Abstract
Psoriasis is chronic inflammatory skin disarray that may drastically affect the feature of life of an affected person. Emulgel is a drug delivery systems found effective in topical delivery. Mometasone is a medium-potency synthetic corticosteroid with anti-inflammatory, antipruritic, and vasoconstrictive properties. We used physiochemical properties of drug, and an assessment of the irrelevance to the final formulation, the chemical and physical stability of drug and compatibility of the active with potential excipients. A suitable method of analysis of drug was UV spectrophotometric. Mometasone furoate showed maximum absorption at a wavelength of 242 nm. Various formulations (F1-F8) were developed by using Carbopol 940 for local release of Mometasone furoate for the treatment of topical infections by using penetration enhancer Tween 80. Developed formulations of Mometasone furoatewere evaluated for the physiochemical parameters such as drug content, viscosity, spread ability, in vitro diffusion. Viscosity studies of various formulations revealed that formulation F4 is better compare to others. Release of drug from mometasone furoate emulgel was significantly slower, which confirmed that slight prolonged drug release rate. Incorporation of carbomer affected the release rate of the drug. By increasing the amount of carbomer, the release rate of the drug decreased, which could be related to the increased rigidity of the formulation, followed by its decreased permeability for the drug. In vitro drug release from the semisolid preparation of mometasone furoate emulgel optimized formulation F4 shows significantly improved in drug release rate as compare to marketed preparation.
Keywords
Psoriasis, Emulgel, Treatment, Evaluation
Introduction
Psoriasis has genetic and life manner triggers; the treatment guidelines involve continuous monitoring and lifelong care for the patients. Knowledge of the disease trigger factors and their part in precipitating the psoriasis is quiet significant in the disease management. Care should be taken to avoid these psoriasis triggers. In recent years, new biological therapies have been introduced and numerous existing treatments have been superior giving new anticipate to people with psoriasis (Saraswat et al., 2007). Superiority of life in a disease whether it’s pre-treatment or post-treatment speaks a lot about its all-round impact on patients. Psoriasis has depressingly effects on quality of life. Psoriasis is a lifelong, chronic, and recurrent disease. In a patient surveys conducted by the National Psoriasis Foundation between 2001 and 2008 in the USA, 33% of patients with soft disease and 60% of patients with moderate-to-severe psoriasis accounted that their disease extensively affect their everyday life. Psoriasis can be as devastating as many others ever medical or psychiatric conditions. The physical, psychological and social dimensions of life are negatively precious by the psoriasis and can be greater than those consequential from life threatening illnesses such as myocardial infarction (Voorhees, 2009). The skin, called cutis in Latin (term cutaneous derived from it), is the main organ of the body counting more than 10% of the total body mass. Its big surface area of approximately two square meters considered important for the survival as it is in straight contact with the environment provided that multifunctional activity against varying conditions. For pharmaceutical technologist’s approach skin presents tremendous opportunities for drug delivery and overcoming the barricade function of it has become the essence in the design of contemporary drug delivery systems. Absorption throughout skin usually takes place either by transepidermal route or by trans follicular route. Trans epidermal pathway can be described as dispersal across the skin. Stratum corneum is the major confrontation encountered all along this pathway. Trans epidermal permeation pathway first absorbs partitioning into the stratum corneum followed by diffusion across this tissue. Most of the drugs and material disperse across the stratum corneum via the intercellular lipoidal route. Trans follicular absorption takes place throughout skin appendage. Hair follicle and connected sebaceous gland present in skin cooperatively referred to as skin appendages. This is a minor route of absorption (Benson, 2005). Unbound corticosteroids irritated cell membranes and bind with high similarity to definite cytoplasmic receptors. Inflammation is reduce by diminishing the discharge of leukocytic acid hydrolases, anticipation of macrophage addition at inflamed sites, interference with leukocyte adhesion to the capillary wall, decrease of capillary membrane permeability, reduction of harmonize apparatus, inhibition of histamine and kinin release, and interference with the formation of scar tissue. The anti-inflammatory proceedings of corticosteroids are thought to engross phosphor lipase A2 inhibitory proteins, lipo cortins, which manage the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes. Mometasone furoate has been revealed in vitro to display a binding similarity for the human glucocorticoid receptor which is approximately 12 times so as to of dexamethasone,7 times that of triamcinolone acetonide, 5 times that of budesonide, and 1.5 times that of fluticasone. Emulgel are also called as gellified emulsions. Emulsion in gel have emerged as one of the most interesting topical drug delivery system as it has dual release systemic emulsion and gel. Emulgel are emulsion, either oil-in-water or water-in-oil type, which are gelled by mixing with a gelling agent. Most pharmaceutical drugs are lipophilic compounds, which are practically insoluble in water. So to overcome this limitation an emulsion based approach is being used so that hydrophobic drug can be easily administered to the skin. An emulsion may be defined as a dispersion of two or more mutually insoluble liquids, one in other. The liquid are typically water and oil. A gel is apparently solid, jelly like material formed from a colloidal solution. By weight gels are mostly liquid, yet they behave like solid due to the addition of jelling agent. The presence of gelling agent in water phase coverts a classical emulsion in to an Emulgel. Emulsion possesses a certain degree of elegance. Gels for dermatological use have several favorable properties such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, non-staining, longer shelf-life, water soluble bio-friendly, transparent, pleasing appearance. Rationales of emulgel as topical drug delivery systems, numbers of medicated products are applied to the skin or mucous membrane that either enhances or restores a fundamental function of skin or pharmacologically alters an action in the underlined tissues. Such products are referred as topical or dermatological products. Many widely used topical agents like ointments, creams lotions have many disadvantages. They are sticky in nature causing uneasiness to the patient when applied, have lesser spreading coefficient so applied by rubbing and they also exhibit the problem of stability. Due to all these factors within the major group of semisolid preparations, the use of transparent gels has expanded both in cosmetics and in pharmaceutical preparations. In spite of many advantages of gels a major limitation is in the delivery of hydrophobic drugs. So to overcome this limitation an emulsion based approach is being used so that even a hydrophobic therapeutic moiety can be successfully incorporated and delivered through gels.
MATERIALS AND METHODS
Preformulation
Preformulation study is the first step in the rational development of dosage form of a drug substance. It can be defined as an investigation of physical and chemical properties a drug substance alone and when combined with excipients. The overall objective of preformulation testing is to generate information useful to the formulator in developing stable & bioavailable dosage forms which can be mass produced. Obviously, the type of information needed depend on the dosage form to be developed.
Solubility
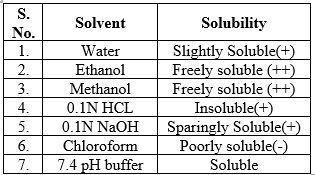
Solubility of the drug was determined by taking some quantity of drug (about 1-2 mg) in the test tube separately and added the 5 ml of the solvent (water, ethanol, methanol, 0.1N HCL, 0.1N NaOH, chloroform and 7.4 pH buffer). Shake vigorously and kept for some time.
Solubility of mometasone furoate
Melting point
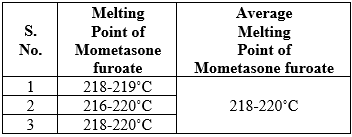
It is one of the parameters to judge the purity of drugs. In case of pure chemicals, melting points are very sharp and constant. Since the drugs contain the mixed chemicals, they are described with certain range of melting point.
Melting point of the mometasone furoate
Partition coefficient
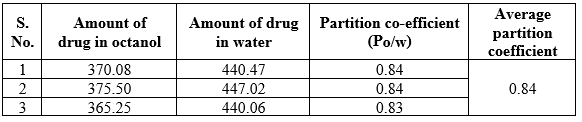
It is a measurement of a drug’s lipophilicity and an indication of its ability to cross cell membrane is the oil/water partition coefficient in system such as octanol /water and chloroform/water. The partition coefficient is defined as the ratio of un-ionized drug distributed between the organic and aqueous phases at equilibrium. It does provide a mean of characterizing the lipophilic /hydrophilic nature of the drug.
Partition coefficient of the Mometasone furoate
Partition coefficient of the Mometasone furoate
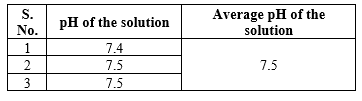
Determination of pH (1 w/v solution in water)
About 100mg of the Powder was taken and dissolved in 100ml of distilled water with sonication and filtered. The pH of the filtrate was checked with standard glass electrode
Identification test using FTIR Spectroscopy
Infra- red spectrum is an important record which gives sufficient information about the structure of a compound. This technique provides a spectrum containing a large number of absorption band from which a wealth of information can be derived about the structure of an organic compound. The region from 0.8 µ to 2.5 µ is called Near Infra-red and that from15 µ to 200µiscalled Far infra-red region.
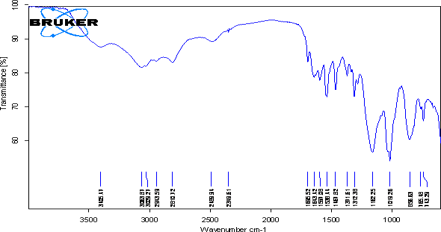
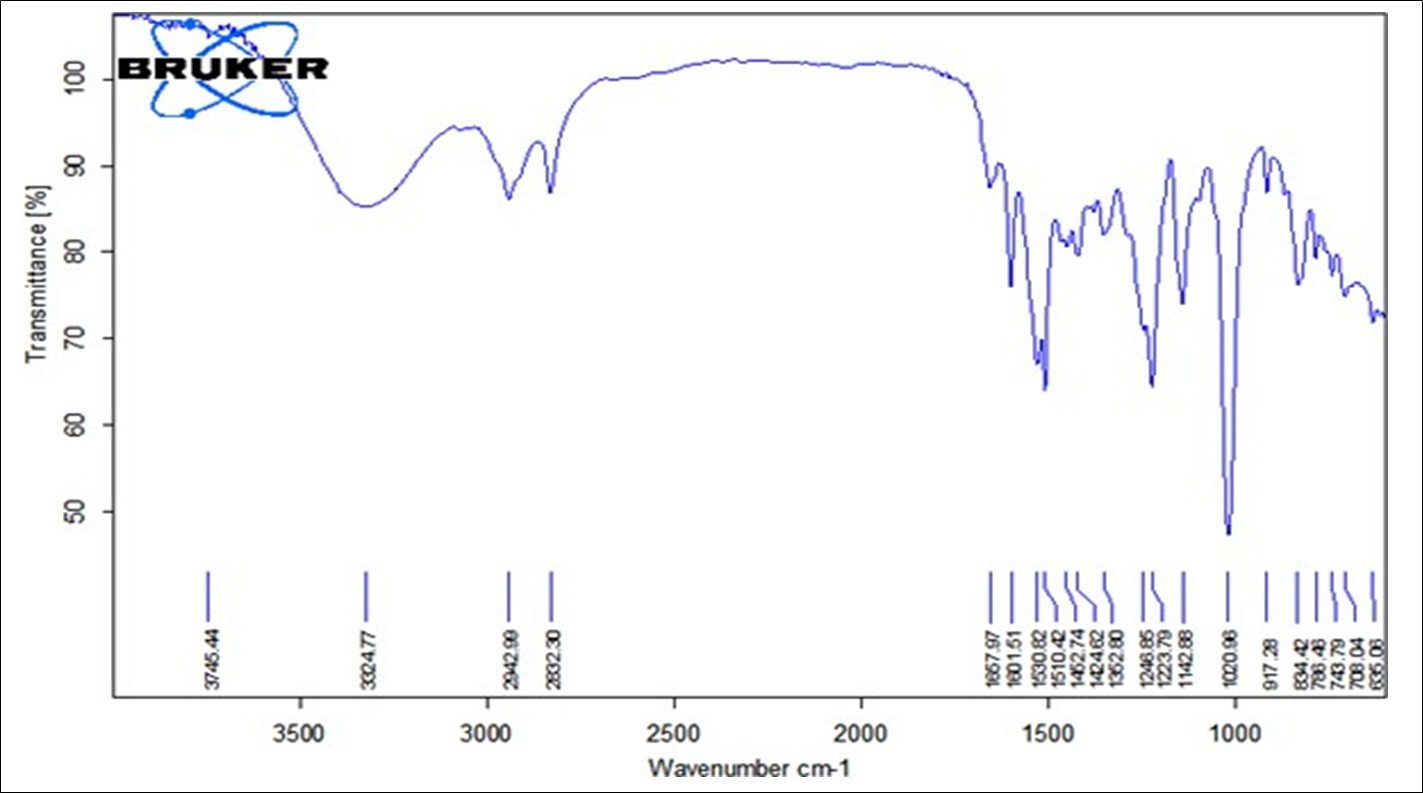
Figure: FT-IR Spectrum of Pure Drug and Excipients
Loss on drying
The moisture in a solid can be expressed on a wet weight or dry wet basis. On a wet weight basis, the water content of a material is calculated as a percentage of the weight of the weight solid. The term loss on drying is an expression of moisture content on a wet weight basis.

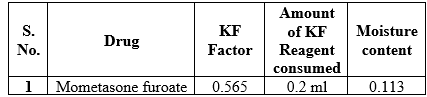
Moisture content determination
Karl Fischer coulometry is a micro-method and is particularly suitable for samples with low water content, from 10µg upto10mg. Here, there quire diodine is generated electrochemically in the titration vessel by anodic oxidation from iodide contained in the coulometric reagents. The amount of consumed electric charge is used to calculate the consumption of iodine and therefore the amount of water in the sample.
Table 6: Moisture content determination
Determination of ?max of mometasone furoate
The ?max of mometasone furoate was determined by running the spectrum of drug solution in double beam ultraviolet spectrophotometer.
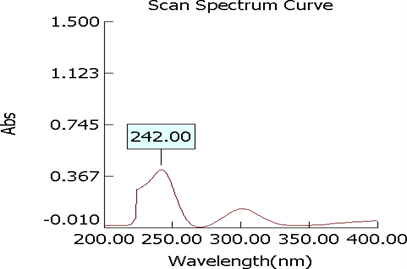
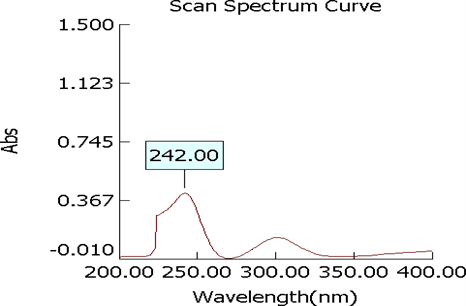
Figure 3: Standard calibration curve of mometasone furoate
Table : Calibration curve of Mometasone furoate
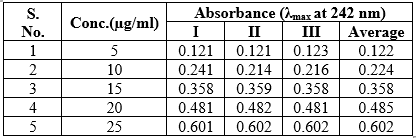
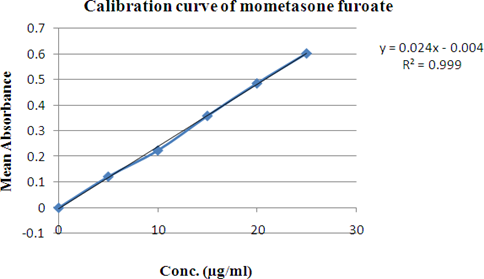
Figure 4: The linear regression analysis for standard curve
Compatibility studies of drug and excipients
In the compatibility testing program, blends of drug and excipients are prepared by triturating the drug with Individual excipients.
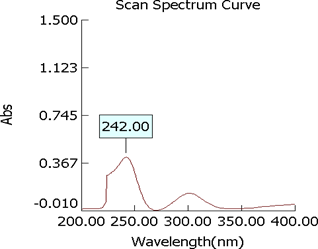
Figure 5: U.V. Graph of standard Mometasone furoate
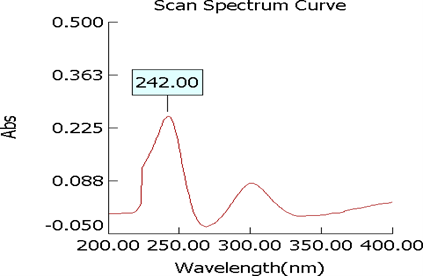
Figure 6: U.V. Graph of standard mometasone furoate+ all excipients
PREPARATION AND CHARACTERIZATION
Formulation development
Different formulations F1, F2, F3, and F4,were prepared using Carbopol 934 as gelling agent whereas formulations F5, F6, F7 and F8 were prepared by dispersing HPMC in heated distilled water (80°C), and the dispersion was cooled and left overnight. The pH of all the formulations was adjusted to 6-6.5 using tri ethanolamine (TEA). The oil phase of the emulsion was prepared by dissolving Span 20 in light liquid paraffin, while the aqueous phase was prepared by dissolving Tween 20and ethanol in purified water. Methyl paraben was dissolved in propylene glycol and mixed with aqueous phase. Drug was dissolved in oil phase. Permeation enhancers were dissolved in the oily phase. Both the oily and aqueous phases were separately heated to 70° to 80°C, then the oily phase was added to the aqueous phase with continuous stirring until it got cooled to room temperature. The obtained emulsion was mixed with the gel in 1:1 ratio along with the addition of a few drops of glutaraldehyde followed by gentle stirring to obtain the Emulgel.
Table 11 : Different formulas of Mometasone furoate emulgel (%w/w)

Evaluation of emulgel
Physical Characteristic
The Physical Characteristic was checked for emulgel formulations (colour, clogging, homogeneity and texture) and observations were shown in Table 8.1.
Determination of pH
The pH of the emulgel was determined by digital pH meter. One gram of gel was dissolved in 25 ml of distilled water and the electrode was then dipped in to gel formulation for 30 min until constant reading obtained. And constant reading was noted. The measurements of pH of each formulation were replicated two times.
Wash ability
Formulations were applied on the skin and then ease and extent of washing with water were checked manually and observations were shown in Table8.1.
Extrudability study
The emulgel formulations were filled into collapsible metal tubes or aluminiumcollapsibletubes.Thetubeswerepressedtoextrudethematerialandtheextrudabilityof theformulation was checked.
Spreadability Principle:
An important criterion for emulgel gels is that it must possess good spreadability. Spreadability is a term expressed to denote the extent of area to which the gel readilyspreadsonapplicationtoskin.Thetherapeuticefficacyofaformulationalsodepends on its spreading value. A special apparatus has been designed to study the spreadability of the formulations. Spreadability is expressed in terms of time in seconds taken by two slides to slip of from formulation, placed between, under the application of a certain load. Lesser the time taken for the separation of two slides, better the spreadability.
Viscosity
The measurement of viscosity of the prepared gel was done using Brookfield digital Viscometer. The viscosity was measured using spindle no. 6 at 10 rpm and 250C. The sufficient quantity of gel was filled in appropriate wide mouth container. The gel was filled in the wide mouth container in such way that it should sufficiently allow to dip the spindle of the Viscometer. Samples of the gels were allowed to settle over 30 min at the constant temperature (25±/10C) before the measurements.
In-vitro drug release studies using the prehydrated cellophane membrane
Preparation of cellophane membrane for the diffusion studies:
The cellophane membrane approximately 25 cm x 2cm was taken and washed in the running water. It was then soaked in distilled water for 24 hours, before used for diffusion studies to remove glycerin present on it and was mounted on the diffusion cell for further studies.
Diffusion Studies:
The in-vitro diffusion of drug from the different gel preparations were studied using the classical standard cylindrical tube fabricated in the laboratory; a simple modification of the cell is a glass tube of 15 mm internal diameter and 100 mm height. The diffusion cell membrane was applied with one gram of the formulation and was tied securely to one end of the tube, the other end kept open to ambient conditions which acted as donor compartment. The cell was inverted and immersed slightly in 250 ml of beaker containing neutralizing phthalate buffer, freshly prepared (pH 5.4) as a receptor base and the system was maintained for 2 hrs at 37±0.5oC. The media was stirred using magnetic stirrer. Aliquots, each of 5 ml volume were withdrawn periodically at predetermined time interval of up to 12 hrs and replaced by an equal volume of the receptor medium. The aliquots were suitably diluted with the receptor medium and analyzed by UV- Vis spectrophotometer at 260.0 nm using neutralizing phthalate buffer as blank.
RESULTS AND DISCUSSION
Characterization of emulgel
Gels were evaluated for their clarity, pH, viscosity, spreadability, skin irritation test, in vitro diffusion studies using standard procedure. All studies were carried out in triplicate and average values were reported.
Results of psycho rheological characteristic
The Psychorheological Characteristic was checked for emulgel formulations (color, clogging, homogeneity and texture). Formulations were applied on the skin and then ease and extent of washing with water were checked manually.
Table 12: Psychorheological Characteristic
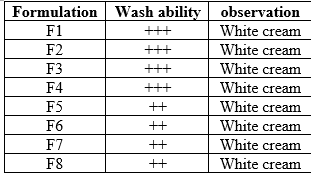
Washability -Excellent: +++,Good:++,Average:+, Poor: -
Results of extrudability study
The emulgel formulations were filled into collapsible metal tubes or aluminium collapsible tubes. The tubes were pressed to extrude them aterial and the extrudability of the formulation was checked.
Results of Spreadability
The weight was removed and the excess of the emulgel formulation adhering to the slides was scrapped off. The lower slide was fixed on the board of the apparatus and one end of the upper slide was tied to a string to which 20 gram load could be applied 50 with the help of a simple pulley. The time taken for the upper slide to travel the distance of 6 cms and separate away from lower slide under the direction of the weight was noted. The experiment was repeated and the average of 6 such determinations was calculated for each emulgel formulation Ahmad (2008).
Where, S= Spreadability (gcm/sec)
m= weight tied to the upper slide (20 grams) l=length of glass slide (6cms).
t= time taken is seconds.
Table 13: Extrudability and Spreadability study
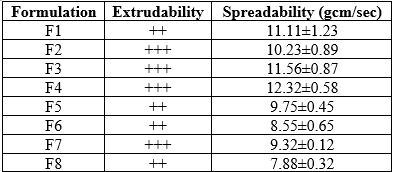
Excellent:+++,Good:++,Average:+, Poor:-
Figure 1: Graph of Spreadability study
Results of Viscosity
The measurement of viscosity of the prepared gel was done using Brookfield digital Viscometer. The viscosity was measured using spindle no. 6 at 10 rpm and 250C. The sufficient quantity of gel was filled in appropriate wide mouth container. The gel was filled in the wide mouth container in such way that it should sufficiently allow to dip the spindle of the Viscometer. Samples of the gels were allowed to settle over30 min at the constant temperature (25±/10C) before the measurements.
Determination of pH
The pH of the gel was determined by digital pH meter. One gram of gel was dissolved in 25 ml of distilled water and the electrode was then dipped in to gel formulation for 30 min until constant reading obtained. And constant reading was noted. The measurements of pH of each formulation were replicated two times.
Table 14: Viscosity and pH
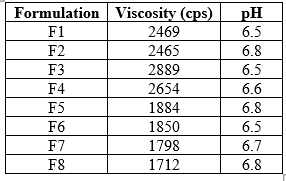
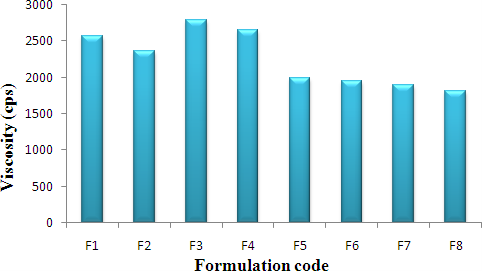
Figure 2: Graph of Viscosity (cps)
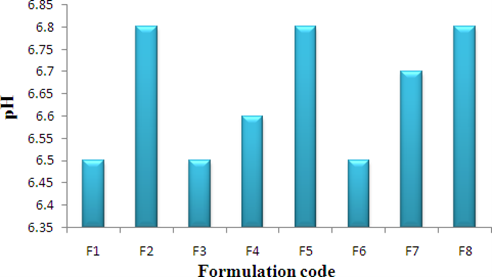
Figure 3: Graph of pH
In-vitro drug release studies of formulation F1-F8
Table 15: % Cum. drug release of formulation F1-F8
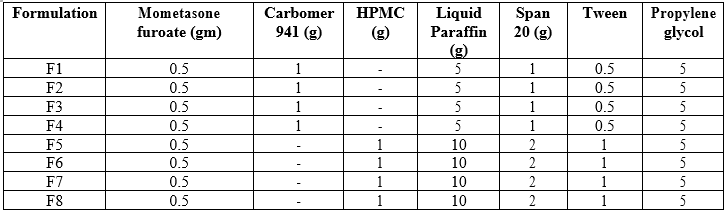

Figure 8.4: Graph of% Cum. Drug release of formulation F1- F8
Table 8.5: In-Vitro Drug Release Data for optimized formulation F4
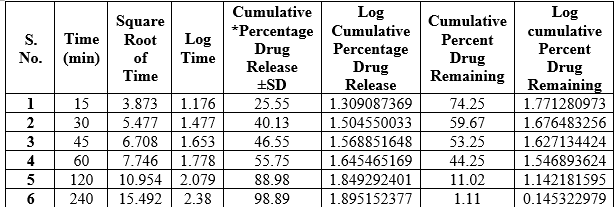
*Average of three determinations
Zero order release Kinetics of Optimized formulation F4
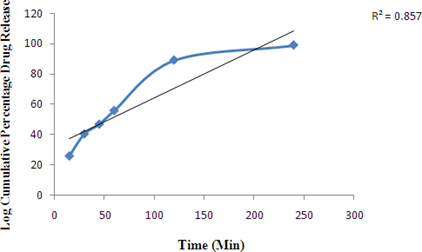
Figure 5: Graph of Zero order release Kinetics of Optimized formulation F4 First order release Kinetics of Optimized formulation F4
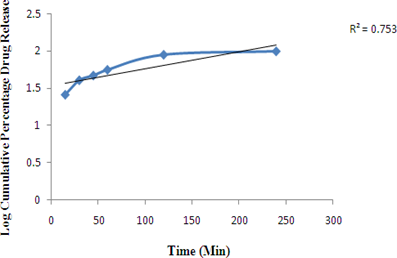
Figure 6: Graph of First order release Kinetics of Optimized formulation F4
In vitro drug release from the semisolid preparation of Mometasone furoate emulgel optimized formulation F4 shows significantly improved in drug release rate as compare to marketed preparation. It was concluded that developed formulations deliver the drug for the treatment of fungal disease. Hence it could be concluded that the carbomer based semisolid preparation would providing local onset of action without need of any device for their application on skin. The preparation of emulgel has potential advantages over marketed preparation as they improved patient compliance rapid local onset of action for longer period with cost effectiveness. The pediatric and geriatric populations are the primary ones whose problems are easily targeted.
SUMMARY AND CONCLUSION
Many advantages of gels a major limitation is in the delivery of hydrophobic drugs. So to overcome this limitation an emulsion based approach is being used so that even a hydrophobic therapeutic moiety can enjoy the unique properties of gels. When gels and emulsions are used in combined form the dosage form is referred as Emulgel in recent years, there has been great interest in the use of novel polymers. A suitable method of analysis of drug was UV spectrophotometric. Mometasone furoate showed maximum absorption at a wavelength of 242 nm. Various formulations (F1-F8) were developed by using Carbopol 940 for local release of Mometasone furoate for the treatment of topical infections by using penetration enhancer Tween 80. Developed formulations of Mometasone furoatewere evaluated for the physiochemical parameters such as drug content, viscosity, spread ability, in vitro diffusion. Viscosity studies of various formulations revealed that formulation F4 is better compare to others. Release of drug from Mometasone furoate emulgel was significantly slower, which confirmed that slight prolonged drug release rate. Incorporation of carbomer affected the release rate of the drug. By increasing the amount of carbomer, the release rate of the drug decreased, which could be related to the increased rigidity of the formulation, followed by its de-creased permeability for the drug. In vitro drug release from the semisolid preparation of Mometasone furoate emulgel optimized formulation F4 shows significantly improved in drug release rate as compare to marketed preparation. It was concluded that developed formulations deliver the drug for the treatment of fungal disease. Hence it could be concluded that the carbomer based semisolid preparation would providing local onset of action without need of any device for their application on skin. The preparation of emulgel has potential advantages over marketed preparation as they improved patient compliance rapid local onset of action for longer period with cost effectiveness. The pediatric and geriatric populations are the primary ones whose problems are easily targeted.
REFERENCES
- Saraswat A, Agarwal R, Katare OP, Kaur I, Kumar B. A randomized, double blind, vehicle controlled study of a novel liposomal dithranol formulation in psoriasis. J Dermatol Treatment, 2007; 18: 40-45.
- Voorhees A V, Feldman S R, Koo J Y M, Lebwohl M G, Menter A. The psorias is and psoriatic arthritis pocket guide: Treatment options and patient management. 3rded, publ. National Psoriasis Foundation, Portland, USA, 2009.
- Cevc G, Gebauer D. Hydration-Driven Transport of Deformable Lipid Vesicles through Fine Pores and the Skin Barrier. Biophysical Journal, 2003; 84: 1010–1024.
- Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Deliv Rev, 2004; 56: 675-711.
- Lopes LB, Speretta FF, Bentley MV. Enhancement of skin penetration of vitamin Kusing mono olein-based liquid crystalline systems. Eur J Pharm Sci, 2007; 32:209-215.
- Benson H A E. Trans dermal drug delivery: penetration enhancement techniques. Curr Drug Deliv 2005; 2:23-33.
- Vyas S P, Khar R K. Controlled drug delivery system. 1st ed: Vallabh Prakashan. 2005. p. 417-426.
- Khan, A.; Sharma, P.K.; Visht, S. & Malviya, R., (2011). Niosomes as colloidal drug delivery system: A review. Journal of Chronotherapy and Drug Delivery, Vol. 2, No. 1, pp. 15-21
- Azeem, A.; Anwer, M.K. & Talegaonkar, S., (2009). Niosomes in sustained and targeted drug delivery: some recent advances. J Drug Target, Vol. 17, No.9, pp. 671-689, ISSN: 1061-186X.
- Handjani-Vila, R.M.; Ribier, A.; Rondot, B. & Vanlerberghe, G., (1979). Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmetic Sci, Vol. 1, pp. 303-314.
- Perrett, S.; Golding, M. & Williams, W.P., (1991). A simple method for the preparation of liposomes for pharmaceutical application and characterization of liposomes. J Pharm Pharmacol, Vol. 43, pp. 154-161, ISSN: 2042-7158.
- Gopinath, D.; Ravi, D.; Rao, B.R.; Apte, S.S.; Renuka, D. & Rambhau, D., (2004). Ascorbyl palmitate vesicles (Aspasomes): formation, characterization and applications. Int J Pharm, Vol. 271, No. 1-2, pp. 95-113, ISSN:0378-5173.
- Yoshida, H.; Lehr, C.M.; Kok, W.; Junginger, H.E.; Verhoef, J.C. & Bouwstra, J.A., (1992). Niosomes for oral delivery of peptide drugs. J Control Release, Vol. 21, No. 1-3, pp. 145-153, ISSN: 0168-3659.
- Yoshioka, T.; Sternberg, M.; Moody, M. & Florence, A.T., (1992). Niosomes from Span surfactants: Relations between structure and form. J Pharm Pharmacol, Vol. 44, pp.1044-1044, ISSN: 0022-3573.
- Cevc, G., (1996). Transfersomes, liposomes and other lipid suspensions on the skin: permeation enhancement, vesicle penetration, and trans dermal drug delivery. Crit Rev Ther Drug Carrier Syst, Vol. 13, No. 3-4, pp. 257, ISSN:0743-4863.
- Cevc, G.; Blume, G.; Schätzlein, A.; Gebauer, D. & Paul, A., (1996). The skin: a pathway for systemic treatment with patches and lipid-based agent carriers. Adv Drug Deliv Rev, Vol. 18, No. 3, pp. 349-378, ISSN: 0169-409X.
- Agarwal, R.; Katare, O.P. & Vyas, S.P., (2001). Preparation and in vitro evaluation of liposomal/ niosomal delivery systems for anti-psoriatic drug dithranol. Int J Pharm, Vol. 228, No.1, pp.43-52, ISSN: 0378-5173.
- Baillie, A.J.; Coombs, G.H.; Dolan, T.F. & Laurie, J., (1986). Non-ionic surfactant vesicles, niosomes, as a delivery system for the anti-leishmanial drug, sodium stibogluconate. J Pharm Pharmacol, Vol. 38, No. 7, pp. 502-505, ISSN: 0022-3573
- Abdelkader, H.; Wu, Z.; Al-Kassas, R. & Alany, R.G., (2012). Niosomes and discuses for ocular delivery of naltrexone hydrochloride: morphological, rheological, spreading properties and photo-protective effects. Int J Pharm, Vol. 433, No. 1-2, pp. 142-148, ISSN: 0378-5173.
- Ruckmani, K.; Jayakar, B. & Ghosal, S.K., (2000). Nonionicsurfactant vesicles (niosomes) of cytarabine hydro chloride for effective treatment of leukemias: Encapsulation, storage, and in vitro release. Drug Dev Ind Pharm, Vol.26, No. 2, pp. 217-222, ISSN: 0363-9045.
- Pardakhty, A.; Varshosaz, J. & Roulholamini, A., (2007). In vitro study of poly oxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm, Vol. 328, pp. 130-141, ISSN: 0378-5173.
- Jain, C.P.; Vyas, S.P. & Dixit, V.K., (2006). Niosomal system for delivery of rifampicin to lymphatics. Indian J Pharm Sci, Vol. 68, No. 5, pp. 575, ISSN: 0250-474X.
- Sahin,N.O. Niosomes as nano carrier systems. Nano materials and nano systems for biomedical applications 2007. p. 67-81.
- Ogiso, T.; Niinaka, N. & Iwaki, M., (1996). Mechanism for enhancement effect of lipid disperse system on percutaneous absorption. J Pharm Sci, Vol.85, pp. 57-64, ISSN: 1520-6017.
- Fang, J.Y.; Hong, C.T.; Chiu, W.T. & Wang, Y. Y., (2001). Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm, Vol. 219, No. 1-2, pp. 61-72, ISSN: 0378-5173.
- Javadzadeh, Y.; Shokri, J.; Hallaj-Nezhadi, S.; Hamishehkar, H. & Nokhodchi, A., (2010). Enhancement of percutaneous absorption of Finasteride by co solvents, co surfactant and surfactants. Pharm Dev Technol, Vol.15, No. 6, pp. 619-625, ISSN: 1083-7450.
- Hofland, H.; Bowuwstra, J.A.; Ponec, M. Boddé, H.E.; Spies,F.; Coos Verhoef, J.; Junginger H. E., (1991). Interactions of non-ionic surfactant vesicles with cultured keratinocytes and human skin in vitro: a survey of toxicological aspects and ultra structural changes in stratum corneum. J Control Release, Vol. 16, No. 1, pp. 155-167, ISSN: 0168-3659.
- Ana Cristina Gomes Barros Salgado, Alexandra Maria Nunes Nogueira daSilva, Marta Cristina Jorge Cabral Machado, Maria Aida da Silva Costa Duarte, Helena Margaridade Oliveira Marques Ribeiro, Development, stability and in vitro permeation studies of gels containing mometasone furoate for the treatment of dermatitis of the scalp, Brazilian Journal of Pharmaceutical Sciences, 2010, 46, (1),110-114.
- Kirti Kumari, UVS. Sara and Monika Sachdeva, Formulation and Evaluation of Topical Hydro gel of Mometasone Furoate using Different Polymers, International Journal of Pharmaceutical and Chemical Sciences,2013, Vol. 2(1),89-100.


 Abishek jhariya *
Abishek jhariya *
 Nazneen Dubey
Nazneen Dubey
























 10.5281/zenodo.13371575
10.5281/zenodo.13371575