Abstract
Digestible sensing capsules are quickly becoming a vital technological advancement with the potential to significantly influence clinical settings, nutrition, and overall health. Due to their non-invasive nature, these ingestible gadgets are highly appealing to consumers. Due to the increasing availability of Smartphone’s with Internet connection, users and doctors may easily monitor and evaluate the data generated by this technology online. The results offer priceless insights into the condition of the gut and its ailments, as well as the effects of nutrition, pharmaceutical supplements, and environmental modifications on the digestive system. Such ingestible sensors are special because they can access every single gastrointestinal tract organ through their transit through the gut lumen. Such ingestible sensors are special because they can access every single gastrointestinal tract organ through their transit through the gut lumen. Consequently, the capacity to collect photos and track luminal fluid as well as the components of every gut segment, such as electrolytes, enzymes, metabolites, hormones, and microbial populations, are provided by ingestible sensors. As a result, it is possible to gather a staggering amount of information on each person's functionality and health status using important gut indicators. An outline of the gut's structure is provided in this review, along with a discussion of both existing and new digestible technology. The paper aims to give a thorough review of ingestible sensing capsules from the perspectives of body physiology and technology, as well as to provide specifics on each.
Keywords
Wireless capsules, Smart pills, Telemetry, Small intestine, Colon, Swallowable biomarker
Introduction
The FDA approved the first ingestible sensor (IS) technology in 2015 (1), then in 2019(2), it approved a second sensor variation. This technology records oral medicine adherence. The ability of these medical devices to record distant, real-time data on drug ingestion sometimes along with concurrent physiological data—is a significant achievement (3). These innovative digital technologies may enable continuous treatment adherence support in addition to precise oral dose ingestion confirmation (4). The WHO global strategy states that digital technology will play a role in global health in the future. Global security, health promotion, and the provision of services to the most vulnerable segments of the population can all be enhanced by digitalization (5). A digital pill is a pharmaceutical dosage form that has an ingestible sensor inside of it. It can also be referred known as a smart pill or an ingestible sensor (6). When it has been consumed, the sensor starts to communicate medical data. Digital medicine includes both the data supplied by the pill's sensor and the technology that goes into making the pill. The sensor's function is to determine whether or not the patient is taking their medication (a method referred to as "compliance")(7). Using ingestible devices is a safe and non-invasive way to access some of the target bodily fluids. This paradigm will make the ability to directly access the gut environment while food is moving through the gastrointestinal (GI) tract. There are many different biomarkers and therapeutic targets that can be assessed since the mucous membrane of the gut facilitates the easy and rapid passage of substances within the gut. Some the interesting substances that are exchanged within the GI tract are enzymes, metabolites, and electrolytes (8).

INGESTIBLE PILL
MATERIALS REQUIRED
Materials required for ingestible sensors are
-
-
- Power supplies
- Telecommunication
- Microcontrollers and processors
- Power switches
- Cladding
- Sensors
Power Supplies
The power consumption of ingestible devices must be kept low due to the length of operation and the requirement to send data in order to collecting it. Silver-oxide coin batteries are used in a lot of commercially marketed edibles. These batteries have an 8 mm diameter and 2.5 mm thickness and they have a 3 V voltage at about 80 mAh with an acceptable pulsed current of 70 mA. While having a far higher energy density than silver oxide, Li ion batteries are not recommended because of health hazards if they come into contact with stomach fluid. They increase the pH of the stomach liquid if the sealant is removed, which might lead to serious illnesses.
Telecommunication
Commercial transmitter chips are frequently utilized in communications, and in order to protect patient privacy, data encoding is suggested. Furthermore, recent efforts have been made to increase the transfer carrier frequency in order to enhance data rate transmission (9).
Microcontrollers and Processors
The job of transferring commands to and from sensors and transmitters falls to microcontrollers and processor units. They play a considerably bigger role in camera capsules since they have to compress the images in order to use the bandwidth efficiently and convey the data safely. Custom designs are possible based on the requirements.
Power Switches
The ingestible devices typically turned on before use employing magnetic sensing switches.
Cladding
Ingesting electronic devices must have claddings built of materials that are both biocompatible and able to survive the severe environments of the gastrointestinal tract (10).
Sensors
The main elements defining the operation of the ingestible electronic capsule are the sensors, which are used to detect either chemical or physical aspects. The many ingestible sensing capsules that use various kinds of sensors and their driver unit will be covered in more detail when the technologies are discussed in the Application section (11).
MECHANISM

SENSING TARGETS
ORAL CAVITY
Medical professionals visually inspect the oral cavity in order to look for lesions, ulcers, cold sores, cuts, and/or inflammation. For diagnostic purposes, the mouth provides the easiest and most invasive access to biological fluids released, and saliva is typically used to collect DNA samples (12).
OESOPHAGUS
Various imaging capsules, optical coherence tomography, and endoscopic systems have been developed to evaluate the presence of inflammation and lacerations in the esophageal wall, as it is a very important tissue. In addition, the mucosa of the esophageal is observed in order to diagnose conditions where the mucosa's quality could indicate issues such eosinophilic esophagitis (13).
STOMACH
The balance of the stomach juice is essential. It is crucial to keep an eye on several key factors in the stomach, including pH, metabolite concentrations, enzyme synthesis, and the production of electrolytes like bicarbonate. Checking the mucosa's quality visually is essential while looking for stomach ulcers and other conditions. Finding bacteria like Helicobacter pylori, the cause behind ulcers, is an essential phase in the medical diagnostic process (14).
SMALL INTESTINE
Electrolyte, gas, and metabolite balances are useful indications to evaluate the activity of each specific small intestinal segment. Imaging helps determine the integrity of the mucosa and whether there are some lacerations or inflammations. The microbial community of an individual's small intestine can be better understood by the use of taxonomic profiling and bacterial counts (15).
COLON
The results of this research can be used to diagnose a variety of issues, which includes as infections (caused by bacteria, viruses, or parasites), insufficient nutrient absorption, and in serious cases, cancer. It is possible to look for blood traces in the colonic material in addition to lipids, proteins, and fibers. The colon's electrolyte breakdown plays an important part in deciding how well it works. Colonoscopies are an essential tool to inspect the mucosa of the colon and looking for lesions and inflammation. Along with other metabolites, the three major SFCAs butyrate, acetate, and propionate are significant indicators of the colon's health. The study and sequencing of this region's micro biome genes are becoming more and more important for treating various health issues, especially in connection with the idea of leaky colon (16).

OTHERS
TEMPERATURE SENSING CAPSULES
Ingesting temperature sensors are frequently used to measure body temperature in order to assess heat stress in patients, industrial personnel, and maybe even soldiers in the field. Thermostat-based temperature sensors are found in capsules like Vital Sense's CorTemp (23 mm in length, 8.6 mm in diameter), which are manufactured by Mini Mitter Co., Inc and are used to detect core body temperature. Recently, temperature sensors have been incorporated into the majority of newly manufactured products (17).
MEDICATION MONITERING PILLS
The Proteus Digital drug intake tracking tablet is a relatively recent addition to the market. This pill tracks the quantity consumed and serves as a reminder or signal to consumers that their medication has to be taken. The Proteus pills are not active unless they come into contact with acidic electrolyte. Once this happens, they send a signal to a small body patch that is powered by batteries. The data is then sent via Bluetooth to a nearby Smartphone (18).
PH MONITORING SYSTEM
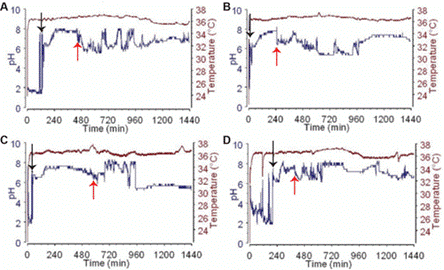
As HCl is secreted into the stomach, the gastric juice is extremely acidic. The transit of the capsule into the alkaline duodenum is indicated by a sudden pH rise (>3 pH units) from the gastric baseline. The sudden decrease in pH of one or more pH units is commonly used to identify the ileocecal junction, or the point where the ileum and colon meet. Early in 2010, Given Imaging Inc. purchased the Bravo pH monitoring system. The test is carried out while the patients continue with their regular daily activities and diets. The capsule lifetime is typically several days. As the gold standard for monitoring stomach reflux, pH testing are now more widely accepted, assisting medical professionals in the diagnosis and treatment of gastro esophageal reflux disease (GERD). Functional nonnuclear dyspepsia, colonic diseases, and small bowel dysfunctions are among the other conditions that can be diagnosed using the pH detecting capsules(19).
PH MONITORING SYSTEM
INGESTIBLE SENSORS SAFETY AND RETENTION
Reports of capsule retention in healthy users are rare. Depending on the type of food consumed, the passage of the electronic capsule in healthy persons may take several weeks; however, for capsules smaller than 11 mm in diameter and 28 mm in length, it has been considered 100% safe overall. Patients with digestive issues, however, have a significantly greater retention rate. For ingestible devices, the most serious potential adverse event is bowel obstruction from capsule retention. The retention rate is 0.33%, according to a report from the manufacturers of electronic capsules. This is based on 6000 individual device trials with 20 protracted capsule retention incidents. For patients who have had several medical procedures performed in their digestive system, capsule retention can also be a significant problem. It is recommended that patients with pacemakers avoid using capsules because of the potential for mutual interference (20).
ADVANTAGES
- They can monitor various health parameters like temperature and pH level
- They provide non – invasive way to gather internal data without need for surgery
- They can improve patient compliance with medication and monitors its activity
- They detect physiological changes in the body.
DISADVANTAGES
- It is costly to manufacture
- The approval and regulation is complex
- Its technology may have limitation which may affect the usefulness
- Collecting and transmitting internal health data raises significant privacy concerns regarding data security and confidentiality.
SIDE EFFECTS
- Gastrointestinal discomfort
- Nausea
- Obstruction
- Diarrhoea
- Vomiting
- Irritation
- Allergic reactions
- Hypersensitivity
- Itching
- Difficulty in swallowing
- Battery malfunction
- Interaction with medication
- Injury
- Ejection (rare)
REFERENCES
- 510(K) Premarket notification: ingestible event marker K150494, Proteus digital health feedback device.U.S Food and drug administration.
- 510(K) Premarket notification: ingestible event marker K183052, ID-cap system. U.S Food and drug administration.
- Browne SH, Behzadin Y, Littlewort G. Let visuals tell the story: medication adherence in patience with type 2 diabetes captured by a novel ingestion sensor platform.JMIR Mhealth Uhealth.Dec 31,2015
- Hafezi H,Robertson TL, Moon GD,Au-Yeung KY,Zdedlick MJ,Savage GM. An ingestible sensor for measuring medication adherence.IEEE Trans biomed Eng.Jan 2015.
- Global strategy on Digital Health 2020-2025 world health organization;Geneva,Switzerland 2021
- Batra,S;Baker,RA;Wang,T;Forma,F;DiBiasi, F;Peters-Strickland,T(2017). Digital health technology for use in patients with serious mental illness: systemic review of the literature. Medical Devices: Evidence and Research.
- Wamsley, Laure (November 14, 2017). FDA Approves First Digital Pill that can Track Whether You have taken it.
- Johnson, L. Physiology of the Gastrointestinal Tract, Fifth ed.; Academic Press: New York, 2012
- Litovitz, T. L. Button Battery Ingestions - a Review of 56 Cases JAMA, J. Am. Med. Assoc. 1983.
- Sina Nejati, Devendra Sarnaik, Sarath Gopalakrishnan, Venkat Kasi, Akshay Krishnakumar, Samuel Hyde, Robyn McCain, Kinam Park, Jay S. Johnson, Rahim Rahimi. Electronic?Free Traceable Smart Capsule for Gastrointestinal Microbiome Sampling. Advanced Materials Technologies 2024,
- Mariam Elgabry. Biocrime, the Internet-of-Ingestible-Things and Cyber-Biosecurity. 2023,
- Keller, S.; Ridinger, J.; Rupp, A. K.; Janssen, J. W. G.; Altevogt, P. Body Fluid Derived Exosomes as a Novel Template for Clinical Diagnostics J. Transl. Med. 2011
- Atkins, D.; Kramer, R.; Capocelli, K.; Lovell, M.; Furuta, G. T. Eosinophilic Esophagitis: the Newest Esophageal Inflammatory Disease Nat. Rev. Gastroenterol. Hepatol. 2009
- Hur, S. J.; Lim, B. O.; Decker, E. A.; McClements, D. J. in vitro Human Digestion Models for Food Applications Food Chem. 2011
- Sommer, F.; Backhed, F. The Gut Microbiota - Masters of Host Development and Physiology Nat. Rev. Microbiol. 2013
- Lau, W. L.; Kalantar-Zadeh, K.; Vaziri, N. D. The Gut as a Source of Inflammation in Chronic Kidney Disease Nephron 2015
- McKenzie, J. E.; Osgood, D. W. Validation of a New Telemetric Core Temperature Monitor J. Therm. Biol.2004
- Bettinger, C. J. Materials Advances for Next-Generation Ingestible Electronic Medical Devices Trends Biotechnol. 2015
- Kwiatek, M. A.; Pandolfino, J. E. The Bravo pH Capsule System Dig. Liver Dis.2008
- Demosthenous, P.; Pitris, C.; Georgiou, J. Infrared Fluorescence-Based Cancer Screening Capsule for the Small Intestine IEEE Trans. Biomed. Circuits Syst. 2016,


 Manjula.T*
Manjula.T*
 Nirosha.C
Nirosha.C
 Nandhini.D
Nandhini.D
 Kokila.M
Kokila.M
 Revathi.V
Revathi.V




 10.5281/zenodo.13333392
10.5281/zenodo.13333392