Abstract
Transdermal drug delivery systems (TDDS), also known as “patches,” are dosage forms designed to deliver a therapeutically effective amount of drug across a patient’s skin. In order to deliver therapeutic agents through the human skin for systemic effects, Transdermal delivery provides a leading edge over injectables and oral routes by increasing patient compliance and avoiding first pass metabolism respectively. With the advancement in technology Pharma industries have trendified all its resources. Earlier we use convectional dosage form but now we use novel drug delivery system. One of greatest innovation of novel drug delivery is transdermal patch. The advantage of transdermal drug delivery system is that it is painless technique of administration of drug.
Keywords
Transdermal drug delivery systems (TDDS), Diffusion, First-generation TDS, Second-generation TDS, Polymer Matrix, Permeation enhancers, Iontophoresis, Microneedle.
Introduction
Transdermal patch (Skin patch) uses a special membrane to control the rate at which the liquid drug contained in the reservoir within the patch can pass through the skin and into the Bloodstream. Some drugs must be combined with substances, such as alcohol, that increase their ability to penetrate the skin in order to be used in a skin patch. Drugs administered through skin patches include scopolamine (for motion sickness), nicotine (for quitting smoking), estrogen (for menopause and to prevent osteoporosis after meno pause), nitroglycerin (for angina), and lidocaine to relieve the pain of shingles (herpes zoster). Molecules of insulin and many other substances, however, are too large to pass through the skin. Patches applied to the skin eliminate the need for vascular access by syringe or the use of pumps. Transdermal patches were developed in the 1970s and the first was approved by the FDA in 1979 for the treatment of motion sickness. It was a three-day patch that delivered scopolamine. In 1981, patches for nitroglycerin were approved, and today there exist a number of patches for drugs such as clonidine, fentanyl, lidocaine, nicotine, nitroglycerin, oestradiol, oxybutinin, scopolamine, and testosterone. There are also combination patches for contraception, as well as hormone replacement.Depending on the drug, the patches generally last from one to seven days.
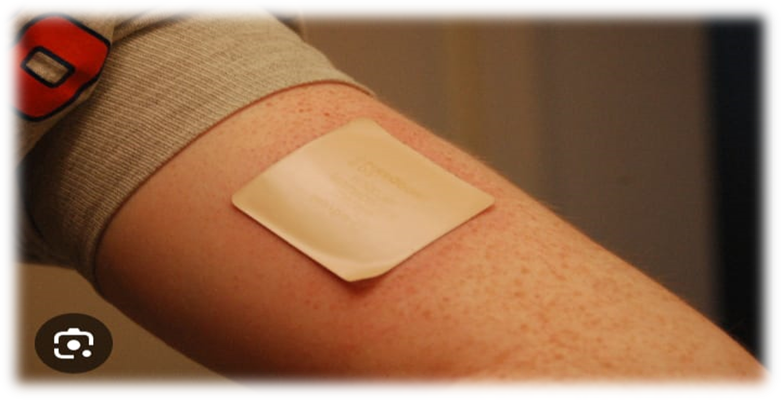
Figure 1: Transdermal Patches
2. Basic Components Of TDDS
- Polymer matrix/drug reservoir
- Membrane
- Drug Permeation enhancers
- Pressure-sensitive adhesives (PSA)
- Backing laminates
- Release liner
- Other excipients like plasticizers and solvents
2.1 Polymer matrix/drug reservoir
Polymers are the backbone of TDDS, which control the release of the drug from the device. A polymer matrix can be prepared by dispersion of drug in a liquid or solid state synthetic polymer base. Polymers used in TDDS should have biocompatibility and chemical compatibility with the drug and other components of the system, such as penetration enhancers and PSAs. Additionally, they should provide consistent and effective delivery of a drug throughout the product’s intended shelf-life, and should be safe. The following criteria should be preferred in selecting the polymer to be used in the transdermal system
(i) Molecular weight, glass transition temperature and chemical functionality of the polymer should be such that the specific drug diffuses properly and gets released through it
(ii) The polymer should be stable, nonreactive with the drug, easily manufactured and fabricated into the desired product, and should be inexpensive.
(iii) The polymer and its degradation products must be nontoxic or nonantagonistic to the host.
(iv) The mechanical properties of the polymer should not deteriorate excessively when large amounts of active ingredients are incorporated into it.
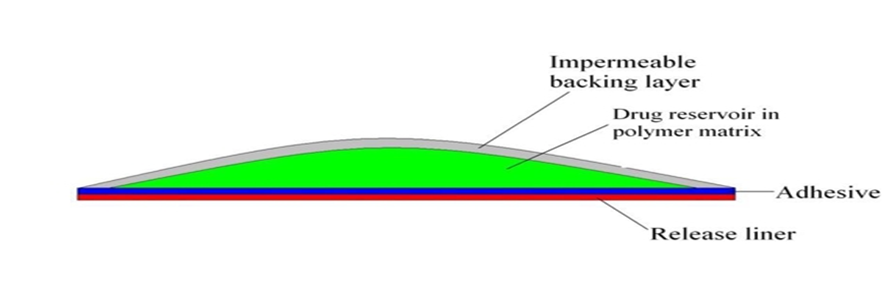
Figure 2: Matrix diffusion controlled film.
2.2 Membrane
A membrane may be sealed to the backing to form a pocket to enclose the drug-containing matrix or used as a single layer in the patch construction. The diffusion properties of the membrane are used to control availability of the drug and/or excipients to the skin. For example, ethylene vinyl acetate, silicone rubber, polyurethrane, etc. are used as a rate-controlling membrane.
2.3 Drug
For successfully developing a TDDS, the drug should be chosen with great care. Transdermal patches offer many advantages to drugs that undergo extensive first-pass metabolism, drugs with narrow therapeutic window or drugs with a short half-life, which cause noncompliance due to frequent dosing. There are some examples of drugs that are suitable for TDDS, like Nicardipine hydrochloride, Captopril, Atenolol, Metoprolol tartrate, Clonidine, Indapamide, Propranolol hydrochloride, Carvedilol, Verapamil hydrochloride and Nitrendipine, etc.
2.4 Permeation enhancers
One long-standing approach for improving TDD uses penetration enhancers (also called sorption promoters or accelerants), which increase the permeability of the SC so as to attain higher therapeutic levels of the drug candidate. Penetration enhancers interact with structural components of the SC thus modifying the barrier functions, leading to increased permeability. Three pathways are suggested for drug penetration through the skin: polar, nonpolar and polar/nonpolar. The enhancers act by altering one of these pathways
The key to altering the polar pathway is to cause protein conformational change or solvent swelling. The key to altering the nonpolar pathway is to alter the rigidity of the lipid structure and fluidize the crystalline pathway (this substantially increases diffusion). The fatty acid enhancers increase the fluidity of the lipid portion of the SC. Some enhancers (binary vehicles) act on both polar and nonpolar pathways by altering the multilaminate pathway for penetrants.The methods employed for modifying the barrier properties of the SC to enhance the drug penetration (and absorption) through the skin can be categorized as
(1) chemical
(2) physical methods of enhancement.
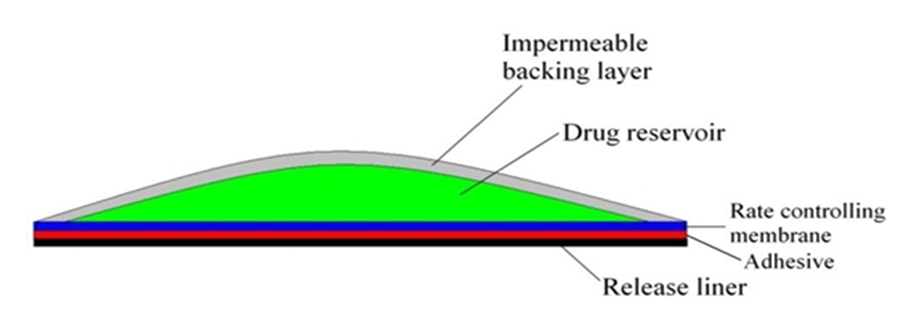
Figure- 3: Membrane permeation controlled film.
2.5 PSA (pressure sensitive adhesion)
PSAs are the material that adhere to a substrate, in this case skin, by application of light force and leave no residue when removed. They form interatomic and intermolecular attractive forces at the interface, provided that the intimate contact is formed. To obtain this degree of contact, the material must be able to deform under slight pressure, giving rise to the term “pressure sensitive.” Adhesion involves a liquid-like flow, resulting in wetting of the skin surface upon the application of pressure, and, when the pressure is removed, the adhesive sets in that state. A PSA wets and spreads onto the skin when its surface energy is less than that of the skin. After the initial adhesion, the PSA/skin bond can be built by stronger interactions (e.g., hydrogen bonding), which will depend on skin characteristics and other parameters. Widely used PSA polymers in TDDS are polyisobutylene-based adhesives, acrylics and silicone-based PSAs, hydrocarbon resin, etc. The PSA can be located around the edge of the TDDS or be laminated as a continuous adhesive layer on the TDDS surface. The PSA should be compatible with the drug and excipients, as their presence can modify the mechanical characteristics of the PSA and the drug delivery.
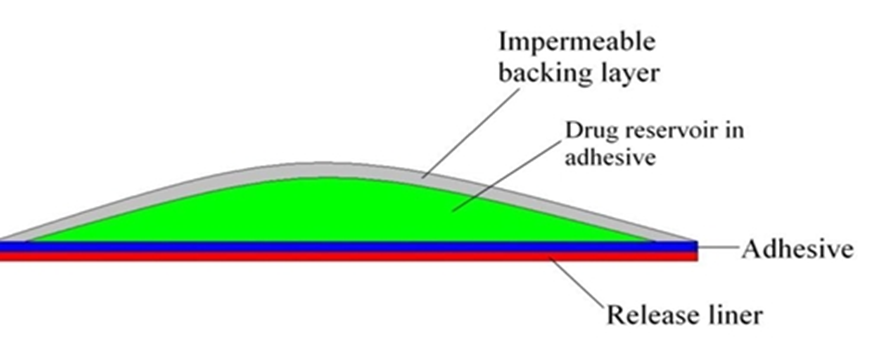
Figure 4 : Adhesive diffusion controlled film.
2.6 Backing membrane:
Backing materials must be flexible while possessing good tensile strength. Commonly used materials are polyolefin’s, polyesters, and elastomers in clear, pigmented, or metallized form. Elastomeric materials such as low-density polyethylene conform more readily to skin movement and provide better adhesion than less compliant materials such as polyester. Backing materials should also have low water vapour transmission rates to promote increased skin hydration and, thus, greater skin permeability. In systems containing drug within a liquid or gel, the backing material must be heat-sealable to allow fluid-tight packaging of the drug reservoir using a process known as form-fill-seal. The most comfortable backing will be the one that exhibits lowest modulus or high flexibility, good oxygen transmission and a high moisture vapour transmission rate. Examples of some backing materials are vinyl, polyester films, Polyester-polypropylene films, Polypropylene resin, Polyethylene resin, Polyurethylene, Ethylene-vinyl acetate, Aluminized plastic laminate.
2.7 Release Liner:
Release liner is a protective liner for the TDDS patch that is removed prior to the application on the skin. Typically, it consists of a base layer which may be non-occlusive (e.g. paper fabric) or occlusive (e.g. polyethylene, polyvinylchloride) and a release coating layer of silicon.
2.8 Other excipients:
Various solvents such as water, ethanol, isopropylmy-ristate, isopropyl alcohol, and dichloromethane are used alone or in combination to prepare the drug reservoir Propylene glycol, ethanol are used as co solvents along with the permeation en-hancer. Plasticizers like diethyl phthalate, dibutylpthalate, glycerol, triethyl citrate, polyethylene glycol 400, eudraflex and propylene glycol provide plasticity to the trans-dermal patch.
3. Classification of Transdermal patches:
- Single layer drug in
- Multi -layer drug in adhesive
- Vapour patch
- Reservoir system:
- Matrix system
- Drug-in-adhesive system
- Matrix-dispersion system
- Micro reservoir Controlled TDDS
3.1.Single layer drug in adhesive:
In this type the adhesive layer contains the drug. The adhesive layer not only serves to adhere the various layers together and this type of layer is responsible for the releasing the drug to the skin. The adhesive layer is surrounded by a temporary liner and a backing.
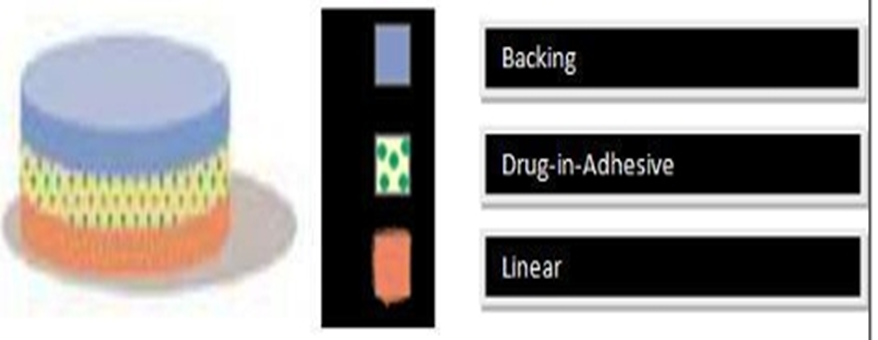
Figure 5: Single-layer drug-in-adhesive.
3.2. Multi -layer drug in adhesive:
This type is also similar to the single layer but it contains a immediate drug release layer which is different from other layer which will be a controlled release along with the adhesive layer. The adhesive layer is responsible for the releasing of the drug. This patch also has a temporary liner layer and a permanent backing.

figure- 6: Multi layer drug -in-adhesive.
3.3.Vapour patch:
In this type of patch the role of adhesive layer not only serves to adhere the various layers together but also serves market, commonly used for releasing of essential oils in decongestion. Various other types of vapor patches are also available in the market which are used to improve the quality of sleep and reduces the cigarette smoking conditions.
3.4. Reservoir system:
In this system the drug reservoir is embedded between the two layers; an impervious backing layer and a rate controlling membrane. The drug releases only through the rate controlling membrane, which can be micro porous or non porous. In the drug reservoir compartment, the drug can be in the form of a solution, suspension, gel or dispersed in a solid polymer matrix. Hypoallergenic adhesive polymer can be applied as outer surface polymeric membrane which is compatible with drug.
3.5. Matrix system:
In this type the drug reservoir is formed by dispersing the drug in an adhesive polymer and then spreading the medicated adhesive polymer by solvent casting or melting on an impervious backing layer. On top of the reservoir, unmediated adhesive polymer layers are applied for protection purpose.

Figure 7: Drug reservoir-in-adhesive.
Matrix-dispersion system
In this type the drug is dispersed homogenously in a hydrophilic or lipophilic polymer matrix. This drug containing polymer disk is fixed on to an occlusive base plate in a compartment fabricated from a drug impermeable backing layer. Instead of applying the adhesive on the face of the drug reservoir, it is spread along with the circumference to form a strip of adhesive rim.
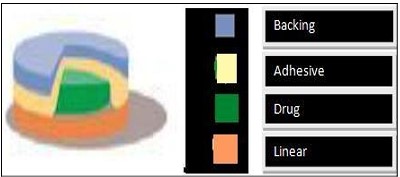
Figure 8: Drug matrix-in-adhesive.
3.6. Microreservoir Controlled TDDS:
This drug delivery system is a combination of reservoir and matrix-dispersion systems. The drug reservoir is formed by first suspending the drug in an aqueous solution of water-soluble polymer and then dispersing the solution homogeneously in a lipophilic polymer to form thousands of unreachable, microscopic spheres of drug reservoirs. The thermodynamically unstable dispersion is stabilized quickly by immediately cross linking the polymer in situ. A Transdermal system therapeutic system thus formed as a medicated disc Positioned at the center and surrounded by an adhesive rim.
4. Factors affecting on transdermal bioavailability
Three major factors affecting the transdermal bioavailability of the drug through the transdermal route.
4. 1.Physicochemical factors
Skin hydration: When exposed to water, the skin's permeability greatly increases. Adequate hydration plays a crucial role in enhancing skin permeation. Consequently, humectants are employed in transdermal delivery.
Temperature and pH: The drug permeability experiences a ten-fold increase due to temperature fluctuations. As the temperature decreases, the diffusion coefficient decreases as well. Weak acids and weak bases dissociate based on the pH and pKa values. The concentration of the drug in the skin depends on the proportion of the unionized drug. Consequently, drug penetration is significantly influenced by both temperature and pH.
Diffusion coefficient: The ability of a drug to permeate tissues is influenced by the diffusion coefficient of the drug. When the temperature remains constant, the diffusion coefficient is determined by various factors, such as the characteristics of the drug itself, the properties of the surrounding medium through which it diffuses, and the potential interactions occurring between the drug and the medium. In simpler terms, the penetration of a drug is affected by the way it interacts with the environment it diffuses through, along with the inherent properties of the drug itself.
Drug concentration: The flow rate is directly related to the difference in concentration between the two sides of the barrier, and this difference will be greater if there is a higher concentration of the drug across the barrier.
Molecular size and shape: Drug absorption is inversely proportional to molecular weight; small molecules penetrate faster than large ones.
4.2.Biological factors:
Skin condition: The unbroken skin functions as a protective barrier, but a variety of substances, such as acids and alkalis, can still pass through the skin cells and enter the body Solvents like methanol and chloroform can remove the lipid portion of this layer, creating artificial pathways through which drug molecules can effortlessly travel.
Skin age:
It has been observed that the skin of adults and young individuals is more easily penetrated than that of older individuals, although the difference is not significant. Children are more susceptible to toxic effects due to their larger surface area relative to body weight. As a result, powerful steroids, boric acid, and hexachlorophene have caused severe adverse reactions.
Skin metabolism: Skin metabolism plays vital role in the metabolism of steroids, hormones, chemical carcinogens, and some drugs. So skin metabolism regulates the efficacy of drugs permeated through the skin.
Regional skin site:
Thickness of skin, composition of the outermost layer of the stratum corneumand density of appendages (hair follicle and sweat gland) varies from site to site. These factors significantly affecting the penetration of drug molecules.
Blood supply: Changes in peripheral circulation can significantly affect on which substance are absorbs through the skin.
Species difference: The skin thickness, density of appendages, and keratinization of skin vary from species to species, which affects the penetration.
4.3. Environmental factors:
Sunlight: Sunlight has the ability to cause thinning of the walls of blood vessels, resulting in bruising even with minor trauma in areas exposed to the sun. Additionally, one of the prominent effects of sunlight on the skin is the occurrence of pigmentation changes, with freckles being the most notable manifestation.
Cold season: Often results in itchy, dry skin. Skin s act by increasing oil production to compensate for the weather dryeffects. Good moisture will help ease symptoms of dry skin. Also, drinking lots of water can keep your skin hydrated and looking radiant.
Air pollution: Dust can block pores and increase the amount of bacteria on the face and skin surface, leading to acne or pimples. This affects drug delivery through the skin. Invisible chemical pollutants in the air can interfere with the skin’s natural defense system, breaking down the skin’s natural oils that normally trap moisture in the skin and keep it supple.
Effect of heat on transdermal patch: Heat causes high absorption of drugs injected through the skin. Patients should be advised to avoid exposing the patch application area to external heat sources such as hot water bags or hot water bottles. Even a high body temperature can increase the amount of drug applied to the skin.
5. Advantage and Disadvantages :
5.1 Advantages:
- They can avoid gastrointestinal drug absorption difficulties covered by gastrointestinal pH, enzymatic activity and drug interaction with food, drink and other orally administration drug.
- They can substitute for oral administration of medication when the route is unsuitable as with vomiting and diarrhea.
- To avoid the first pass effect e.g. Transdermal Nitroglycerin. It is rapidly metabolized by the liner when taken orally.
- They are noninvasive, avoiding the inconvenience of parenteral therapy.
- They provided extended therapy with a single application, improving compliance over other dosage forms requiring more frequent dose administration e.g. Tradermal clonidine day.
- The activity of drugs having a start half life is extended through the reservoir of drug in the therapeutic delivery system and its controlled release.
Drug therapy may be terminated rapidly by removal of the application from the surface of the skin.
5.2 DISADVANTAGES:
- Some patients develop contact dermatitis at the site of application from one or more of the system components, necessitating discontinuation.
- Only potent drugs are suitable candidates for transdermal patch because of the natural limits of drug entry imposed by the skin's importability.
- Some drugs e.g. scopolamine transdermal patch placed behind the ear, it is uncomfortable.
Long time adhere is difficult.
- Methods of Preparation of TDDS:
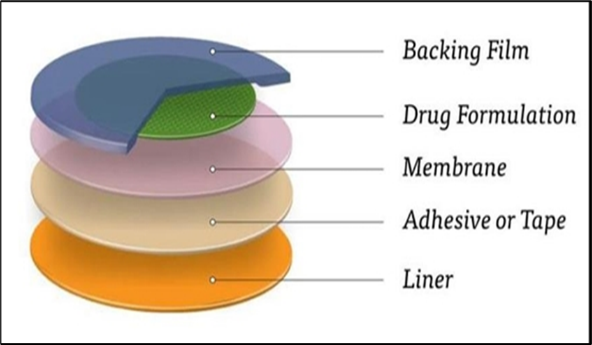
Figure 9: Drug matrix-in-adhesive.
i. Asymmetric TPX membrane method
ii. Circular Teflon mould method.
iii. Mercury substrate method.
iv. By using “IPM membranes” method
v. By using “EVAC membranes” method
6.1. Asymmetric TPX Membrane Method:
These are prepared by using the dry or wet inversion process. In this TPX is dissolved in a mixture of solvent and non- solvent additives at 60°C to form a polymer solution. The polymer solution is kept at 40°C for 24 hrs and cast on a glass plate. Then casting film is evaporated at 50°C for 30 sec, then the glass plate is to be immersed immediately in coagulation bath (temperature mantained at 25°C). After 10 minutes of immersion, the membrane can be removed, air dry in a circulation oven at 50°C for 12 hrs.
6.2. Circular Teflon Mould Method:
It was discovered by Baker and Heller in 1989. Polymeric solution in various proportions is used as an organic solvent. Then that solution is divided in two parts. In one parts calculated amount of drug is dissolved & in another part enhancers in different concentration are dissolved, and then two parts mixed together. Then plasticizer is added into the drug polymer solution. The total contents are to be stirred for 12 hrs and then poured into a circular Teflon mouldThe solvent is allowed to evaporate for 24 h. After which a dried film formed & that is to be stored for another 24 h at 25±0.5°C in a desiccators containing silica gel before evaluation to eliminate aging effects.
6.3. Mercury Substrate Method:
In this method drug & plasticizer get dissolved in polymeric solution. It stirred for 10- 15 min to produce homogenous dispersion then it is poured into levelled mercury surface, covered with inverted funnel to control solvent evaporation.
6.4. By Using “IPM Membranes” Method:
In the mixture of water & polymer (propylene glycol containing Carbomer 940 polymer) drug get dispersed and stirred for 12 hrs in magnetic stirrer. The dispersion is to be neutralized and made viscous by the addition of triethanolamine. If the drug solubility in aqueous solution is very poor then solution gel is obtained by using Buffer pH 7.4. The formed gel will be incorporated in the IPM membrane.
6.5. By Using “EVAC Membranes” Method:
For the preparation of TDS, 1?rbopol reservoir gel, polyethelene (PE), ethylene vinyl acetate copolymer (EVAC) membrane is needed as rate control membrane. If the drug is insoluble in water then use propylene glycol for gel preparation. Drug is dissolved in propylene glycol, carbopol resin will be added to the above solution and neutralized by using 5% w/w sodium hydroxide solution The drug is placed on a sheet of backing layer covering the specified area. A rate controlling membrane will be placed over the gel and the edges will be sealed by heat to obtain a leak proof device.
7. Evaluation Parameters:
The evaluation methods for transdermal dosage form can be classified into following type ? Physicochemical evaluation
? In vitro evaluation
? In vivo evaluation
? Stability studies
7.1 Physicochemical evaluation:
Interaction Studies:
Excipients are integral components of almost all pharmaceutical dosage forms. The stability of a formulation amongst other factors depends on the compatibility of the drug with the excipients. The drug and the excipients must be compatible with one another to produce a product that is stable, thus it is mandatory to detect any possible physical or chemical interaction as it can affect the bioavailability and stability of the drug.
Thickness of the Patch:
The thickness of the drug loaded patch is measured in different points by using a digital micro- meter and determines the average thickness and standard deviation for the same to ensure the thickness of the prepared patch.
Weight Uniformity:
The prepared patches are to be dried at 60°c for 4hrs before testing. A specified area of patch is to be cut in different parts of the patch and weigh in digital balance. The average weight and standard deviation values are to be calculated from the individual weights.
Folding Endurance:
A strip of specific are is to be cut evenly and repeatedly folded at the same place till it broke. The number of times the film could be folded at the same place without breaking gave the value of the folding endurance.
Percentage Moisture Content:
The prepared films are to be weighed individually and to be kept in a desiccators containing fused calcium chloride at room temperature for 24 hrs. After 24 hrs the films are to be reweighed and determine the percentage moisture content from the below mentioned formula. Percentage moisture content = [Initial weight- Final weight/ Final weight] ×100
Percentage Moisture Uptake:
The weighed films are to be kept in a desiccator at room temperature for 24 hrs containing saturated solution of potassium chloride in order to maintain 84% RH. After 24 hrs the films are to be reweighed and determine the percentage moisture uptake from the below mentioned formula. Percentage moisture uptake = [Final weight- Initial weight/ initial weight] ×100
Water Vapour Permeability (WVP) Evaluation:
Water vapour permeability can be determined with foam dressing method the air forced oven is replaced by a natural air circulation oven. The WVP can be determined by the following
formula: WVP=W/A
Where, WVP is expressed in gm/m per 24hrs,
W is the amount of vapour permeated through the patch expressed in gm/24hrs and A is the surface area of the exposure samples expressed in m2.
Drug Content:
A specified area of patch is to be dissolved in a suitable solvent in specific volume. Then the solution is to be filtered through a filter medium and analyse the drug contain with the suitable method (UV or HPLC technique). Each value represents average of three different samples.
Content Uniformity Test:
10 patches are selected and content is determined for individual patches. If 9 out of 10 patches have content between 85% to 115% of the specified value and one has content not less than 75% to 125% of the specified value, then transdermal patches pass the test of content uniformity. Bu if 3 patches have content in the range of 75% to 125%, then additional 20 patches are tested for drug content. If these 20 patches have range from 85% to 115%, then the transdermal patches pass the test.
Uniformity of Dosage Unit Test:
An accurately weighed portion of the patch is to be cut into small pieces and transferred to a specific volume volumetric flask, dissolved in a suitable solvent and sonicate for complete extraction of drug from the patch and made up to the mark with same. The resulting solution was allowed to settle for about an hour, and the supernatant was suitably diluted to give the desired concentration with suitable solvent.
7.2 In Vitro Evaluation:
In vitro drug release studies:
The paddle over disc method (USP apparatus V) can be employed for assessment of the release of the drug from the prepared patches. Dry films of known thickness is to be cut into definite shape, weighed, and fixed over a glass plate with an adhesive. The glass plate was then placed in a 500-mL of the dissolution medium or phosphate buffer (pH 7.4), and the apparatus was equilibrated to 32± 0.5°C. The paddle was then set at a distance of 2.5 cm from the glass plate and operated at a speed of 50 rpm.
In vitro skin permeation studies:
An in vitro permeation study can be carried out by using diffusion cell. Full thickness abdominal skin of male Wistar rats weighing 200 to 250g, equilibrated for an hour in dissolution medium or phosphate buffer pH 7.4 before starting the experiment and was placed on a magnetic stirrer with a small magnetic needle for uniform distribution of the diffusant. The temperature of the cell was maintained at 32 ± 0.5°C using a thermostatically controlled heater
7.3 In Vivo Evaluation Studies:
In vivo Evaluation:
In vivo evaluations are the true depiction of the drug performance. The variables which cannot be taken into account during in vitro studies can be fully explored during in vivo studies. In vivo evaluation of TDDS can be carried out using:
? Animal models
? Human volunteers
? Biophysical models
• Animal models:
Considerable time and resources are required to carry out human studies, so animal studies are preferred at small scale. The most common animal species used for evaluating transdermal drug delivery system are mouse, hairless rat, hairless dog, hairless rhesus monkey, rabbit, guinea pig etc. Various experiments conducted lead us to a conclusion that hairless animals are preferred over hairy animals in both in vitro and in vivo experiments.
• Human models:
The final stage of the development of a transdermal device involves collection of pharmacokinetic and pharmacodynamic data following application of the patch to human volunteers. Clinical trials have been conducted to assess the efficacy, risk involved, side effects, patient compliance etc. Phase I clinical trials are conducted to determine mainly safety in volunteers and phase II clinical trials determine short term safety and mainly effectiveness in patients. Phase III trials indicate the safety and effectiveness in large number of patient population and phase IV trials at post marketing surveillance are done for marketed patches to detect adverse drug reactions.
• Biophysical Models:
Models based on steady-state mass balance equation, solution of Fick’s second law of diffusion for the device, stratum corneum and viable epidermis, as well as linear kinetics have been described in the literature.
Some of the unresolved issues include the barrier function of the skin with age, skin metabolism, in-vivo functioning of penetration enhancers etc…
CONCLUSION:
During the past decade, the number of drugs formulated in the patches has hardly increased, and there has been little change in the composition of the patch systems. Modifications have been mostly limited to refinements of the materials used. The reason is the only a limited number of drugs fit the molecular weight, and potency requirements for transdermal absorption. These so-called “active” transdermal technologies include iontophoresis (which uses low voltage electrical current to drive charged drugs through the skin), electroporation (which uses short electrical pulses of high voltage to create transient aqueous pores in the skin), sonophoresis (which uses low frequency ultrasonic energy to disrupt the stratum corneum), and thermal energy (which uses heat to make the skin more permeable and to increase the energy of drug molecules Regulatory bodies will also require data to substantiate the safety of the device on the skin for either short or long term use. Thus, for any of these novel drug delivery technologies to succeed and compete with those already on the market, their safety, efficacy, portability, user-friendliness, cost effectiveness and potential market has to be addressed.
REFERENCES
- Soni Ankita et al., “Article Reviewing Transdermal Drug Delivery System”, Journal of Drug Delivery and Therapeutics, 2022; 176-180.
- N.Chaithanya et al., “A Review Article of Transdermal Drug Delivery System (TDDS)”, International Journal of Research in Engineering, Science and Management, 2019; 111-116.
- Delly Ramadan et al., “Enhancement Strategies for Transdermal Drug Delivery Systems. Current Trend & Application”, Drug Delivery and Translational Research, 2022; 12: 759-791.
- Hanumanaik et al. “Design, Evaluation and Recent Trends in Transdermal Drug Delivery Systems: A Review”, CNS Drugs, 2021; 3(8): 2393-2406, UPSR.
- Deependra Singh et al., “Recent Advancesof Transdermal Drug Delivery Systems”, Journal of Pharmaceutical Negative Results, 2022; 13: 4452-4459.
- Sadab, Sahu S., Patel S., Khan R., Khare B., Thakur BS, Jain A, and Jain P.K., “A Comprehensive Review: Transdermal Drug Delivery System: A Tool for Novel Drug Delivery System”, Asian Journal of Dental and Health Sciences, 2022; 2(4): 40–47.
- Purushotham and K. Anie Vijetha, “A Review of Transdermal Drug Delivery Systems," GSC Biological and Pharmaceutical Sciences, 2023; V-22(02): 245-255.
- Bacchav Rishikesh Shankar, Kale Suvarna Bhausaheb, “ABasic Overview of Transdermal Drug Delivery Systems," IJTSRD, 2021; 6(1): 1162-1168.
- Alkilani, A.Z.; Nasereddin et al., “Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems,” Pharmaceutics, 2022; 14: 1152.
- Shankhadips Nandi, Saptarshi Mondal, “Fabrication and Evaluation of Matrix-Type Novel Transdermal Patches Loaded with Tramadol Hydrochloride”, Turk J Pharm. Sci., 2022; V-19(5): 572–582.
- N.Chaithanya et al., “A Review Article of Transdermal Drug Delivery System (TDDS)”, International Journal of Research in Engineering, Science and Management, 2019; 2: 111-116.
- Nidhi Sharma, “A Brief Review on Transdermal Patches”, Organic and Medicinal Chemistry International Journal, June, 2018; 7: 001-005.
- Dr. Abdul Mannan et al., “A Review Article on Transdermal Drug Delivery System”, IJIRT, June, 2022; 9: 185-192.
- Kharat Rekha Sudam and Bathe Ritesh Suresh, “A Comprehensive Reviewon: Transdermal Drug Delivery Systems”, International Journal of Biomedical and Advance Research, 2016; 7(4): 147-159.
- Aundumbar Digambar Mali, Ritesh Bathe and Manojkumar Patil, “An Updated Review of Transdermal Drug Delivery Systems”, International Journal of Advances in Scientific Research, 2015; 1(06): 244-254.
- Rajesh Mujoriya and Kishor Dhamande, “A Review on Transdermal Drug Delivery Systems,” Research J. Science and Technology, 2011; 3(5): 227–231.
- Himanshi Tanwar and Ruchika Sachdeva, “Transdermal Drug Delivery System: A Review,” International Journal of Pharmaceutical Science and Research, 2016; 7(6): 2274–2290.
- Swati Hardainiyan et al., “A Review on the Recent Innovations in Transdermal Drug Delivery for Herbal Therapy,” Journal of Biomedical & Pharmaceuticals Research, 2014; 3(3): 88–101.


 D. Rama Brahma Reddy *
D. Rama Brahma Reddy *









 10.5281/zenodo.14505870
10.5281/zenodo.14505870