Abstract
Among the most recent developments in Skillful DDS, gel allows for the dual control of the emulsion's and gel's appearance, making it ideal for a variety of practical applications. Accuracy standards: An oil sorting device utilizing liquid paraffin and propylene glycol, a surfactant consisting of Tween 80 and arrive at 80, and the utilization of pseudo ternary coordinate graphs were utilized in the development of this smaller-than-common emulsion w/o. Using a variety of methods, the high-level emulsion orchestrate was mixed into the gel mixture containing Carbopol-934, HPMC-5, and HPMC15+ Carbopol-934. What happened: Real look, cure material, consistency, spreadability, extrudability, pH, and in vitro steady release ponders were all shown for the built up gel. At the end of 5 hours, the in vitro cure appearance of the high-level nuances appeared to be 97.82% when defined with F9 and 1:3 HPMC15+ Carbopol-934. A lot of people wouldn't have believed it was feasible, but improved organization made a huge difference. Conclusion: Ibuprofen was found to be an acceptable gel for productive vehicle co-ordinator in order to attain predominate sorting consistency. Medications: ibuprofen, gel, carbopol-934, HPMC-5, HPMC15+ carbopol-934, liquid paraffin, tween 80, length 80, and propylene glycol are all part of the articulations.
Keywords
variety of methods, Knee Osteoarthritis, Insights and Future Directions.
Introduction
There has been a tremendous acceleration in the past twenty years in the field of pharmaceutical research and development. The confusing portions of modern medicine development structures are made-do-with-open-hate consistency and ampleness. Researching newer features of correspondence on the body for the introduction of medicines has been an ever more absurd framework. By avoiding first-pass planning and increasing understanding consistency, transdermal vehicles provide a significant advantage over injectables and oral courses, respectively.Since oral medication administration recalls the pleasure and support of prescription obsession for the body within an obligingly strong reach by appearance of an extraordinary piece at common reaches, transdermal administration has surpassed oral administration as the primary creative evaluation district in cure development. This is due to the fact that the medication fixation inside the body follows a zenith and box profile, leading to a more basic possibility of stunning effects areas of strength for or; a gigantic degree of sedate is lost close to the objective organ and close to believed is expected to screen treatment to avoid going too far away. To avoid hepatic "in any case pass" hepatic removal (HEPE), maintain consistent and effective drug levels in the body, and circumvent the difficulties and risks associated with intravenous treatment blend, transdermal medication alliance through flawless skin is a clear and safe alternative. Table 1.1 keeps an eye out for the key areas and harms that can be overlooked when treating the skin to calm it down.

Summary of TDDS Benefits and Drawbacks (Table 1.1)
It is considered a good choice to provide medication through the skin rather than limiting it to oral consumption. Patients often forget to take their prescription, and the most shocking thing is that the first thing that comes to mind is the obvious fatigue from swallowing tablets, particularly if they need to take a tiny amount daily. Additionally, avoiding the unpredictable gastrointestinal (GI) effects and partial first-pass inactivation by the liver is possible by avoiding the GI region. Blood level spikes and the formation of a box are reliably predicted by verbal evaluation structures after the clear and consistent consumption of a prescription over several hours or days. Although the gastrointestinal tract's capacity is to provide ingested texture suitable for retention, the skin's ability is to prevent substances from entering the body, therefore there is a small catch to the transdermal route that makes it less than ideal. The corneum, the outermost and most protective layer of the skin, is home to the skin's greatest anticipation. Keratinized and smoothed out remnants of formerly truly isolated epidermal cells make up the stratum corneum. Being both hygroscopic and impervious to water, it is a versatile and unusual layer. Lipids abound in the intercellular region. While the corneum layer is typically about 10 microns thick, it can be as thick as 600 microns on the soles and palms of the hand.
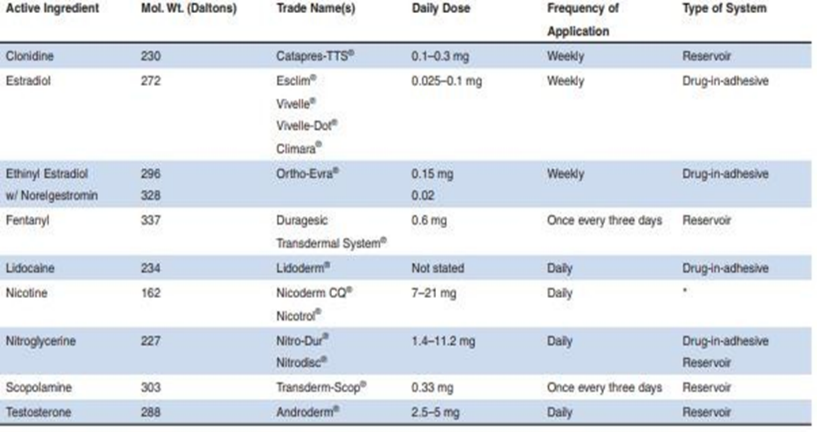
Features of Multiple Transdermal Drug Delivery Systems (Table 1.1)
Abuse of drugs and skin
Understanding the main and biochemical components of human skin, as well as the factors that contribute to the deterrent limit and the rate of prescription assistance entering the body through the skin, is essential for TDDS, which aims to accomplish head drug through skin application on glorified skin. As shown in Figure 1.1, the skin actually consists of two layers: the epidermis and the dermis or corium, which are penetrated by hair follicles and organ canals. In a typical adult, the skin covers an area of about 2 square meters, making it one of the most expansive organs in the body. From the inside out, the massive stratum corneum (hypodermis), the dermis (connective tissue layer), and the tiny epidermis (inner cell layer) make up the skin. The heart pumps about one-third of the blood throughout the body via this complex organ. The epidermis is approximately 150 μm thick and is produced by epithelial basal cells that are actively involved in cell function. The dermal layer is responsible for bringing improved skin cells from the basal ayer all the way up to the skin's surface during cell division. The epidermis does not have any veins, therefore in order to be aware of its centrality, enhancements and incidental effects must disperse over the dermal-epidermal junction. Layers germinativum (base layer), spinosum (spinous layer), granulosum (granular layer), lucidum (layer on the outside), and corneum (layer on the interior) make up the epidermis. It is common to refer to the skin lacking SC as the pragmatic epidermis due to the fact that SC cells are dead. In transdermal penetration of most particles, the SC is thought of as the rate- restricting aversion. A lipophilic structure including fifteen to twenty layers of corneocytes packed with keratin (terminally restricted keratinocytes) makes up the SC. The lipids of this extracellular structure are indisputable in many ways: (1) they provide a really stable orchestrate and spread pathway from the skin surface to the SC premise; (2) the action of phospholipids, free unsaturated fats, and ceramides is unique among biomembranes; (3) the SC lipids exist as multilamellar sheets despite the lack of polar bilayer-forming lipids; and (4) the mainly submerged, long-chain hydrocarbon tails collaborate with a generally determined, interdigitated orchestrate and the difference in gel stage layer spaces, as opposed to the more typical (and more fluid and vulnerable) liquid glasslike film structures. In its dry state, the SC has a thickness of 10-15 μm; when it is hydrated, it expands and can reach a thickness of 40 μm. In the SC, the keratin-rich corneocytes (the "squares") are implanted inside the intercellular lipid-rich structure (the "mortar"), which can be conceptualized as a long-term plan. Originating from mesoderm, the dermis is a layer of connective tissue that provides a stable foundation for the epidermis. A dense network of connective tissue, including layers of collagen and elastin, as well as pliable tissue at the deeper layers, makes up the dermis or corona.In the dermis are located the intricate networks of veins, lymphatics, nerves, sebaceous glands, sweat glands, and hair follicles.
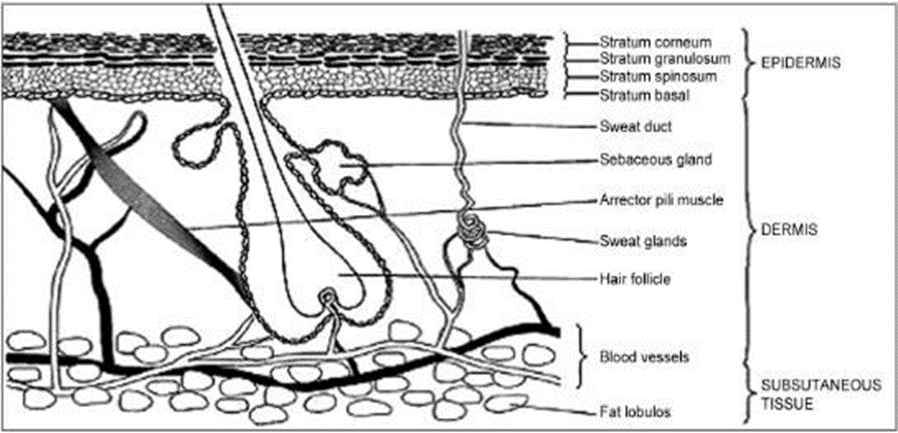
This figure presents a cross-sectional image of human skin, illustrating the many cell layers and appendages included.
Routes Of Penetration
Essentially, a medication molecule can traverse the perfect SC in one of three ways: via skin cells (shunt routes), intercellular lipid gaps, or a transcellular route [Figure 1.2]. The creation of a customized medication to absorb by a combination of these channels is guided by the particle's physiochemical characteristics.3
The path of the appendages
Facilitated immersion into the sweat organs and over the hair follicles with their associated sebaceous organs is achieved via the transappendageal courses, which are commonly referred to as shunt courses. Over the SC counteraction, skin persons provide an unending channel explicitly. Careful evaluations have cast doubt on the long-held belief that the follicles comprise approximately 0.1% of the skin's surface area. Based on their findings, Otberg et al. concluded that these individuals should be the primary focus of calm vehicle examinations for determining follicular number, opening width, and volume. The safe-haven yields a follicular infundibula of 13.7 mm2/cm2, meaning that 13.7% of the surface area of the shelter is occupied by follicles. In an unusual twist, a related study fundamentally implied that the long-term perspective on the follicles contributing around 0.1% of the SC is essential for the skin of the lower arm.
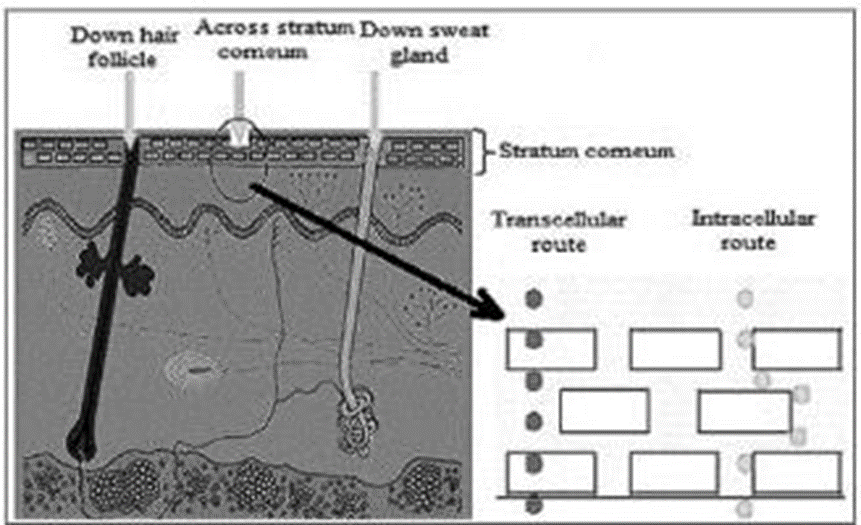
Figure 1.2: Routes of drug entry through the skin
Transcellular route
Corneocytes are the entry point for drugs via the transcellular route into the skin. Hydrophilic drugs can pass through corneocytes that have highly hydrated keratin. Keeping entering and diffusing through the keratin blocks and, more importantly, into and over the intercellular lipids is essential for the transcellular pathway.
Path between cells
Peaceful diffusion over the stable lipid bilayer characterizes the intercellular pathway. As a fundamental deterrent, this course might serve two purposes:
(I) rather than the logically fast method for the transcellular course, the interdigitating concept about the corneocytes produces a tangled pathway for intercellular medicine immersion when assessing the "pieces and mortar" display of SC.
(ii) Converging bilayers may be present in the intercellular space. Therefore, a sedate has to be painstakingly incorporated into and distributed via recycled liquid and lipid spaces. In general, this is thought of as the main widely recognized pathway for non-harmful, neutral particles to penetrate the skin.
Thinking About When Developing a Transdermal Solution
The skin's excellent barrier function against subatomic particles prompted careful planning of this transport process. Elective courses were sought after, and there are a few of instances where the verbal course, the main core of medical use approaches, isn't utilitarian. Although administering the drug intravenously sidesteps some of these requirements (such as gastrointestinal and hepatic setup), its noticeable and troublesome nature (especially for consistent connection) has prompted the search for alternative frameworks, and almost all approved openings have been assessed for their actual control as alternative medication transport courses. However, there are a few distinct advantages to the transdermal mode: (1) the skin provides a generously sized and rapidly permeable surface area (1-2 m2) for support; and (2) applying a settle-like device to the skin surface is a safe (and thus easily understood) way to allow for continuous intervention (i.e., structure repositioning, clearing, or replacement). In recent years, thanks to advancements in technology, the benefits of TDDSs have grown. These pave the way for constant release, which is important for medications with short half- lives that need oral or intravenous administration, and for controlled input, which is especially important for medications with weak recovery histories. It goes without saying that TDD progress must be diligently "kept up with"; nevertheless, medications with high verbal bioavailability and inconsistent dose schedules, as seen by patients, do not support such precautions. Simply put, transdermal connection isn't the way to go for rapid bolus-type treatment inputs. Instead, it's usually expected to provide moderate, sustained calm improvement over long periods of time. As a result, block-impelling prescriptions or those (like engineered materials) that require chronopharmacological regulators aren't practical at this time. Anyhow, TDD is still linking to a vast array of medications that are as of yet incomprehensible. From a broader viewpoint, the entry into this issue is the credibility of the SC. A good "settle assessed" location should keep the standard sedate segment inside the 10 mg range, according to the wonderful diffusional square introduced by the layer. Transdermal medications have become important areas of support for pharmacologically patching blood concentrations within the ng/ml range or lower, and this necessity is driving the necessary degree for an appropriate transdermal unused child on the market. Not all particles that pass the "quality" test will have the major physicochemical qualities; SC is incredibly strict about the sort of molecules that can be gotten past its outer layer.
Factors Affecting Transdermal Drug Delivery
Disease of the skin
Even though the skin acts as a barrier, substances such as acids and soluble bases are able to penetrate it and attack the underlying cells. Solvents such as chloroform and methanol remove the lipid section, creating fake shunts that allow stable particles to pass freely, thereby opening the horny layer's confused thick pathway.
Skin aging Skin permeability is higher in adults and younger individuals compared to those with more organized bodies. however there isn't any exciting prerequisite. Because there is more obvious surface area per unit of body weight, children appear to have harmful impacts. As a result, hexachlorophene, boric harming, and solid steroids have all had quite helpful side effects.
Physical and chemical variables Skin hydration
Everywhere you look, when water hits the skin, it swells the tissues, creases the skin, and makes you more susceptible to drug particles penetrating the skin.5
Skin pH and temperature
Wearing special clothing on the body prevents significant changes in temperature and entrance rates, which cause changes to the part rate and a shrinking scrambling coefficient, respectively. The lipid bilayer is quickly disregarded by the fair-unionized particles, which are pH-dependent; small acids and bases are distributed to varying degrees according on their pH and pKa or pKb values.So, the pH is directly related to the party of unionized steady in the associated organization, which will end up the persuasive film point.
Standard components Light from the sun
Vei
n dividers could have been more delicate, causing enlargement, with minimal damage inside the sun-exposed region, according to light. Sunlight lentigo or spots can also cause pigmentation, the most noticeable change in a sun-exposed collection.6
Order of cured plan for the skin
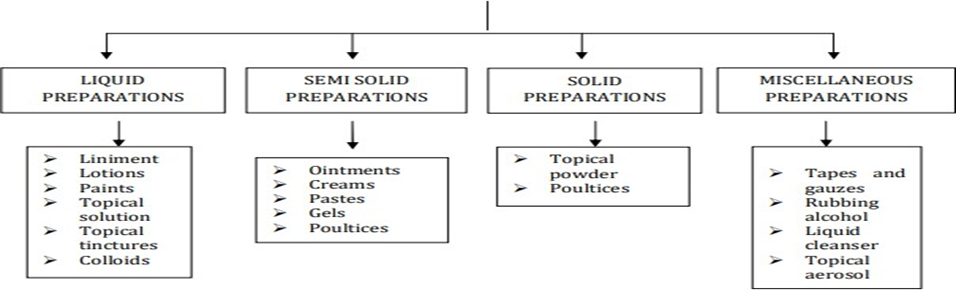
The classification of Medicated Formulations for the Skin is shown in Figure 1.3.
As a result of its superior precutaneous osmosis and ease of administration, gel is quickly becoming the best of these options. 'Gel' became a popular term in the late 1800s to describe a semisolid material as determined by pharmacological rather than nuclear tests. Gels 8 are described by the U.S. Patent as having a semisolid structure with dispersing inorganic particles or large normal particles surrounded and permeated by liquid. The inorganic particle forms a "spot of card" structure with three layers. The two-part structure of a gel is as follows: large ordinary particles are dispersed throughout the strong organ, randomly subsided into flexible strands, and inorganic particles are not degraded at all; they are just dispersed. Transdermal gels have seen increased application in both the field of eminence care and treatment redirection strategies within the large group of semisolid guides of movement. Colloid materials that are typically completely liquid are trapped in a gel by surface tension and a macromolecular bond of strands formed by a small amount of a gelating substance. A specific prescription vehicle can link to any part of the body through the skin, including the eyes, the rectal area, the vagina, and the skin as a whole. The skin is a pioneering organ in terms of skin affiliation and the leader in terms of skin quiet car structure, among the earliest open organs in the human body. To either push forward or mend a basic limit of the skin, or to pharmacologically trade an action within the highlighted tissues, large quantities of mucous or skin-friendly substances are applied. Products for the skin or dermatology are recommended for such concerns.9 Gel sedate vehicle structures have made use of hydrophilic polymers such as sodium alginate, carbopol 934P, and hydroxy propyl methyl cellulose (HPMC) for topical application. In practical gel definition, a preoccupation ranging from 1 to 5% of these polymers' sub-atomic bits is utilized, leading to an improvement in grades. Due to their rough texture, they might leave a washable film on the skin in addition to a very revolutionary result. High-molecular-weight polymers (such as sodium alginate, carbopol 934P, and HPMC) are non-toxic and do not penetrate the skin.
An Illustration of Gels It is possible to depict gels using two methods that are based on:
a) The nature of the colloid orchestrate First, inorganic gels; second, conventional gels
b) Thinking about how dissolvable
I. Gels in a liquid state ii. Gels that are not in a liquid state According to the interface preparation proposed by Faucci, quiet gels can be roughly coordinated taking into account their affiliation microstructure. 10
A. A polymer compound with a covalently attached structure that is entirely disorganized.
B. As a matter of fact, constrained polymer preparation is invariably seen outside of the presence of these loci.
Except for gel mesophases encased in inorganic muds,
C. exhibits well coordinated lamellar structures.
1) Advancement with Covalent Support
Gel frameworks that have been covalently cross-lined are structures that cannot be reversed. They often have one of two types of hydrophilic polymer attachments. An infinite gel coordinate is generated in the first proximity joining technique by non-straight copolymerization of two or more monomer species, one of which is fundamentally trifunctional.The residual microstructure of this gel is completely messed up since the path and position that each polymer chain takes during the response are clashing. It is possible to anticipate the gel point for copolymerization using modified Carothers conditions, which involve equimolar groups of two monomer species:
It is written as Xn = 2/2-Pfav.
Where Xn is the quantity and the normal degree of polymerization P stands for the fragmented change, and fav for the global assistance of the monomers involved.
Assuming that the necessary adjustment for gelation (PG) is equal to 2/fav, the gel direct approaches when Xn ↠’ ∠ž .
2) A properly built framework
Reversible designs are a hallmark of fully formed gel networks. A transition between the sol and gel phases can be triggered by changes in components such as temperature and molecule concentration. The typical generic polymers, such as proteins and polysaccharides, along with some man-made collaborators, form these gels. The specific arrangement of polymer chains in a joining zone is determined by a manufactured design of the repeating unit. Sulfated galacatose progressions from twofold helicles, some of which extend into a multi-helicles useful zone, are the building blocks of sulfated polysaccharides like match agar and carrageenans. Additionally, the closeness of a few related advances generates partners that actually helix enhance in epic package deals of chains, demonstrating that steric fit is essential for urge movement. In order to increase polymer chains, other sector combining necessitates the close vicinity of multivalent particles. Previously, Pawel et al. suggested an egg field display for the production of calcium alginate gels, wherein the ionized carboxy groups formed by the polyguluronate orchestrate of alginic damage obediently bind to the calcium cations. Areas are formed within the interstices using expressed polysaccharide chain segments.
Thirdly, Get Your Gels Coordinated
Under controlled conditions, soil soils, silica, and alumina form inflexible gels called lyogels. When smectite-containing mud (and related minerals including bentonite, hectorite, and loponite) comes into contact with water, it forms a gel through an immediate process of interlayer growth followed by osmotic extension. The mud particles that resemble plates are grouped into a so-called "cubic cardhouse" structure. This structure is supported by attractive forces and completed with the application of an electrical double layer. An astonishingly well-planned lamellar stack pass piece exhibit was suggested for the gel made with 10% w/w cetosteryl alcohol and 0.5?trimide surfactant using small-factor X-shaft spreading (SAXS), which is a clear combination of surfactant and long-chain clean alcohols in water. On the other hand, surfactant communities are crucial to the microstructure of Brij 96 gel. When using SAXS at a 40-60% w/w in water association, a hexagonal liquide-glasslike gel structure was noticed; nevertheless, a more favorable lamellar shape was evaluated at greater fixation (70-80% w/w).
Gel Forming Substances 11
The straightforward connection, which is critical in the context of gels, is brought about by polymers. The time it takes to extract polymers out of gel is:
1.Collagen and gelatin are examples of standard polymers. 2. Agar, alginate acid, sodium or potassium carageenan, tragacanth, gelatin, guar gum, cassia tora, xanthan, and gellum gum are examples of polysaccharides.
2. Polymers with Semiconductivity A Subset of Cellulose: Hydroxypropyl cellulose, Hydroxypropyl (methyl) cellulose, Hydroxyethyl cellulose, Methylcellulose, and Carboxymethyl cellulose
3. Polymers Shown — Carbomer - Carbopol 940, Carbopol 934 — Poloxamer — Polyacrylamide — Polyvinyl alcohol — Polyethylene and compounds derived from it
4. Solid, non-living things — Aluminum hydroxide — Bentonite
5. Beneficial surfactants: cebrostearyl alcohol (Brij - 96). 12
There are a lot of reasons why the skin-drug relationship for effective cutaneous and percutaneous medicinal drug delivery is becoming more important:
By manipulating stomach pH, enzymatic enhancement, and medication interactions with food and drink, they are able to circumvent gastrointestinal quiet maintenance issues.
When the oral delivery of the medication fails, they might step in to fill the void. Overall, the hidden passage of medicinal substance through the essential and entrance route after gastrointestinal assistance, perhaps avoiding inactivation via belly-related and liver- designed pathways, is vital to prevent the initial bypass effect. They possess a serene consistency and are harmless. You can efficiently remove them from your skin because they are not as smooth. †“ wise with money. Partitioning using verbal package deal structures leads to a reduction in components. Restricted impact with little delayed effects.
• Assist in increasing the bioavailability of drug and decreasing section repeat. †¢Rest easy vehicle profiles.
Limitations
If the transdermal improvement is projected to be more than 10 mg/day throughout the therapy period for patching, the medicinal medication must have a couple of excellent physicochemical properties for penetration through the stratum corneum. The skin's impermeability limits the normal utmost scopes of drug section, therefore only moderately powerful drugs are good candidates for TDDS. A significant number of patients have contact dermatitis in the region of usage for a system component that has to be addressed.
The shape of the skin changes as we get older, starting with one area and moving on to the next, from one person to another, and from one person to another's body.
Medication Delivery Device 13
By using the formula dQ/dt = Ps (Humbler circle - Cr), we can rationally determine the speed of entry over several layers of skin tissues over productive utility. Here, dQ/dt is the velocity of interruption over multiple layers. Medications gathered within the donar coordinate form a smaller circle. Cr implies that the treatment is concentrated inside the receptor arrangement. In this context, "Ps" refers to the skin's fragility coefficient. The main focus is on the straightforward distribution that is entering as a pharmaceutically lucrative development, for instance, Where Kc is the crew coefficient of the penetrant particles, Ps is equal to KcDs/hs. hs is the industry standard for measuring the thickness of skin and pores. For the trustworthy kingdom scrambling of attack moles, Ds is the obvious diffusivity. Suppose Circle >>> Cr, then the situation is shaped PsCd = dq/dt
Methods for Strategy 14
The dispersing method involves dissolving the polymer in water for two hours until it is completely dissolved. Then, additional intentional decorations are added and mixed well until a uniform mass is obtained. Using the cold process, which operates at a temperature of around 50 degrees Celsius, the ornaments are fused together to form a uniform mass. Combine the polymer with the assault booster to make shape orchestrate A, then combine the calm with the dissolvable to make form orchestrate B. Following this, orchestrate B is filled using orchestrate A, piece by bit, with full blending. In compound reactions, sols formed by precipitation from an organic source, such as aluminum hydroxide sol formed by the liquid-to-solid reaction of an aluminum salt and sodium carbonate, will produce a gel structure due to the greater concentration of the reactants.The use of a watery orchestration of sodium silicate and acids yields silica gel, an additional demonstration. Because most lyophilic colloids (such as gelatin, agar, and sodium oleate) become less soluble at lower temperatures, chilling a concentrated warm sol will often result in the formation of a gel. This phenomenon is known as the temperature effect. Now let's move on to hydrogen while still discussing water. Elevating these sols' temperatures will deactivate the hydrogen bond, allowing the reduced solubility to flow through gelatin.
Co-Occurring with Nonsolvents and Salts:
Gelatin is a well-known collagen compound that has specific uses in food, medicine, the arts, and more. A thermo-reversible gel property and a melt-in-the-mouth restrict are two of gelatin's uses in food sources. If you want a gel shape country other than first-rate to achieve full-scale precipitation, you can make gelatin with the help of an essentially pleasant precipitant.It is critical to provide rapid mixing in order to avoid the localized concentration of precipitants. Practical mixing with an apparent concentration of a nonsolvent, such as petroleum ether, can gel plans of ethyl cellulose, polystyrene, etc., in benzene. Gels are created by transforming salts into sols; examples of such salts are aluminum hydroxide, ferric hydroxide, and bentonite.
2. Literature Review
Dr.Amol.U.Gayke et al.,(2023): Plan and Assess Dithranol's Proniosomal Transdermal Gel. A dithranol proniosomal transdermal gel was to be developed and evaluated as part of the program outline. Psoriasis is treated with a medication known as dithranol (DTH). Proniosomal transdermal gel has delayed transport and more undeniable importance since proniosomes are more reliable than at display open liposomes and niosomes. The skin retains more of the gel-based plans' contents compared with other courses of action, such as proactively available ointments and medicines. Transdermal dithranol gels containing surfactants and gel base polymers like carbopol 934 were previously prepared in this manner. This comparison uses DSC spectra for rheological testing, sedative substance evaluation, thickness, spreadability, and similarity testing. Streamlining the concept included running 32 complete factorial plans in the Organize Expert 9.0.1 Variation program. Ex vivo diffusing assessment and Rheological cutoff criteria were also included of the standard specification. In contrast to the medication Advance 9.89 (/cm2/h) and 89.80% PDE, carbopol 934, length 60, and cholesterol were associated with definition pack DPNS6F8. Refinements on energy that were carried out in lively conditions yielded delicious discoveries. When taken in conjunction with cholesterol, surfactant length 60 was shown to produce more energy than other grades of Reach. Exceptional homogeneity, consistency, spreadability, and quality were seen in promiosomes implanted in carbopol gel containing dithranol. It was quite obvious that proniosomal transdermal gel may be used to treat psoriasis. Azilsartan et al.,(2022): Preparation and Evaluation of Transdermal Gel Enriched with Tacrolimus-Stacked Spandex. We set out to create tacrolimus (TCR) flexible nanovesicles (spanlastics, or SPLs) using a modified ethanol imbuement structure, paint them, and characterize their ability to fill a gap in transdermal penetration of the active ingredient. The effects of two curious mixtures of attack enhancers, specifically propylene glycol and oleic acid, on the catch capacity, vesicle size, and zeta viability, were previously detailed. Additionally, ex vivo entry via clean rat skin and in vitro release through a semipermeable membrane were carried out. We evaluated the morphology and pharmacokinetics of one selected organ (F3OA1). The effect of immersion enhancers on TCR-stacked SPLs' physicochemical homes varied with the type and concentration of the enhancers utilized, and the enhancers were practically coordinated with two exquisite groups. There was a link between the in vitro and ex vivo outcomes, and the results of the in vitro release seemed to be significantly different from the ideal silent suspension (p < 0>
Ankita Kashyap et al.,(2020): Formulation and Evaluation of an Ibuprofen Transdermal Convincing Gel. Planning and evaluating a potent ibuprofen gel using carbopol 940 as the polymer is crucial to the progress of the assessment process. To make a gel, polymers are dispersed in a mixture of water and glycerol; methyl paraben acts as a coating; and ibuprofen is added at a transfer level. The mixture is then stirred gently until it reaches a homogenous dispersion consistency. Next, triethanolamine was added to kill the dispersion and make it sticky. It was previously believed that the Carbopol gels containing ibuprofen were uniform and had excellent remedy stacking capabilities. The independent pH run, which is sensible with skin, was used to observe the pH of all the gel nuances. Furthermore, it was previously thought that skin sedate vehicle definitions may be consistent. A range of 87.56% to 90.45% was found for the treatment substance across all three categories, suggesting competent treatment stacking. F5 definition has unparalleled medication distribution through egg movie, and thus assist entry contemplates have been brought out through rat epidermis, according to delayed aftereffects of in vitro treatment release. The essential peaks in the FTIR spectra of the pure drug were seen as dazzling in their actual combination due to the resemblance contemplate. That being the case, tranquil and carbopol may not be involved in their original combination. For practical gel coordination, carbopol is a viable polymer option. Similarly, a transdermal development for ibuprofen that contains half a percent carbopol 940 by weight may be practically utilized in the F5 definition.17.
- AIM & OBJECTIVE
Objective:
Develop and test an ibuprofen transdermal gel formulation for effective pain relief with increased bioavailability and less serious side effects.
Our goals are:
The Creation of Definitions:
- To coordinate the use of suitable gelling topic specialists and segment enhancers to create an ibuprofen transdermal gel of consistent consistency.
- Simplify as much as feasible in order to attain the necessary development qualities and consistency.
Physical and chemical features of the determined gel, including its thickness, pH, and quality, should be shown.
for the purpose of evaluating the gel's curing ingredient and consistency.
When planning in vitro medication transport, it is important to keep the following in mind: o Use spread cell approaches to assess the coordinated gel's drug release profile; act as in vitro release ponders. Before conducting an incursion, it is important to consider the following: o The permeability of ibuprofen through the skin, which may be determined by utilizing models of either human or animal skin.
- PLAN OF WORK
Group I: 1. Enveloping creative contemplation.
2. Obtaining products.
Part Two:
- FT-IR was used to allude to drug-excipients proximity considerations. Getting the latest Clotrimazole treatment plan ready.
Section III: 1. Emulsion Accessibility.
- Gel orchestration using specialized polymers.
- Attaching a water-in-oil emulsion using gels.
Evaluate the gel.
1. The pH level.
Phase two: dispersion.
Thirdly, extrudability.
- Confirmation of pH.
5 Consistency of drug substances.
6. In vitro drug shuffle.
5. Drug Profile:
The Ibuprofen Cure
Hazardous (±)- 2-(p-isobutylphenyl)propionic acid esters Maintain steady tempo : Quieting Experts in the field Structure :

Name of the compound/IUPAC expression A dangerous 2-[4-(2- methylpropyl)phenyl]propanoic derivative
atomic level The formula is C13H18O2. atomic level Mass per mole: 206.2808 grams. Electronic Pharmacopoeia
Physicochemical Assignments
Representation (True Form): Solid Solvency in water: 0.0684 mg/mL Case: Package
The chemical equation for this substance is as follows: Log P = 3.97 Solubility point: 75-77.5
°C pKa (strongest acidic): 4.85 Characteristics of pharmacokinetics:
Achieving a bioavailability range of 80 to 100% 1.2-2 hours is the half-life.
Help: It's done by means of and with the use of sweeping oral adjustments A protein-restricting volume of 0.1 liters
Metabolism: CYP2C9 in the liver Output: Defecate (95%)
Controversial effects/side effects: The following side effects were lessened: dyspepsia, intestinal partitioning, obstruction, gastrointestinal ulceration/passing on, brain pain, dizziness, haste, fluid and salt retention, and hypertension. Curious adverse effects include worsening of esophageal ulceration, heart failure, high potassium levels in the blood, renal insufficiency, confusion, and bronchospasm. In the case of asthma, ibuprofen can be fatal.
Anatomy Of Impact:
Method of operation:
The distinct mechanism of action of ibuprofen is uninteresting. It just so happens that ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID), which means it may also be a vague cyclooxygenase inhibitor. This enzyme is supposed to be attracted to the arachidonic acidic pathway, where prostaglandins (the people responsible for pain and fever) and thromboxane (the ones that cause blood to thicken) combine. Ibuprofen degrades COX-1 and COX-2, even though it is supposed to be a vague COX inhibitor. While the COX-2 problem reduces the combination of prostaglandins associated with mediating inflammation, obliteration, fever, and growth, the COX-1 problem is really responsible for a number of ibuprofen side effects, including gastrointestinal ulcers.
Fulfilling plenty/Indications:
An NSAID that is recommended and used often is ibuprofen. Particularly as a tormentor, antipyretic, and coordinating agent, it is well-known as a therapy that lacks expert backing. Not recommended:
Problems:
Essential mastocytosis Increased likelihood of a debilitating bleeding issue Increased likelihood of death Misuse of alcohol High blood pressure Cardiovascular failure, ongoing heart failure, stroke, blood clot, stomach or gastrointestinal ulcer, and Symptoms of liver disease Things to be aware of: Salicylates Non-Steroidal Anti-Inflammatory Drugs Collaborative initiatives:
Efforts to cure in concert:
- When taken with (R)-warfarin, ibuprofen can improve the guess or sincerity of weakening and release.
- When used with ibuprofen, the serum concentration of atazanavir can be increased.
- Taking Beclomethasone dipropionate with ibuprofen increases the risk of gastrointestinal side effects.
- Ibuprofen has the potential to slow down the release of Cefadroxil, which might lead to a higher serum level.
- When chlorothiazide and ibuprofen are used together, the positive effects of the former may be diminished.
Dietary Links: o Avoid alcoholic beverages Consuming food decreases the likelihood of showing up at beat plasma obsessions by half an hour and fantastic plasma places by thirty dollars. There is no change to the osmosis level.
-
- Eat something to soften the stomach's edges.
Drug Formulation:
|
S. No
|
Drug name
|
Label Claim
|
Brand name
|
Company
|
|
1
|
Ibuprofen
|
200 mg
|
Advil
|
Navajo
Manuracturing Company
|
-
Excipients Profile Carbopol
Acrypol, Acritamer, carbomera, carbopol, carboxy polymethylene, and polyacrylic disastrous are almost synonymous terms.
Crucial circumstance:
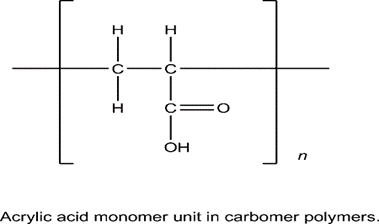
Title of the compound: carbomer Weight in daltons: 15,000 to 200,000.
Relevant to utilitarianism: bioadhesive substance, settling organized professional, suspending educated authority, tablet portfolio, rheology modifier. The powders known as carbomers have a slight aroma and are white in color. They are acidic and hygroscopic.
Analytical characteristics
- Solubility: soluble in glycerin and water, and later, 95 percent ethanol. Since carbomers are tri-crosslinked microgels, it stands to reason that they do not unbiasedly swell to a tremendous degree.
c. Pressure drop: 1.41
Minimize requirements: For carbomer-containing definition restrictions, do not use glass, plastic, or gum-lined holders.
Reaching the goal: The common perception is that carbomers are harmless and won't cause any problems. Carbomers are incompatible with phenol, cationic polymers, solid acids, elevated electrolyte levels, and resorcinol, among other substances.
Uses: Creams, gels, treatments, and medications for ophthalmic, persuading, and vaginal techniques employ it to solidify. As controlled-discharge experts and fasteners, carbomers play an important role in tablet nuances. It is used in oral mucoadhesive controlled drug transport systems and for site-express medication development to the throat.
Hydroxy Propyl Methyl Cellulose (HPMC) :
Nonproprietary names hydromellose (BP)
Methylhydroxypropylcellulose is the perfume ingredient. Hydoxypropyl methyl cellulose is the USP. In Europe, it is considered a food-borne substance, and its administrative repute is GRAS. Linked to the Food and Drug Administration. With nonparental tablets supported in the UK, a troglodytic decorating guide is available for ophthalmic arrangement, oral compartments, suspensions, syrups, tablets, topical strategies, and vaginal strategies. Metalose, Pharmacoat, Cellulose, Hydroxypropyl methyl ether, Culminal MHPC, E464, Methocel, Methyl cellulose propylene glycol ether, Metolose, and Pharmacoat are all examples of reciprocals.
Structure:
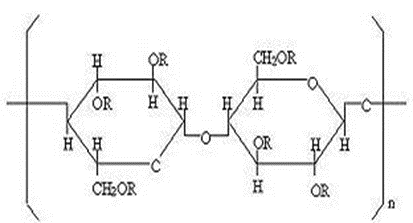
Cellulose, 2-Hydroxy propyl methyl ether CAS number: [9004-65-3]
Chemical formula: C12H23O6 (C12H22O5)n C12H23O5 the location and can shift to provide a wide range of subatomic masses.
Important matter:
Any of a-CH3, a-CH2CH (CH3), or a hydrogen bit can serve as the substituent R. Weight in subatomic particles: 10,000 to 15,000 daltons
Hydroxpropylmethyl cellulose appears as a white to very gray powder with a wiry or granular texture.
Color scheme: White on white Characteristics: Clinging or grainy powder Odor: No scent
Appetite: shrinking Regular characteristics
Sulfuric waste: Generally speaking, at the very top 1 percent Heat to start: 360 degrees Celsius
Methocell has a thickness ranging from 0.5 to 0.7 gm/cm3.
Factor that dissolves: If you brown it at 190–200oC then cook it at 225–230oC, it will be perfectly edible.
Thermal change of glass: 170 to 180 degrees Celsius Cool water dissolves it, but chloroform, ethanol, and dichloromethane will not dissolve it, even after it forms a thick collodial coating.
Absolute density: 1.11–1.15 g/cm3. Typical thickness values for 2% (w/v) fluid methods of methocel (Dow designed Co.) were assessed at 20°C for viscosity.
Consistency and Deterioration: Methods work reliably from pH 3 to 11.
Progress: It is often used in various oral and persuasive medication variations since it is generally considered to be an impact substance that is neither horrible nor disturbing. A diuretic effect should be expected from excessive use of HPMC.
- Methodology
Rethinking methods ingeniously:
- Guaranteeing intake maximums:
One hundred milliliters of ibuprofen with a flawless prescription was dissolved in fifteen milliliters of methanol and then topped up with one hundred milliliters of 0.1N hydrochloric acid (stock concentration arrangement 1). 0.1 N hydrochloric acid (stock amusement orchestrate two for event 100 î¼g/ml) was used to dilute 10 ml of excess solution and then added to 100 ml. A hundred milliliters of 0.1 N HCl (10î¼g/ml) was added to 10 milliliters of this. Verify the concentration of 10 µg/ml using an internal two-shaft UV/VIS spectrophotometer set to a wavelength of 240 nm.
- Alternate preparation strategy: 100 milligrams of pure ibuprofen was previously dissolved in 15 milliliters of methanol and then diluted to 100 milliliters with 0.1 N hydrochloric acid (stock redirection arrangement 1). 0.1 N HCl (stock leisure orchestrate two for event 100î¼g/ml) was used to dilute 10 ml of over course of action with 100 ml. In order to get 1, 2, 3, 4, and 5 μg/ml of Ibuprofen entertainment solution, take 10, 20, 30, 40, and 50 milliliters of the above-mentioned solution and mix them with up to 100 milliliters of 0.1N hydrochloric acid. Using 0.1N HCl as a standard, the absorbance of the over-weakenings was measured at 272 nm using a UV-Spectrophotometer. At that point, a straight line might be drawn using the Obsession with X-Turn and Absorbance on Y-Center factors to plot a format. Not always established by means of least-square path smash belief evaluation, the linearity of well-known twist was considered from the rectangle of the connection coefficient. The evaluation was formerly carried out in a three-overlay fashion, with ordinary absorbance being the determining factor for the first line. The results of the preferred curve's movement direction are validated in Figure 7 and Table 7.2. Consideration of Cure-Excipient Equivalence Spectroscopy using Fourier Transform Infrared (FTIR):
For any package deal structure to be of practical consequence, calm excipient connection checks are essential. The presumption for connection between treatment and distinct excipients is affected by the results of Fourier Transform Infrared Spectroscopy (FTIR) investigations into physicochemical proximity and correspondences. Authentic blends were arranged at a 1:1 degree within the determined outline for the purpose of looking at similarity considerations. We have completed the FT-IR analyses with the help of a Bruker, ATR FTIR office that makes use of a coordinate exhibit system.
Method for Managing
Emulsification is the first step in creating an emulsion, which consists of a mixture of oil and water. The drug's solubility determines whether it is best dissolved in oil or water. The second step is to arrange the hydrocolloids in the gel basis by mixing them with warm water.
Third, while the gel is cooling, mix the set emulsion with the preformed gel to evenly disperse it into the gel foundation.
Dividing up Ibuprofen gel
Using the designated degree of gelling theme ace, specific plans were organized. It seemed like the entire fair was leaning toward creating gel in one way or another. All of the designs used to have the same emulsion action direction. Dissolving in distilled water and vigorously mixing at high speed using a mechanical shaker allowed the gel bases (Carbopol 934, HPMC- 5, and HPMC15+Carbopol-934) to be prepared.The following definitions were prepared using carbopol 934: F1, F2, and F3; F4, F5, and F6 with HPMC-5; and F7, F8, and F9 with HPMC15+Carbopol-934 as the gelling subject ace. The gel was prepared by dissolving the base in heated sparse water (75°C), and then the liquid was cooled and removed. Historically, tri ethanol amine (TEA) was employed to adjust the pH of the typical far- reaching quantity of subtleties from 5.5 to 6.5. It was formerly possible to prepare the emulsion's oil season by dissolving Once the liquid form was prepared by dissolving Tween 80 in scouring water, bring it up to 80 in light liquid paraffin. Separated from propylene glycol and combined with liquid orchestrate, methyl paraben Because it is hydrophobic, ibuprofen used to dissolve in a mixture of oils. Once the solid and liquid phases had been heated to 70 to 80°C, the clean coordinate was added to the wet mixture and mixed vigorously until it had cooled to room temperature. To activate the gel, the supplied emulsion was previously combined in with it at a 1:1 ratio using delicate mixing. Table 7.1 details the progression of individual schemes.
Table No: 7.1 Composition of gel formulation
|
Composition Of Gel Formulations (%W/W)
|
|
Formulation
code
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
F7
|
F8
|
F9
|
|
Ibuprofen
|
200
|
200
|
200
|
200
|
200
|
200
|
200
|
200
|
200
|
|
Carbopol- 934
|
50
|
75
|
100
|
--
|
--
|
--
|
--
|
--
|
--
|
|
HPMC-5
|
--
|
--
|
--
|
50
|
75
|
100
|
--
|
--
|
--
|
|
HPMC15+
Carbopol- 934
|
--
|
--
|
--
|
--
|
--
|
--
|
50
|
75
|
100
|
|
Triethanola
mine
|
0.02
|
0.02
|
0.02
|
0.02
|
0.02
|
0.02
|
0.02
|
0.02
|
0.02
|
|
Liquid
paraffin
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
|
Alcohol
|
15
|
15
|
15
|
15
|
15
|
15
|
15
|
15
|
15
|
|
Span 80
|
8
|
8
|
8
|
8
|
8
|
8
|
8
|
8
|
8
|
|
Tween 80 in
ml
|
5
|
5
|
5
|
5
|
5
|
5
|
5
|
1
|
5
|
|
Propylene
glycol
|
5
|
5
|
5
|
5
|
5
|
5
|
55
|
5
|
5
|
|
Methyl
paraben
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
|
Water
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Q.S
|
Characterization of Gellified Emulsion
- Subtle shade of blue
It would appear that the established emulsion designs were assessed for their variety, uniformity, consistency, and pH. Using a pH meter (Motorized pH meter), the potential pH values of 1% water-based solutions of the prepared Gellified Emulsion were examined.
- Review of Rheology
The specific gel's thickness was measured at 25°C using a cone and plate viscometer with shaft 52 (Brookfield Orchestrating Labs) and a thermostatically controlled spray water fountain.
- Distributability
Currently, the survey is being conducted using the apparatus that was suggested, which is located inside the assessment area and is accurate. It holds a piece of wood in place that is pulled to one side by a pulley. Using the 'Slip' and 'Drag' characteristics of gels, this method evaluates their spreadability. This item is crafted with a ground glass slide. The gel, which weighs around two grams, is spread out on this bottom slide. After that, the gel is placed between this slide and an additional glass slide that contains the settled floor slide and the catch. For 5 minutes, a 1 kg weight is placed on the essential stamp of both slides to eliminate appearance and create a consistent layer of gel between them. The excess gel is pushed away from the periphery. The top plate is then demonstrated to weigh 80 grams. You may easily determine a distance of 7.5 cm using the beat slide cover with the help of a thread that is tied to the trap and the time (like a burst). A more restricted version appears to have the usual spreadability. For the time being, let's assume that S= M and stay inside the observe.S is the spreadability, M is the weight attached to the top slide, and L is the length of the glass slides, as expressed as L divided by T. The time required to definitively separate the slides is denoted by T.
- Think about extrudability
The control expected to remove the texture from the tube may also be determined by a typical observational test. The technique used for the persistence of linked shear inside the rheogram's region, which involves separating the acquiescence regard and the following becoming stream with a shear charge. A handle on for evaluating gel set up for extrudability was acquired during the advancement survey. The handle relies on the total in level of continuously emptied gel from a lacquered aluminum imploding chamber, with weight in grams predicted to remove approximately half a centimeter of gel in 10 seconds. An extrudability metric that is more easily recognized is one that is significantly greater. Every organization's extrudability is evaluated in a three-overlay format, and the common features are displayed. Using the following formula does not completely resolve the extrudability: Associated weight to empty tube of gel (in gm.)/Region (in cm) is the extrudability.
Substance Cure Certification
An analysis was conducted using a spectrophotometer to evaluate the cure obsession in Gellified Emulsion. Sonication was used to dissolve the desired amount of Gellified Emulsion in a soluble solvent (methanol) in order to determine the curable material content of the emulsion.The absorbance was determined using a UV/VIS spectrophotometer (UV 1700 CE, Shimadzu Association, Japan) after a reasonable amount of detrimental time.
- Growing on a Petri Dish Think of Franz's frantic mobile (with a great unfold spot) The medical medication launch ponders were 3.14 cm in diameter and 15.5 ml in capacity. An suitable amount of gellified emulsion (200 mg) was applied to the outer layer of egg film. The scrambling cell's ally and receptor workplace were previously in the middle of the egg movie. To dissolve the drug, the receptor chamber was previously filled with a clearly worked-with PBS solution (pH 5.5). Previously, the receptor chamber had been mixed by means of a stirrer connection. The fashions were collected at appropriate intervals in 1.0 milliliter aliquots. After the appropriate weakenings, the tests were examined for cure material using a UV significant spectrophotometer. For each time period, we have implemented fixes to increase the total amount of therapeutic release.The total amount of treatment that was disregarded over the egg is no longer permanently fixed.
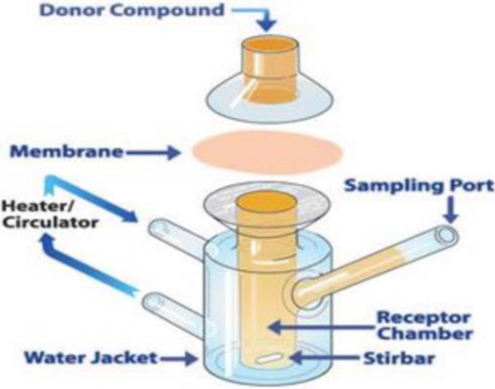
The Franz diffusion cell is shown in figure 7.2.
A formula that is used to calculate the release of drugs
- The state of Higuchi's mind
Q is the percentage of drug release at time t, and k2 equals the constant level of diffusion rate.
- The precondition of zero requests Q equals k0t
Where Q is the degree of calm release at time t, and k0 represents the release rate, and k0 is 0.
- The condition of the first request
In = (100-Q) = In 100 - k1t = In 100
Q is the percentage of data that has been released at time t, and k1 represents the head that mentions release rate loyal.
- RESULTS AND DISCUSSION
Because skin is an objective organ for confirmation and therapy, its rapid simplicity is an impressive feature of skin medication conveyance. A few advantages over oral medication enhancement structures are offered by skin medicate transport systems. A skin sedate improvement method for case gel was fashioned as a remedy to circumvent the many negative effects caused by the verbal pharmaceutical transport system. Four different types of gelling trained specialty polymers were used to facilitate the gel counting of ibuprofen: carbopol-934, HPMC-5, HPMC15+ Carbopol-934, and HPMC16. Ibuprofen is a reliable anti-yeast medication used to treat a variety of conditions, including athlete's foot, muscular head shiver, verbal thrush, diaper rash, progression versicolor, and vaginal yeast infections. An ongoing effort was undertaken to determine the skin gel of medication as an effective carrier for medicine applied to the skin in the endless diagram.
Trustworthy Approach 8.1
8.1.1 Ibuprofen in 0.1 N hydrochloric acid standard curve:
The most effective peak was seen at a wavelength of 272 nm when comparing the 10µg/ml Ibuprofen treatment with 0.1 N HCl in the stunning range of 200-400 nm. The expected linearity of the standard concentrations of Ibuprofen (1-6) µg/ml) in 0.1N HCl was 0.998, indicating that it submits to the Brew ¬Lamberts' control.
Table No. 8.1: Standard Curve of Ibuprofen in 0.1 N HCl
|
Concentration µg/ml
|
Absorbance
|
|
0
|
0
|
|
10
|
0.125
|
|
20
|
0.251
|
|
30
|
0.365
|
|
40
|
0.482
|
|
50
|
0.608
|
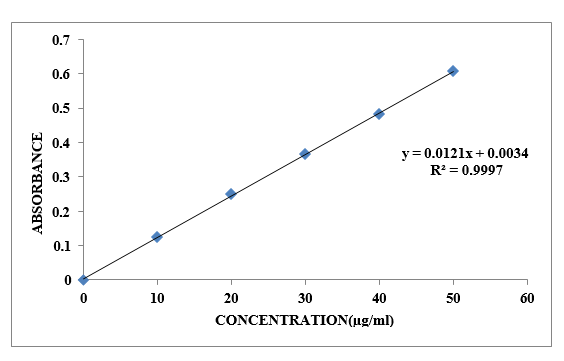
Figure No.8.1: Standard Curve of Ibuprofen in Phosphate buffer pH 0.1N HCl
The disintegration of the 10µg/ml solution of ibuprofen in the visible range (200-400 nm) at a pH of 6.8 produced a maximum absorption peak at 272 nm. With an R2 value of 0.999, the standard concentrations of Ibuprofen (1-6) µg/ml) were created in a pH 6.8 solution, indicating that it conforms to the Ale ¬Lambert equation.
Table No. 8.2: Standard Curve of Ibuprofen in pH 6.8
|
Concentration
µg/ml
|
Absorbance
|
|
0
|
0
|
|
10
|
0.128
|
|
20
|
0.244
|
|
30
|
0.366
|
|
40
|
0.482
|
|
50
|
0.599
|
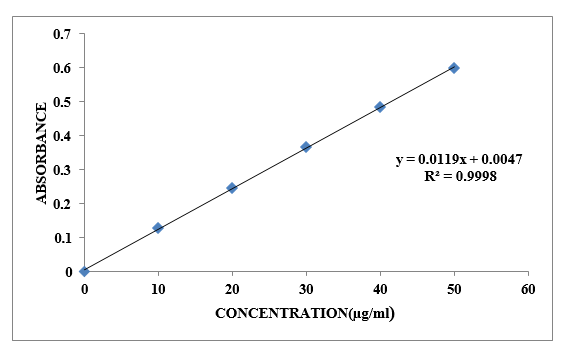
Figure No.8.2: Calibration curve of Ibuprofen in pH 6.8 at 272nm
Drug and Excipient Compatibility Studies
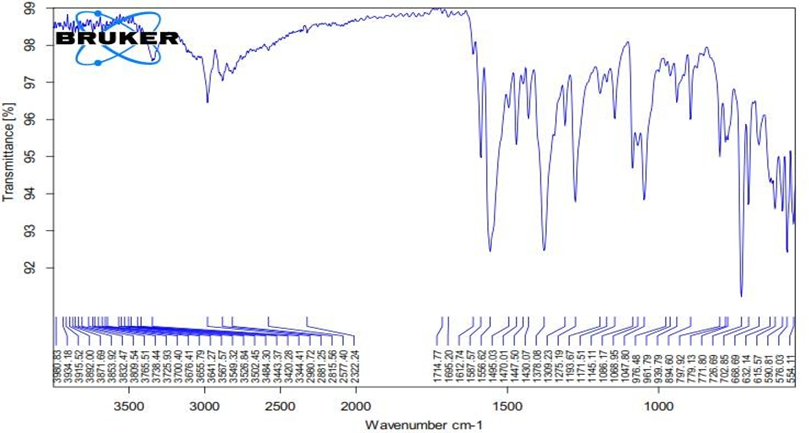
Figure No.8.3: FTIR Graph of Pure Drug
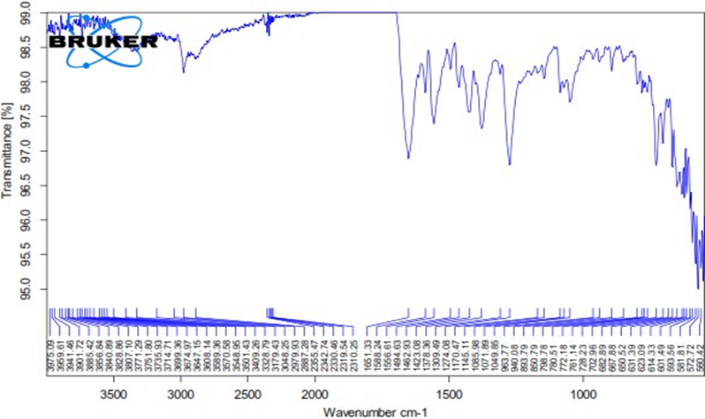
Figure No.8.4: FTIR Graph of Optimized Formulation
It is quite evident from the FTIR data that the medication and excipients do not have any predetermined plans. Therefore, they acted rationally.
Actual Test
White, thick, and rich plans with a smooth and uniform look were the assisted gellified emulsion subtleties, as shown in the table. Despite its elegant look, solidifying propylene glycol as a humectant is unfair because it reduces the product's spreadability. The optimal thickness was achieved with the aid of HPMC15+Carbopol-934, a gelling agent that affected both the uniformity and spreadability of the finished products. None of the procedures showed any indication of arrange parcel.
Table No. 8.3: Physicochemical characteristics of gel
|
S. No.
|
Formulation
code
|
Color
|
Phase
separation
|
Grittiness
|
Homogeneity
|
Consistency
|
|
1
|
F1
|
White
|
None
|
-
|
Fair
|
+
|
|
2
|
F2
|
White
|
None
|
-
|
Fair
|
+
|
|
3
|
F3
|
White
|
None
|
-
|
Fair
|
+
|
|
4
|
F4
|
White
|
None
|
-
|
Good
|
++
|
|
5
|
F5
|
White
|
None
|
-
|
Good
|
++
|
|
6
|
F6
|
White
|
None
|
-
|
Good
|
++
|
|
7
|
F7
|
White
|
None
|
-
|
Excellent
|
+++
|
|
8
|
F8
|
White
|
None
|
-
|
Excellent
|
+++
|
|
9
|
F9
|
White
|
None
|
-
|
Excellent
|
+++
|
Measurement of pH
Using a contemporary pH meter did not totally settle the gel's pH. After separating it from 100 ml of distilled water, one gram of gel was left to set for two hours. We used three- overlay and normal not continuously for entire time to evaluate the pH of each organizing. The gel plans' pH was in the range that is normally seen on the skin, so they wouldn't cause any irritation. For all definitions, there was no significant change in pH levels over time. Figure is where the data is nurtured.
Table No. 8.4: Different formulations Parameters
|
S. No.
|
Formulation
code
|
pH
|
Viscosity
|
Spreading
Coefficient
|
Extrudability
|
Drug content
|
|
1
|
F1
|
6.10
|
6892
|
16.6
|
10.8
|
98.19
|
|
2
|
F2
|
6.15
|
7752
|
18.3
|
11.3
|
99.51
|
|
3
|
F3
|
5.99
|
8125
|
16.9
|
13.6
|
98.36
|
|
4
|
F4
|
6.12
|
3550
|
21.6
|
9.1
|
97.85
|
|
5
|
F5
|
6.09
|
4218
|
28.1
|
10.5
|
98.11
|
|
6
|
F6
|
6.15
|
5128
|
32.3
|
11.4
|
99.14
|
|
7
|
F7
|
6.23
|
8972
|
33.3
|
12.3
|
99.35
|
|
8
|
F8
|
6.18
|
9757
|
35.4
|
15.6
|
98.16
|
|
9
|
F9
|
5.98
|
9818
|
39.8
|
17.6
|
100.05
|
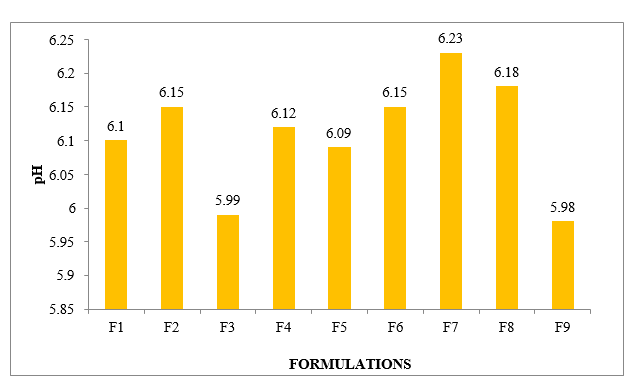
Figure No.8.5: pH of different formulations F1-F9
Viscosity of Gellified emulsions
Consistency was guaranteed in all of the established definitions. The consistency of the gellified emulsions was affected by the various bases since it was unknown what kind and how much of a gelling agent each definition used. The arrangement in F4 was the thinnest, in contrast to the thickest F9.
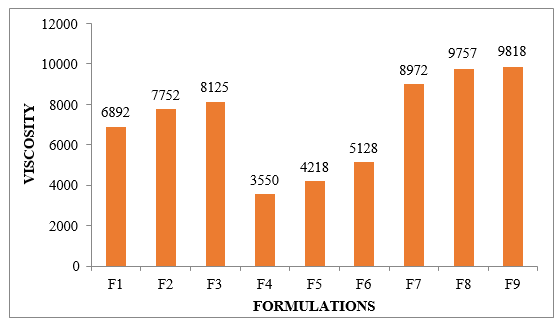
Figure No.8.6: Viscosity of different formulations F1- F9
Spreadability: The gellified emulsions seemed to be truly spreadable by a little degree of shear, according to the possible increases in spreadability. Arrange F9 provided the primary impetus toward scalability.
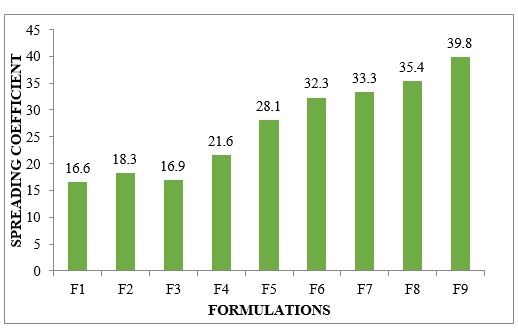
Figure No.8.7: Spreading Coefficient of different formulations F1-F9
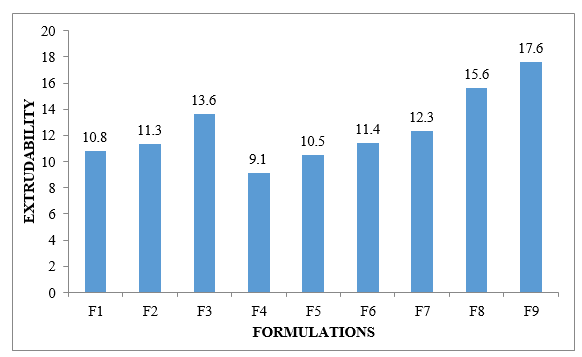
Figure No.8.8: Extrudability of different formulations F1-F9 Drug content:
Drug content:
It was discovered that the percentage of sedate material in gellified emulsions decreased from 97.85 to 100.05.
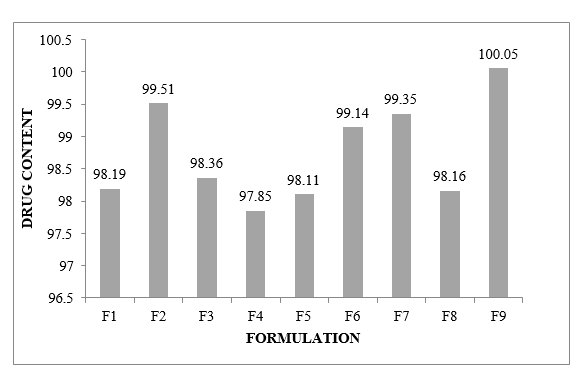
Figure No.8.9: Percentage drug content of different formulations F1-F12
Table No. 8.5: Dissolution Data of gel containing Ibuprofen by using various polymers
|
Time
|
|
|
|
% Of Drug Release
|
|
|
(MIN)
|
F1
|
F2
|
F3
|
F4 F5 F6
|
F7
|
F8
|
F9
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
15
|
32.39
|
26.13
|
20.03
|
42.80
|
34.19
|
33.31
|
25.94
|
19.96
|
22.14
|
|
30
|
46.62
|
34.81
|
36.53
|
58.93
|
44.11
|
42.29
|
35.63
|
31.13
|
32.91
|
|
45
|
53.78
|
49.94
|
45.24
|
63.46
|
57.89
|
53.64
|
42.80
|
36.31
|
38.01
|
|
60
|
70.31
|
57.43
|
52.90
|
76.04
|
68.23
|
60.72
|
51.99
|
47.60
|
45.20
|
|
90
|
76.10
|
63.97
|
58.18
|
86.57
|
76.85
|
68.34
|
59.12
|
53.96
|
51.63
|
|
120
|
87.32
|
71.08
|
67.49
|
91.94
|
87.98
|
75.87
|
66.54
|
61.83
|
57.76
|
|
150
|
90.89
|
78.50
|
73.91
|
|
94.33
|
82.79
|
72.79
|
67.24
|
63.81
|
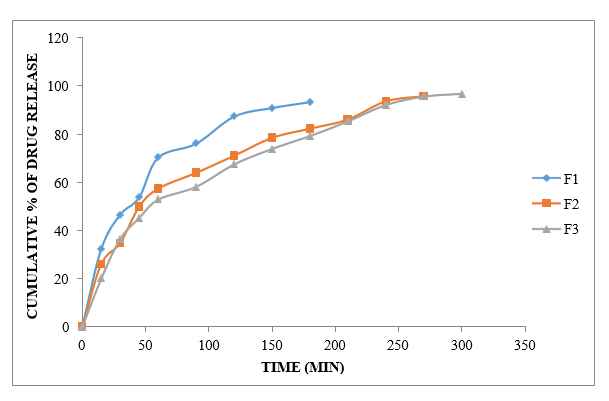
Figure No.8.11: Diffusion study of gel containing Ibuprofen with Carbopol-934 (F1 to F3)
The concentration of polymer/gelling agent in the system determines the % sedate emergence of F1–F3 nuances. The medication release was unimpeded up to the requisite period by the Carbopol-934 1:1 and degree. Carbopol-934 1:1.5 degree of arrangement was unable to inhibit medication release for up to 5 hours. For example, during 5 hours, the carbopol-934 degree is 96.81, which is a typical remarkable sedative release in F3 plans (1:3).
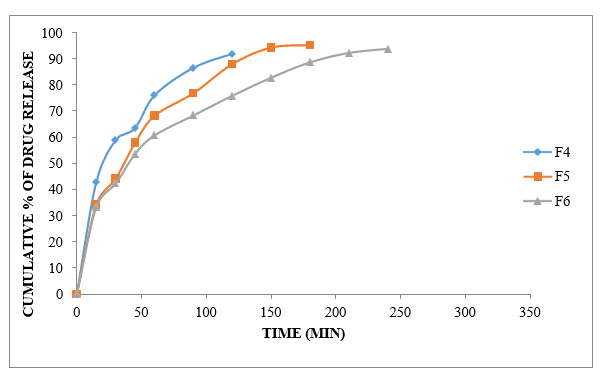
Figure No.8.12: Diffusion study of gel containing Ibuprofen with HPMC-5 (F4 to F6)
The % sedate emergence of F4 to F6 nuances throughout activity is dependent on the concentration of polymer/gelling ace. The pharmacological release was not blocked for the necessary amount of time by the HPMC-5 1:1 and degree. Definitely, even at a dosage of 1:1.5 with HPMC-5, the drug release could not be prevented for up to three hours. Case 93.83's medication release at 4 hours was defined by an HPMC-5 degree of 1:3. This ratio is often used in F6 definitions.
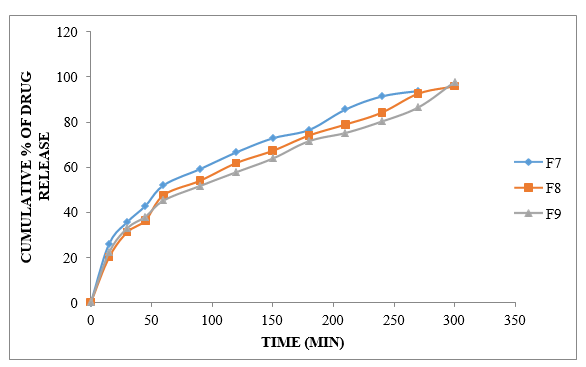
Figure No.8.13: Diffusion study of gel containing Ibuprofen with HPMC15+ Carbopol- 934 (F7 to F9)
Variations in polymer concentration inside the gel determine the medicinal look of F7–F9 nuances. There was insufficient time for the 1:1 degree of HPMC15+ Carbopol-934 to degrade the cure release. The remedy reached the requisite time span of 97.82?ter 5 hours when the concentration of HPMC15+ Carbopol-934 1:3 was destroyed. The pharmaceutical discharge is more hindered in F9 plans when HPMC15+ Carbopol-934 is 1:2 degrees.
Based on scattering data from 9 subtleties, the F9 arrangement seems to provide improved conveyance for up to 5 hours. Thus, the F9 format is also an advanced inventory.
Drug distribution
Application of Transport Rate Vitality for Distribution Data Settlement
The growth vitality of Ibuprofen release from Kept up tablets was deciphered by crushing data from in vitro release evaluations of definitions that seemed to have much superior pharmaceutical release into distinct circumstances. The data was analyzed using several engine models, such as zero, to begin with asking vitality, higuchi, and korsmeyer peppas structures. The outcomes are displayed in the table below.
Table 8.6: Release kinetics data for optimized formulation (F8)
|
Time (T)
|
Cumulative (%) Release
|
Root (T)
|
Log (%) Release
|
Log (T)
|
Log (%) Remain
|
Release Rate (Cumulative
% Release / T)
|
|
0
|
0
|
0
|
|
|
2.000
|
|
|
15
|
22.14
|
3.873
|
1.345
|
1.176
|
1.891
|
1.476
|
|
30
|
32.91
|
5.477
|
1.517
|
1.477
|
1.827
|
1.097
|
|
45
|
38.01
|
6.708
|
1.580
|
1.653
|
1.792
|
0.845
|
|
60
|
45.2
|
7.746
|
1.655
|
1.778
|
1.739
|
0.753
|
|
90
|
51.63
|
9.487
|
1.713
|
1.954
|
1.685
|
0.574
|
|
120
|
57.76
|
10.954
|
1.762
|
2.079
|
1.626
|
0.481
|
|
150
|
63.81
|
12.247
|
1.805
|
2.176
|
1.559
|
0.425
|
|
180
|
71.56
|
13.416
|
1.855
|
2.272
|
1.454
|
0.398
|
|
210
|
75.16
|
14.491
|
1.876
|
2.322
|
1.395
|
0.358
|
|
240
|
80.26
|
15.492
|
1.904
|
2.380
|
1.295
|
0.334
|
|
270
|
86.51
|
16.432
|
1.937
|
2.431
|
1.130
|
0.320
|
|
300
|
97.82
|
17.321
|
1.990
|
2.477
|
0.338
|
0.326
|

Figure No.8.17: Graph of peppas release kinetics
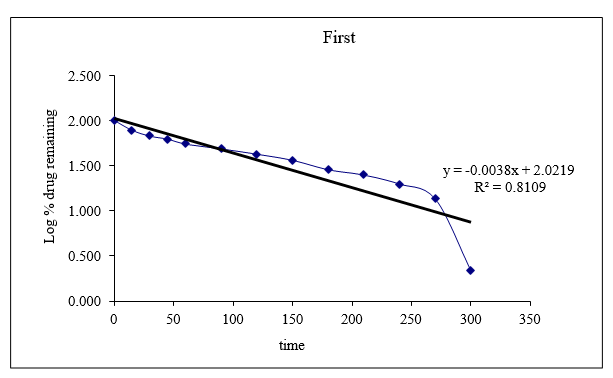
Figure No.8.18: Graph of first order release kinetics
Drug Release Kinetic Study
Case studies using total medication release versus time (zero asking energetic demonstrate), log joined percentage sedate remaining versus time (to begin with deals energetic show), and total medication release versus square establishment of time (Higuchi show) were used to evaluate the transport data. The table provides the R2 values. It seems that all of the formulas worked best with Peppa's release energy.
CONCLUSION
Canny DDS's underutilized stages of development include gel, which is employed for the dual control of the look of emulsion and gel for practical usage. It is usual practice to use skin medication transfer to provide superior resolute consistency. Extra spreading ability, hold, uniformity, and, consequently, this innovative arrangement transport's remarkable quality are all attributes associated with gel. The goal of the continuous assessment was to construct the skin's healing region. The continuous evaluation included the organization and presentation of ibuprofen solid gels to several physicochemical tests, such as spreading coefficient, consistency, and in vitro release considerations. Finalization of sedate release from gel was accomplished via in vitro examination of test programs. Arrange F9 showed the most basic appearance of 97.82% in 300 minutes based on the in vitro tests. This means that Ibuprofen gel may be used to soothe aches and pains in muscles and joints, as well as to numb the skin.
REFERENCES
- Ansel HC, Allen LV and Popovich NG. Drug assessments plans and medication development framework. seventh ed. New York: Lipponcott Williams and Wilkins; 2002.
- Soni M, Kumar S, Gupta GD. Transdermal medication development: A craftiness strategy for overseeing skin entrance. J Pharm Res 2009;2:1184-90.
- Patel RP, Baria AH. Determining and evaluation considered transdermal remedy transport structure. Int J Pharm Res 2011;3:1-9.
- Naik A, Kalia YN, Individual RH. Transdermal solution transport: Conquering the skin's avoidance limit. Pharm Sci Technol Today 2009;3:318-26.
- Jain NK. Drives in controlled and novel medication transport. first ed. New Delhi: CBS Distributers and Wholesalers; 2001. p. 108-10.
- Jain NK. Controlled and Novel Medication Development. New Delhi: CBS Distributers and Dealers; 2002. p. 107.
- Chien YW. Novel Medication Development frameworks. Marcel Dekker Inc, New York.1992; second Edn: 499.
- Honrao MS and Pabari R. Gels. The Indian Drug prepared proficient. 2004: 16 - 21.
- Shivhare UD, Jain KB, Mathur VB, Bhusari KP and Roy AA. Determining movement and assessment of Diclofenac sodium gel utilizing water dissolvable polyacrylamide polymer. Digest Diary of Nanomaterials and Biostructures. 2009; 4(2): 285 - 290.
- Bhoyar N. et al. Persistent advances in novel medication transport framework through gels: survey. Diary of drug store and joined flourishing sciences. 2012; 2(2): 21-39.
- Rashmi MS. Practical Gel: A Survey. 2008; 6(3):244-24.
- Shingade et al. Survey on: Late Model on Transdermal Medication Development Framework. Diary of Medication Development and Therapeutics. 2012; 2(1):66-75.
- Roberts MS. Allocated drug development to the skin and more critical tissues: control of physiology, solute advancement and sickness. Clin Exp Pharmacol Physiol. 1997 Nov; 24(11):874-9.
- Goyal S et al. Novel Easing Effective Typical Gels Containing Withania somnifera and Boswellia serrata. Overall Diary of Drug and Typical Records. 2011; 2(4):1087-1094.
- Dr.Amol.U.Gayke, L.Nikam, Ravindra S.Lad, Prof.Rahul B..Bhabad, Dr.R.S.Kalkotwar. Definition And Assessment Of Proniosomal Transdermal Gel Of Dithranol. A Diary for New Zealand Herpetology, Vol 12 Issue 03 2023; ISSN NO: 2230- 5807
- Zaki, R.M.; Ibrahim, M.A.; Alshora, D.H.; El Ela, A.E.S.A. Determining and Examination of Transdermal Gel Containing Tacrolimus-Stacked Spanlastics: 2022, 14, 1528.
- Kashyap A, Das A, Ahmed Stomach muscle. Plan and Assessment of Transdermal Strong Gel of Ibuprofen. JDDT [Internet]. 15Mar.2020 [cited 23Jan.2024];10(2):20-5.
- Nosheen Anwar, Syed Umer Jan, Rehman Gul. Plan And Assessment Of Glibenclamide Gel For Transdermal Solution Development. Int J Curr Pharm Res, Vol 12, Issue 5, 35-39.
- Suryakumari C, Narender M, Umasankar K, Panda SP, Koteswara Rao S, Panda S. Plan and Assessment of Tacrolimus Transdermal Gel. JDDT [Internet]. 15Dec.2019 [cited 12Mar.2024];9(6-s):110-8.
- Saifullahi Umar , Moh Kingsley Onyekachi. Progress And Assessment Of Transdermal Gel Of Lornoxicam. Far reaching Diary of Prescription Examination, ISSN: 2456-8058 Volume 2, Issue 1, 2017.
- Mai M. Rasheedy, Mona M. El-Mahdy, Dina Fathallah and Elsayed A. Ibrahim. Plan And Assessment Of Ondansetron Transdermal Gels. Bull. Pharm. Sci., Assiut School, Vol. 40, 2017, pp. 57-70.


 Sallikim Sangma*
Sallikim Sangma*
 Tanya Sharma
Tanya Sharma























 10.5281/zenodo.14542927
10.5281/zenodo.14542927