excretion, primarily excreted in urine and, to a lesser extent, in feces [2-4]. Administration is generally advised with breakfast or the initial significant meal of the day to enhance its glucose-lowering efficacy [5-8].
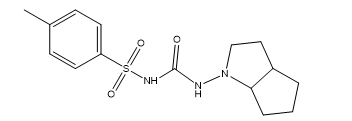
Figure 1: Structure of Gliclazide
The advancement of controlled-release technologies has transformed medicine administration in drug delivery. These systems are engineered to provide pharmaceuticals at a controlled pace over an extended duration, ensuring stable drug concentrations in the bloodstream and tissues [9,10]. These systems are especially advantageous for drugs characterized by rapid gastrointestinal absorption and brief half-lives, such as gliclazide, which frequently necessitate multiple doses. Diverse methodologies, such as bioadhesive systems and mechanisms that prolong stomach emptying, have been devised to address these issues [11,12]. An innovative approach involves the utilization of floating microspheres, a drug delivery technology characterized by a bulk density inferior to that of gastric fluids, enabling the formulation to maintain buoyancy in the stomach for prolonged durations [13]. These microspheres, generally measuring between 1 and 1000 µm, deliver the medicine at a regulated rate, improving solubility, concealing unpleasant tastes, and providing prolonged drug release. Floating microspheres predominantly comprise a blend of pharmaceuticals and polymers that create a gel-like barrier upon interaction with stomach fluid [14-16]. This barrier controls drug release while preserving buoyancy through entrapped air within the hydrated polymer matrix.
The ionotropic gelation method is a prevalent approach for producing floating microspheres. The process entails the cross-linking of polyelectrolytes, such as sodium alginate, in the presence of counter ions to create a gel matrix that wraps the drug and regulates its release rate. This approach is economical and adaptable, rendering it suitable for encapsulating various pharmaceuticals.
This work investigates the formulation and assessment of gliclazide-loaded floating microspheres by the ionotropic gelation technique, emphasizing its capacity to augment drug solubility, regulate release kinetics, and boost patient adherence [17].
MATERIALS AND METHODS
The gift sample of gliclazide was collected and the floating microspheres were prepared using ionotropic gelation technique. It is a process where an ionic polymer interacts with an oppositely charged ion to commence cross linking and create hydrogel beads.
Preparation of Gliclazide-Loaded Floating Microspheres
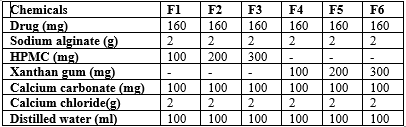
Gliclazide-loaded floating microspheres were prepared using the ionotropic gelation method in six formulations, each with varying ratios of hydroxypropyl methylcellulose (HPMC K100M) and xanthan gum 200M. Sodium alginate served as the base matrix due to its viscosity and binding properties, while calcium chloride (CaCl?) acted as a cross-linking agent to form microspheres. Calcium carbonate (CaCO?) was included to enhance buoyancy by releasing carbon dioxide in the gastric environment [18]. The gliclazide-alginate solution, with different polymer ratios, was extruded into a CaCl? solution, where ionotropic gelation occurred, forming microspheres. These microspheres were then washed, dried, and collected for further evaluation. This method effectively controlled drug release and enhanced the floating behavior of the microspheres, making them a potential delivery system for improved bioavailability of gliclazide in type-2 diabetes treatment.
Evaluation tests of microspheres:
Percentage yield:
The percentage yield of floating microspheres was determined by dividing the actual weight of the product by the total amount of all non-volatile components employed in the manufacturing of the floating microspheres. This value is represented by the following formula [19].
Micromeritic properties:
The micrometric parameters of the prepared microspheres include bulk density, tapped density, Carr's compressibility index, Hausner's ratio, and angle of repose [20].
Bulk and Tapped density:
The bulk and tapped densities were determined using a 50 ml graduated cylinder. A precisely measured quantity of 5g of the material was passed through a glass funnel. The sample put into the cylinder was physically tapped 3 to 100 times in order to calculate the bulk volume (Vb) and tapped volume (Vt) correspondingly. Next, the volume was recorded by tapping, and the bulk density and tapped density were subsequently determined. The density was measured in grams per cubic centimeter (g/cm?3;).
Tapped density=(mass of microspheres(M))/(volume of microspheres after tapping)
Carr's Compressibility Index:
The compressibility index (C.I.) or Carr's index value of microspheres was determined using the following equation.
Hausner's ratio:
The Hausner's ratio of microspheres was calculated by comparing the tapped density to the bulk density using a specific equation.
Angle of repose:
The maximum angle which is formed between the surface of a pile of powder and horizontal surface is called the angle of repose.
Tan ?=h/r
Where, T-angle of repose; h=height of the circle formed by the powder heap; r = radius of heap [21].
Percentage swelling:
The produced beads were immersed in an excess amount of distilled water at a temperature of 37°C for a specified duration. Subsequently, the beads were promptly extracted and measured using an analytical balance. The swelling percentage of the beads was determined using the following formula [22].
% swelling=(swollen beads weight-dry beads weight)/(dry beads weight)×
Buoyancy percentage:
The experiments were conducted utilizing the USP type 2 dissolution test apparatus, namely the rotating paddle configuration. Each formulation, consisting of 100mg of beads, was placed inside a capsule and added to a dissolving medium containing 900ml of 0.1N HCl. The temperature was kept constant at 37 ? C and the paddle turning speed was set at 50rpm. The buoyant and sedimented beads were retrieved individually. The percentage buoyancy (Pb) was determined using the following formula [23].
Drug entrapment efficiency:
Microspheres containing a dosage of 25 mg of the substance Model drug were used for examination. The quantity of medication encapsulated was determined by pulverizing the microspheres. The powder was transferred to a 100 ml volumetric flask and dissolved in a 1.2 pH hydrochloric acid (HCl) buffer solution with a concentration of 0.1N. The buffer solution was made by dissolving 2.0g of sodium chloride (NaCl) in water, adding 7.0ml of hydrochloric acid (HCl), and diluting with water to a total volume of 1 Liter. The solution was filtered using Whatmann filter paper after 24 hours, and the absorbance was measured at 269 nm using spectrophotometry after appropriate dilution. The quantity of medication encapsulated within the microspheres was determined using the subsequent formula [24].
In-vitro release studies:
The drug release rate from floating microspheres was determined using the USP type II (Electro Lab.) dissolving paddle assembly. A measured quantity of buoyant microspheres, with a mass equal to 100 mg of the medicine, were evenly distributed in 900 ml of a solution containing 0.1 N hydrochloric acid (pH=1.2). The temperature of the solution was maintained at 37 ± 0.5°C, and it was agitated at a speed of 50 revolutions per minute. A 5 ml sample was extracted at specific time intervals, filtered, and then an equal volume of dissolving media was added back into the vessel after each extraction to ensure that the concentration of the dissolved substance remained constant. The gathered samples were examined using spectrophotometry to ascertain the concentration of the medication in the dissolving medium [25].
RESULTS AND DISCUSSION
Preparation of floating microspheres:
The sodium alginate solution containing gliclazide was drawn into a 20 ml syringe and carefully added drop by drop into a calcium chloride solution. The microspheres were allowed to form over a period of 30 minutes, after which they were filtered using a funnel. The filtered microspheres were air-dried for 24 hours to ensure complete drying. After this process, the microspheres were fully prepared for further evaluation. Figure 2 illustrates the preparation process, while six different formulations, using varying polymer compositions, are summarized in Table 1.


Fig. 2 : Floating microspheres of gliclazide
Table 1: Various Formulations of floating microspheres
Evaluation Parameters
Percentage yield
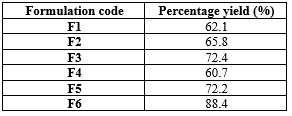
It was found that the % yield increased as the concentration of polymers increased. Polymers play a major role in increasing the yield. The percentage yield of all the six formulations calculated using the specified formula. The F6 formulation has the highest % yield that is 88.4% recorded in (table 2).
Table 2: Percentage yield of floating microspheres
Micromeritic properties:
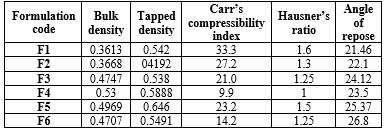
All the micromeritic properties for floating microspheres were calculated according to the standard formulas. Carr's index ranges from 9.9 to 33.3, Hauser's ratio ranges from 1 to 1.6, and the angle of repose ranges from 21.46 to 26.8 degrees as per (table 3) The micromeritic investigations indicated that the microspheres possess good flow characteristics, suggesting that they are spherical and not clumped together. F6 formulation exhibits good flow characteristics within the standard limit ranges.
Table 3: Micromeritic properties of floating microspheres
Percentage swelling
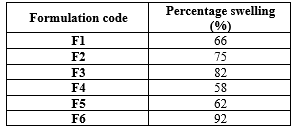
The percentage swelling of the microspheres increased with the higher concentration of polymers in the formulations. This swelling behaviour is primarily attributed to the inclusion of calcium carbonate, which not only imparts buoyancy to the microspheres but also contributes to their expansion. The swelling of the microspheres enhances drug release, as it facilitates the diffusion of the drug from the polymer matrix. The swelling percentage was calculated using the specified formula, with the F6 formulation exhibiting a swelling percentage of 92%, as shown in Table 4.
Table 4: Percentage swelling of floating microsphere
Buoyancy percentage:
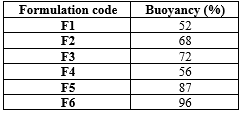
The buoyancy percentage of the floating drug delivery system increased with higher polymer concentrations. This is attributed to the increase in system volume while maintaining a relatively constant density, resulting in enhanced buoyancy of the microspheres. The F6 formulation exhibited the highest buoyancy percentage of 96%, as detailed in Table 5. The buoyancy percentage was determined using a standard calculation formula.
Table 5: Buoyancy percentage of floating microspheres
Drug Entrapment Efficiency:
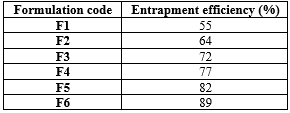
An increase in polymer concentration led to a corresponding rise in drug content, facilitated by the improved dissolution properties of the polymers. Upon administration, the microspheres reach the stomach, where their buoyant nature allows them to float while gradually disintegrating, promoting both rapid and sustained drug release. The drug entrapment efficiency for all formulations was calculated using the specified formula, with the F6 formulation exhibiting the highest entrapment efficiency at 89%, as detailed in Table 6.
Table 6: Drug entrapment efficiency of floating microspheres
In- vitro drug release studies
The controlled release of the drug by the polymer was confirmed through in vitro drug release studies, which demonstrated the highest regression coefficient values for zero-order release kinetics, indicating a consistent release profile. Drug release was found to be dependent on polymer concentration and was calculated using a standard formula. In vitro dissolution studies were conducted on formulations F1 to F3, with formulation F3, containing 300 mg of HPMC K100M, showing a drug release of 69.04?ter 6 hours, as outlined in Table 7.
Table-7: Drug release of floating microspheres
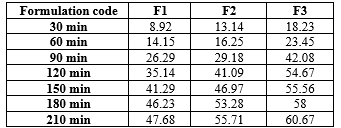
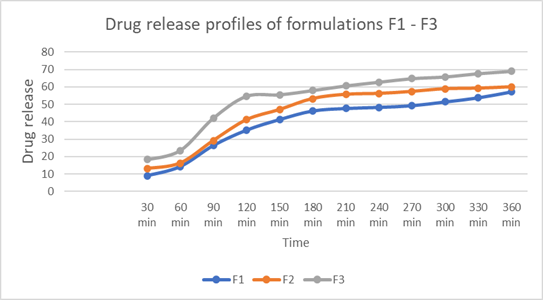
Fig 2 : Drug release of formulations F1 to F3
As said, a higher concentration of polymer leads to sustained release of the medication. Drug release was calculated using specified formula.Formulation 6, which contains 300 mg of Xanthan gum, demonstrated 82.15% drug release after 6 hours, as determined by in vitro dissolution as per data in the table-8.
Table 8: Drug release of formulations F4 to F6


Fig 3: Drug release of formulations F4 to F6
This study successfully developed and evaluated floating microspheres containing gliclazide using the ionotropic gelation method. Six formulations (F1-F6) were created, utilizing natural polymers in varying ratios to improve the solubility and absorption of gliclazide, a sulfonylurea derivative used in the treatment of type-II diabetes. The microspheres demonstrated favorable micromeritic properties, buoyancy, swelling behavior, and drug release profiles. Among the formulations, F6 emerged as the most promising, achieving zero-order drug release with a rate of 82.15%, along with high yield (88.4%), swelling (92%), buoyancy (96%), and entrapment efficiency (89%). This formulation, using natural polymers for the first time, demonstrates the potential of floating microspheres to enhance the therapeutic efficacy of gliclazide as a controlled drug delivery system.
ACKNOWLEDGEMENTS
I would like to express my gratitude to the Sri Venkateshwara College of Pharmacy for providing required chemicals and equipment for the research and my guide for her guidance in completing the research project effectively and efficiently.
REFERENCES
- Hemalatha CH, Vasavi G, Ananda Kumar CH, Sriram N. Formulation and development of gliclazide microspheres for pharmaceutical evaluations. Indian J Appl Pharm. 2014;2:83-92.
- Srikrishna T, Sucharitha S. Design and development of gliclazide floating drug delivery system. J Xi’an Shiyou Univ Nat Sci Ed. 2023;19(2):1649-66.
- McGavin JK, Perry CM, Goa KL. Gliclazide modified release. Indian Drugs. 2002;62(9):1357-64.
- Palmer KJ, Brogden RN. Gliclazide: an update of its pharmacological properties and therapeutic efficacy in non-insulin-dependent diabetes mellitus. Drugs. 1993;46:92-125.
- Hardik Kumar, Rajesh Bhai, Senthil A. Formulation and evaluation of gastro-retentive floating tablets of gliclazide. Int J Res Appl Pharm. 2011;2(4):1368-73.
- Prakash S, Bhandari A, Mishra R, Sharma PK. Development and optimization of floating microspheres of gliclazide. Int J Pharm Sci Res. 2015;6:807-17.
- Prajapati SK, Tripathi P, Ubajdulla U, Vikas. Design and development of gliclazide mucoadhesive microcapsules: in vitro and in vivo evaluation. AAPS PharmSciTech. 2008 Mar;9(1):224-30.
- Vyas SP, Khar RK. Controlled drug delivery: Concepts and advances. 1st ed. New Delhi: CBS Publishers; 2002. p. 196-217.
- Saxena A, Gaur K, Singh V, Kant Singh R. Floating microspheres as drug delivery system. Am J Pharmacol Pharm Sci. 2014;(1):27-36.
- Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci. 1992;81:135-40.
- Garg R, Gupta GD. Progress in controlled gastroretentive delivery systems. Trop J Pharm Res. 2008;7(3):1055-66.
- Chien YW. Rate-controlled drug delivery systems. Indian J Pharm Sci. 1988 Mar-Apr;50:63-5.
- Chugh C, Nanda A. Gastro-retentive drug delivery systems: a review. Int J Pharm Bio Sci. 2017;8:62-8.
- Arora S, Ali J. Floating drug delivery systems: A review. AAPS PharmSciTech. 2006;6:372-90.
- Mukund JY, Kantilal BR, Sudhakar RN. Floating microspheres: a review. Braz J Pharm Sci. 2012;48:17-30.
- Kavitha K, Yadav SK, Tamizh MT. The need for floating drug delivery systems: a review. Res J Bio Pharm Chem Sci. 2010;1(2):396-405.
- Bansal H, Kaur SP, Gupta AK. Microspheres: Methods of preparation and applications; a comparative study. Int J Pharm Sci Rev Res. 2011;10(1):69-78.
- Patil P, Chavanke D, Wagh M. A review on ionotropic gelation method: novel approach for controlled gastroretentive gelispheres. Int J Pharm Pharm Sci. 2012;4(4):27-32.
- Swapna N, Jithan A. Preparation, characterization, and in vivo evaluation of parenteral sustained release microsphere formulation of zopiclone. J Young Pharm. 2010;2:223-8.
- Gadad AP, Naik SS, Dandagi PM, Bolmal UB. Formulation and evaluation of gastro-retentive floating microspheres of lafutidine. Indian J Pharm Edu Res. 2016;50.
- Sammour OA, El-Ghamry HA, El-Nahas HM, Barakat W. Development and characterization of controlled release ketoprofen microspheres. J Appl Pharm Sci. 2012;2:60-7.
- Rane BR, Gujarathi NA, Patel JK. Preparation and in vitro characterization of floating microspheres of nateglinide. Int J Pharm Sci Res. 2012;3:4306-13.
- Patel A, Ray S, Thakur SR. In vitro evaluation and optimization of controlled release floating drug delivery system of metformin hydrochloride. DARU. 2006;14:57-64.
- Sathiyaraj S, Ramya DD, Vedha BN H, Lornoxicam gastro-retentive floating microsphere tablets: design and in vitro evaluation. J Adv Pharm Tech Res. 2011;2:156-62.
- Radha GV, Lakshmi Sravanthi N, Swetha P, Sravani Y, Praveen Kumar P. Formulation and in vitro evaluation of mucoadhesive microspheres of nifedipine. J Appl Pharm Sci. 2012;2:60-7.


 Dr Divya Pingili*
Dr Divya Pingili*











 10.5281/zenodo.13893817
10.5281/zenodo.13893817