Abstract
A novel strategy and more current NDDS technology for topical drug delivery is called Emulgel, and it combines the benefits of both gel and emulsion-controlled release. Emulsion for the treatment of acne, fungal infection and muscle soreness. Emulgel is the term for the combination of emulsion and gel. Emulgel is a clear gel that is used in cosmetic and pharmaceutical products. The issue with gel and emulsion is resolved by emulgel. In contrast to ointments, creams, lotions, and other formulations, gels release drugs more quickly. Gel's limitations when it comes to applying hydrophobic drugs via the skin. Overcoming the limitations of the emulsion-based technique allows even a hydrophobic medicinal moiety to display the special qualities of gels. Various polymers are used to generate emulgel, acting as thickening and emulsifying agents simultaneously. These polymers' gelling capacity produces stable emulsions by reducing surface tension and interfacial tension and simultaneously raising the aqueous phase's viscosity. When taking into account a number of factors, emulgel have significant benefits over both traditional and innovative vesicular systems. The emulgel has a number of advantageous qualities for use in dermatology, including being greaseless, thixotropic, spreadable, emollient, easily removable, water soluble, non-staining, having a prolonged shelf life, clear, and having an attractive appearance
Keywords
Penetration enhancers, Emulgel, Antifungal, Drug Delivery, Nanoparticles, Natural Antifungal Agents.
Introduction
Globally, the prevalence of superficial fungal infections of the skin, hair, and nails has increased. An estimated 40 million individuals in poor and underdeveloped countries have had fungal infections. Due to immune system compromise, fungal infections can spread quickly and become dangerous. Onychomycosis and tinea are frequently caused by dermatophytes. Among the most common fungal infections of the superficial skin are those caused by Candida. When the immune system is compromised, candida can even penetrate deeper tissues and the circulation, resulting in potentially fatal systemic candidiasis.
Several advantages come with topical therapy of fungal infections, such as the ability to target the infection site, lower the chance of systemic side effects, increase treatment efficacy, and promote high patient compliance. Numerous skin infections related to dermatology have been treated with various topical effective antifungal agents. Azole compounds, polyenes, and allylamine/benzylamines are the three primary classes of topical antifungals. Another antifungal substance used topically is ciclopirox. At the moment, traditional dose forms such creams, gels, lotions, and sprays are commercially accessible for these antifungal medications (1).
Mycology
Fungi are amazing creatures that belong to their own kingdom in classification. Fungi are classified as eukaryotes because their cells are larger than those of bacteria, they have a membrane encircling their nucleus, and their molecular processes are quite similar to those of plants and animals. But in contrast to the cells found in mammals, fungi usually invariably have a hard cell wall around their plasma membrane that is made of chitin products. Because fungi do not produce chlorophyll, they are vegetative organisms and cannot be categorized as plants. This non-motile organism's basic structural unit is made up of either a single cell, a chain of cylindrical cells called hyphae, or both. On Earth, common species such as Aspergillus and Candida can be found everywhere. It has been determined that fungi can cause life-threatening illnesses in gardens, playgrounds, homes, hotels, hospitals, and even on the skin and mucous membranes (2).
Type of fungal infection
Superficial fungal infections
Superficial fungal infections and mycoses are frequent, curable illnesses that can present differently in patients with immunosuppression. The primary diseases include infections caused by Malassezia, including pityriasis versicolor, dermatophyte or ringworm infections, and superficial candidiasis of the mouth, skin, or genital tract. Despite the fact that they exhibit typical clinical changes, direct microscopy or the culture of appropriate samples usually improve diagnosis. Depending on the location and extent of the infection, treatment mostly consists of brief courses of topical application or prolonged oral administration of azole (imidazole/triazole) or allylamine antifungals (3).
Dermatophyte Infection
A disorder known as dermatophytosis, or more popularly, tinea, is caused by fungus known as dermatophytes invading keratinized structures in people and animals. There are three primary genera of these fungus. T. rubrum is the most common species in the world, along with Trichophyton, Microsporum, and Epidermophyton. We still don't fully understand these fungi's pathogenicity processes. Studies on the keratinolytic proteases (keratinases) that dermatophytes produce have been conducted, but it is unclear how these fungi control how these proteases are used to get nutrients from the stratum corneum substrate that they invade, or if these proteins have any other functions related to adhesion and immunomodulation (4).
Subcutaneous Mycoses
There are many fungi in the environment that are not very infectious, and these fungi have been linked to subcutaneous mycoses. Traumatic implantation allows these organisms to penetrate into the subcutaneous tissues.
Systemic Fungal Infection
About 90% of HIV patients experience both infectious and non-infectious skin illnesses, and as the immune system deteriorates, the frequency and severity of these conditions rise. The introduction of highly effective antiretroviral therapy completely changed the prognosis of mycoses and other diseases associated with AIDS, allowing patients to live longer and have an acceptable quality of life. Cutaneous manifestations are thought to be good clinical predictors for the immunological condition of the patient with AIDS. The systemic mycoses that are more common in the seropositive population—that is, patients with HIV/AIDS—are described in this article. These include aspergillosis, sporotrichosis, histoplasmosis, coccidioidomycosis, blastomycosis, and para coccidioidomycosis (5).
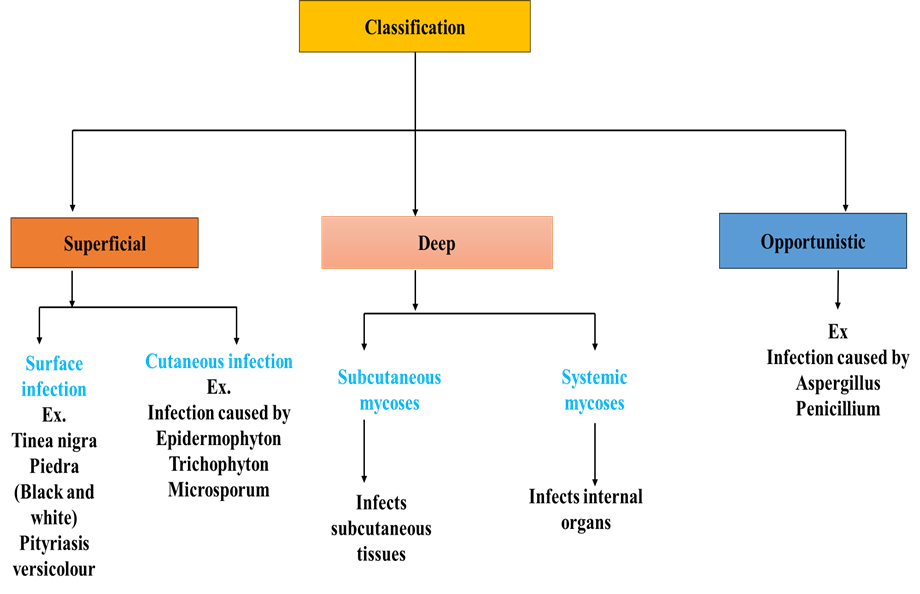
Figure 1: Classification of fungal infection
Etiology of fungal infection
There are many different kinds of fungi that can cause fungal diseases, or mycoses. Organic stuff that has decomposed and soil are among the environments that contain these microbes. Some fungus can infect people and other animals, even though the majority are benign. An understanding of the elements that contribute to the development and transmission of fungal infections is necessary for the research of their etiology. Key information regarding the etiology of fungal infections is provided here(6).
Fungal Pathogens
Certain infections can be caused by distinct fungal species. Candida species, which cause candidiasis, Aspergillus species, which cause aspergillosis, Trichophyton species, which cause ringworm or dermatophytosis, Cryptococcus neoformans, which cause cryptococcosis, and Histoplasma capsulatum, which cause histoplasmosis, are a few prevalent fungal pathogens (7).
Opportunistic Infections
Infections can result from fungi that are often benign or only occasionally present in healthy individuals when they decrease an individual's immunity. We refer to these infections as opportunistic. Aspergillus and Candida infections in immunocompromised people are two examples (8).
Transmission
Fungal infections can spread in a number of ways, such as by the inhalation of fungal spores, direct contact with animals or people who are afflicted, eating contaminated food, and exposure to polluted settings. During birthing, some fungus can also be passed from mother to child.
Risk Factors: Fungal infections are more likely to occur when certain conditions are met. These include long-term antibiotic usage, corticosteroid therapy, diabetes, cancer, immunosuppression (e.g., HIV/AIDS, organ donation), malnourishment, and specific genetic disorders.
Environmental Factors: Depending on the environment and geographic region, different fungal diseases may be more or less common. For instance, certain fungal infections are more prevalent in areas with a specific climate or kind of soil.
Nosocomial Infections: Nosocomial infections, or fungal infections contracted in medical environments, can be quite problematic, particularly in hospitals where there are immunocompromised patients.
Prevention: Preventive measures for fungal infections include maintaining good hygiene, avoiding contact with contaminated sources, using antifungal medications in high-risk patients, and implementing infection control practices in healthcare settings (9).
Pathogenicity and virulence:
There are essentially two sorts of fungi: opportunistic fungi and truly pathogenic fungi, depending on their virulence and pathogenicity. The fungi's capacity to develop at 37 °C and adjust to the conditions inside the host tissues aids in their establishment and dissemination of infection. The fungus-specific factors that cause fungal pathogenicity are unique. The virulence and pathogenicity of different fungi are significantly influenced by their byproducts, poisons, and enzymes. Proteases and aspartic acid proteinase are produced by Aspergillus species. The toxins that Aspergillus species generate are gliotoxin and restriction. As part of the normal human flora, Candida spp. are opportunistic fungi that can cause serious diseases ranging from urinary tract infections and candidemia to superficial skin and nail infections.(7)
Mode of transmission
The precise route of transmission for a given fungal infection varies depending on the type of fungal infection. The following are some typical routes by which fungal diseases can spread (10)
Direct Contact: Direct contact with an infected person or animal can result in the transmission of fungal infections. For instance, skin-to-skin contact with an infected person or coming into contact with contaminated surfaces can spread skin fungal illnesses like ringworm, which is brought on by dermatophytes.
Indirect Contact: When a non-infected individual comes into contact with contaminated surfaces or things, certain fungal spores can survive on them and spread. When it comes to fungi that cause illnesses like athlete's foot or nail infections, this form of transmission is especially important.(11)
Airborne Transmission: Respiratory infections can result from breathing in some fungal spores that become airborne. For example, Aspergillus spores are frequently present in the environment and can infect those who are vulnerable, especially those with weakened immune systems.(12)
Ingestion: Fungal infections in the gastrointestinal tract can result from consuming contaminated food, such as some meals tainted with mold.
Vector-Borne Transmission: Certain fungi can propagate with the use of insects or animals as vectors. For example, scrapes or bites from animals can transmit the fungus that causes "sporotrichosis".(13)
Nosocomial Transmission: Fungal infections can spread from one patient to another in healthcare settings, particularly when infection control procedures are not properly followed.(14)
Soil Contact: When people or animals come into touch with contaminated soil, some fungi that live in the soil can spread to them. For instance, after disturbing contaminated soil, the spores of the fungus Blastomyces and Histoplasma can be inhaled and induce diseases.
Vertical Transmission: Rarely, a mother's fungal infections may pass from her to her unborn child during pregnancy or childbirth.
Sign and Symptom of superficial fungal infection:
Common fungal infections affecting the skin, hair, and nails are called superficial fungal infections, sometimes referred to as ringworm infections or dermatophytosis. They are brought on by dermatophytes, which are different species of fungi. The symptoms and indicators of superficial fungal infections are as follows.(15)
Red, Circular Rash: A red, circular skin rash is one of the telltale symptoms of superficial fungal infections. The rash frequently resembles a ring due to its elevated margins and distinct center. Because of this, the ailment is frequently called "ringworm," despite the fact that worms are not the cause.
Itching: Usually, the afflicted area is highly painful and irritating. Itching can range in severity from moderate to excruciating.
Scaling and Peeling: It's possible for the skin inside the ring to get dry, scaly, and peel. The rings outside edges may be elevated and more noticeable.
Blisters and Pustules: The rash may occasionally turn into tiny pustules or blisters that are filled with pus or liquid.
Hair Loss (in scalp infections): Affected scalps can cause brittle hair that breaks easily or falls out, which can result in hair loss.
Nail Changes (in nail infections): Onychomycosis, a superficial fungal nail infection, can alter the look of the nails. The nails could get brittle, discolored, and thickened. In extreme circumstances, the nails could break off or disintegrate from the nail bed.
Location: Depending on the kind of fungus present, the infection's site may change. The scalp, feet (athlete's foot), groin (jock itch), and body are frequently impacted.
Spread: Direct contact or contact with contaminated surfaces, such as shared towels or clothing, can result in the infectious spread of superficial fungal infections.
Diagnosis of fungal infections
Fungal infections are difficult to diagnose, and many infections are only discovered after death. Furthermore, the process of isolating fungi from clinical samples is unreliable and can be exacerbated by the existence of fungi that are prevalent in the environment or colonizing commensal organisms, which can lead to false-positive results. Due to the immunocompromised nature of many patients with systemic fungal infections, serological assays that look for antibodies have low sensitivity and specificity. Increased use of routine high resolution computed tomography (CT) scanning, PCR for the detection of RNA or DNA, and ELISA testing for the presence of circulating galactomannan, a component of the fungus cell wall in Aspergillus, are among the diagnostic techniques for aspergillosis.(15, 16)
Treatment And Management of Fungal Infections
The kind, severity, and state of health of the patient all influence how fungus infections are treated and managed. A fungus can infect the skin, nails, mouth, throat, and genital areas, among other parts of the body. I'll give a basic rundown of the available choices for managing and treating common fungal infections here.(17)
Antifungal Medications: The majority of fungal infections are treated primarily with antifungal medications. They are available in a number of forms, including as intravenous formulations, oral tablets, ointments, and topical creams. The kind and location of the infection determine which antifungal drug is best. Clotrimazole, Miconazole, Econazole, Ketoconazole, Amphotericin B, Terbinafine, Itraconazole, and Nystatin are a few antifungal medications that are frequently used. To guarantee that the infection is totally eradicated and to stop the emergence of treatment resistance, it is imperative that you adhere to the recommended dosage and time.
Topical Treatment: For fungal infections of the skin, nails, or mucous membranes, topical antifungal creams, lotions, or powders are often used. These are applied directly to the affected area and can be effective for mild to moderate infections.
Oral Antifungal Medications: Oral antifungal medications may be required for fungal infections that are more severe or extensive. When topical therapies are insufficient or for systemic infections, they are frequently recommended.
Nail Infections: Onychomycosis, a fungal infection of the nails, can be difficult to cure. Long treatment regimens may occasionally be necessary as part of the treatment, which may include both oral and topical antifungal medicine.
Intravenous Antifungals: Antifungal drugs may need to be injected intravenously in a hospital environment for severe systemic fungal infections.
Proper Hygiene: Maintaining proper hygiene is crucial to stopping the spread and recurrence of fungal infections. A clean, dry area should be maintained, and exchanging personal belongings like socks, shoes, or towels should be avoided.
Avoiding Irritants: Steering clear of any allergies or irritants might hasten the healing process for skin problems.
Managing Underlying Conditions: Fungal infections can be more common in people with certain medical problems, such as diabetes, HIV/AIDS, or immune system diseases. Recurrent infections can be avoided by properly managing these underlying disorders.
Lifestyle Changes: Changes in lifestyle may be advised in certain situations. For example, people who get recurring vaginal yeast infections might be recommended to wear loose clothing or modify their diet to consume less sugar.
Mechanism of antifungal drugs:
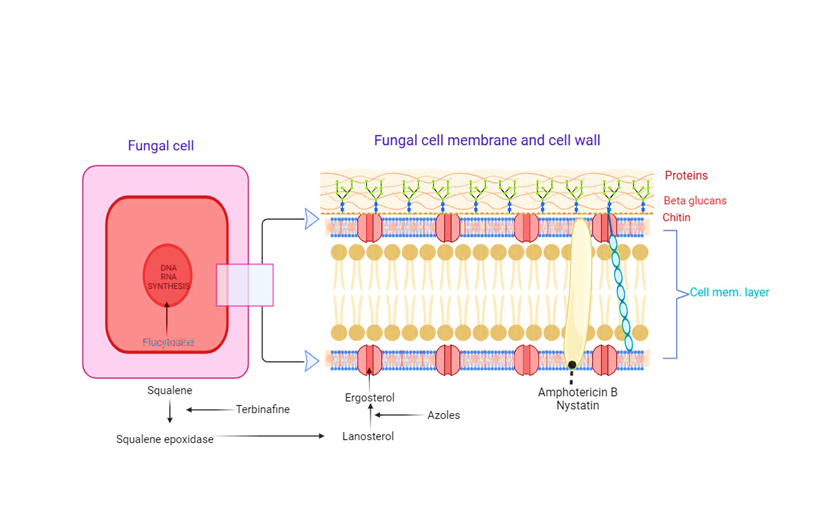
Figure 2: Mechanism of antifungal drug
Skin
The skin, which is the body's outermost covering, serves as a barrier to keep the outside world out. Numerous epithelial and connective tissues that serve as the skin's protective layer make up the skin. The skin, which weighs between 4.5 and 5 kg and measures around 1.7 square meters, is the largest organ in the body and the first to enter after birth. On the eyelids, where it is thickest, it is 0.5–4 mm. The skin of the face is the thinnest and thickest; its typical thickness is between one and two millimetres.(18)

Figure 3: Layer of skin
A. Anatomy of skin
human skin is mainly divided into three layers:
a. Epidermal layer
b. Dermal layer
c. Hypodermal layer
Epidermal layer
The epidermal layer, which is the outermost layer of skin, serves as the body's physical and biological barrier to the outside world. It also keeps the body's internal equilibrium stable, inhibits the loss of water, and blocks the entry of allergens and irritants. The primary constituents of the epidermis, keratinocytes, which make up the stratified squamous epithelium, create the keratin protein. Tyrosine, an amino acid, is bundled into cellular vesicles called melanosomes and transferred into the cytoplasm of keratinocytes, where it is converted into the pigment melanin.(19)
The layer of epidermis are:
- Stratum Germinativum (Growing layer)
- Malpighion Layer (pigment Layer)
- Stratum Spinosum (prickly cell layer)
- Stratum Granulosum (Granular Layer)
- Stratum Lucidum
- Stratum Corneum (Horny layer)
Dermis
The non-descript area between the subcutaneous fatty region and the epidermis is called the dermis. It is mostly made up of a dense network of structural protein fibers, such as collagen, reticulum, and elastin, encased in a semi-gel matrix of pulverized material mucopolysaccharides. The network or gel structure of the cells is what gives the skin its elasticity. The fibrous tissue spreads out and combines with the subcutaneous fat-containing tissue beneath the dermis. One essential component of cutaneous metabolism is protein synthesis.
Subcutaneous Tissue (hypodermis)
The superficial fascia, a film of fat-rich areolar tissue that connects the dermis to the underlying anatomy, makes up this layer. Only the surface area has large arteries and veins.
Physiology of Skin
With its 15% of the total weight of an adult, the skin is the biggest organ in the body. The mucosal lining of the urogenital, digestive, and respiratory tracts joins forces with it to create a capsule that divides the internal body structure from the surrounding environment. A normal square for a 70 kg human with 1.8 m2 of skin covers 10 hair follicles, 12 nerves, and 15 sebaceous glands. There are three 92-centimeter-long blood arteries and 100 sweat glands. Sweat and fatty acid released by sebum have an impact on the pH of the skin's surface, which ranges from 4 to 5.6. Skin temperature ranges from 30 to 40 degrees based on the surrounding environment. It carries out a multitude of essential tasks, including as preventing the body from losing too much water and aiding in thermoregulation, in addition to providing defence against external physical, chemical, and biological threats. The mucous membrane that lines the surface of the body is continuous with the skin.(20)
Permeation through the Skin
Trans -epidermal absorption
The trans-epidermal route involves two points of entry: the intercellular spaces, which facilitate the passive transport of small molecules, the active transport of polar and ionic compounds, as well as the endocytosis and transcytosis of macromolecules, and the intracellular spaces, which facilitate the transport of molecules within or between cells. There are tight connections or other comparable conditions between the cells. The major pathway for the permeate (0/w log k larger than 2) to flow through stratum carenum by both routes is determined by the partition coefficient (log k). Nonetheless, it is generally accepted that the principal route and the main obstacle to the penetration of the majority of medications is the indirect intercellular channel.
Trans- follicular absorption
The appendageal route includes passage through sebaceous glands that are connected to hair follicles and sweat glands. These paths are referred to as "shunt" routes because they avoid passing through the stratum corneum. Given that it only makes up 0.1% of the skin's surface area, this pathway is regarded as being of minimal value. Furthermore, the skin's appendages have an impact on percutaneous absorption because of their secretions, which change the stratum corneum's lipid and water content and, in turn, alter how molecules are absorbed.
Topical Delivery of Antifungal Drugs:
The main reason for a great deal of morbidity and hospital admissions is skin infection. The human skin is fortunately equipped with defence mechanisms that prevent the entry of harmful microorganisms. Furthermore, under some conditions, the skin's barrier function is compromised, allowing infections to enter the skin (21). The three most noticeable layers of the human body's skin are the dermis, hypodermis, and epidermis. Skin is an organized organ. It is the best location to apply medications that have a localized or systemic effect. The stratum corneum, the topmost layer of the epidermis, is made up of dead and keratinized cells that function as a unique barrier to prevent different medications from passing through the skin. A necessary step for maintaining the intended therapeutic concentration is skin penetration. For medications to have a therapeutic effect, they must therefore deeply penetrate the skin's layers. medication delivery by topical application is largely dependent on the physicochemical characteristics of the medication and the formulation of an efficient carrier system.(1)
Conventional Approaches
The primary classes of antifungal medications are polyenes, azoles, allylamines, and benzylamines. These antifungal medications are sold commercially as creams, gels, lotions, and sprays in standard dosage forms.(22) Its poor permeability and inaccessibility to target the drug in the necessary concentration at the precise site of action for an extended period of time is the main reason for the failure of conventional therapy. Additionally, it is discovered that conventional dose forms are unable to protect normal tissues, which can have negative effects such dermal atrophy linked to corticosteroids. To prevent metabolic breakdown, the medicine must also be shielded from the skin's metabolic environment. Hazardous metabolites may intensify side effects, while the presence of inactive metabolites may limit the effectiveness of the medication and necessitate repeated administration. Furthermore, one of the limitations of the standard formulation was that it did not take into account the need for the medicine at the best site of action. In this regard, a particular mechanism is needed to target the medication in the most effective way. After taking into account all of these problems with traditional dose forms, it is advised that a new method for topical medication delivery be developed.(23)
Novel Approaches to Topical Therapy
Topical treatment of fungal infections was difficult due to the low penetration of antifungal medications into the various layers of the skin. A selective delivery system that improves the penetration of the bioactive moiety into the skin, localizes the drug at the site of action, and lowers percutaneous absorption is needed to address the issues with dermato-pharmacotherapy, such as its limited local activity. Several strategies have been investigated for the effective delivery of bioactive molecules.(24)Innovative methods of formulation have been devised and investigated to enhance the permeability throughout the stratum corneum. PNPs, SLNs, and NLCs are examples of nanoparticulate drug carriers that have been the subject of much study.(25) The vesicular technique is also becoming more insightful. Invasomes, ethosomes, and transferosomes are a few of the recently studied carriers to guarantee skin targeting of antifungal medications.When compared to traditional dose forms, all of these nanocarriers have the capacity to topically deliver the medication efficiently. Numerous studies have examined these nanocarriers for the topical administration of antifungals, antivirals, and antibacterials.(21, 26)
Nanoparticulate Carriers
Since they make it easier for loaded medications to penetrate the skin, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NSCs) are the nanoparticulate carrier systems for topical treatment of skin-related fungal infections. Lipid pellets, which are particle matrices in the form of SLN, can be created by combining lipids with surfactants. It is made by creating a microemulsion and employing high homogeneity.
The SLNs have certain drawbacks: only a small number of medications can dissolve in the right lipids, and these lipids may crystallize into more stable structures, which would force the medication out of the particles. NLCs are a new class of lipid particles that were created to address some of the drawbacks of SLNs. Different solid lipid mixes are combined with liquid oils to create NLCs. These carriers offer the benefit of a reduced toxicity risk. When stratum corneum comes into contact with tiny lipid particles, it may improve the medications' ability to penetrate the skin.(27)
Vesicular Carriers (Liposomes, Ethosomes, Niosomes and Transferosomes)
Colloidal vesicular carriers such as liposomes or niosomes have been extensively applied in drug delivery systems. Topical drug administration has been initiated since long time to accomplish several functions on different skin levels (skin surface, epidermis, dermis and hypodermis). But several limitations have been associated with the conventional topical preparations e.g., low percutaneous penetration because of the barrier function of the stratum corneum, the outermost layer of the skin and absorption to the systemic circulation. The scientific reports now –adays offer several systems that can be able to deliver drugs through the skin. Topical drug delivery means the application of drug to skin for localized effect and in transdermal drug delivery system (TDDS) skin is used as a potential route for the delivery of systemic action of drug(28)
Numerous lipids and surfactants can be used to create vesicles; they are usually made up of non-ionic surfactants (niosomes, spanlastics) or phospholipids (liposomes, ethosomes, transferosomes, and transethosomes). The fundamental problem with these formulations is that small changes could transform the preparation intended for local targeting into a systemic one. They have been used topically in a range of formulations to enhance permeability or medication targeting to a specific layer of the skin.
Gelling Systems-polymeric Carriers
Microsponge and Nanosponge
This carrier method uses both microsponge and nanosponge to deliver the antifungal medication. Composed of macroporous beads with a diameter ranging from 10 to 25 ?m, this microsponge allows for the regulated release of topical medicines. This type of technology is helpful because it allows for more formulation flexibility, better stability, and suitable drug entrapment. According to the study's findings, microsponge and nanosponge systems are non-toxic, non-allergic, non-mutagenic, and non-irritating.
Amphiphilic Gels
Non-ionic surfactants make up the amphiphilic gels, in which the gelation of one surfactant induces the gelation of another. Amphiphilic gels are also utilized as topical and transdermal medication and vaccine carriers; the idea behind their use was that the gels' surfactant properties would improve the active agents' penetration of the skin. Since the gels' surfactants are non-ionic, the skin won't become irritated when the gels are applied topically or applied transdermally.(29)
Microemulsions
Microemulsions are clear or translucent, isotropic, thermodynamically stable systems made of water, oil, and surfactant—often in conjunction with a cosurfactant for topical and transdermal medication delivery. Droplet sizes vary between 0.1 and 1.0 ?m. Improved medication solubility, thermodynamic stability, optical clarity, ease of production, and low cost are benefits of microemulsion. Microemulsions frequently have benefits over conventional topical and transdermal drug delivery systems because of their physicochemical characteristics. Microemulsions can be used as liquid membrane carriers to move hydrophilic materials across lipoidal media or lipophilic materials through aqueous media. Because of their exceptional biocompatibility, microemulsions are a suitable delivery mechanism for topical and transdermal applications. The oils and surfactants that are part of the microemulsion formulation help the medications to penetrate the stratum corneum more effectively.(30)
Nano emulsion
An aqueous phase and an oil phase make up the two immiscible phases of a heterogeneous system known as a nanoemulsion. The submicron droplet size spans from 5 to 200 nm. These days, using nanoemulsions topically and transdermally are commonplace.(31)
Emulgels
Topical medication delivery systems also employ gelled emulsions called emulgels. Both an emulsion and a gel release control system are present in the emulgels. When the emulsion is mixed with gel, it also becomes more stable. These emulgels offer significant advantages over both traditional and new vesicular systems in a number of areas. Thixotropic, greaseless, easily spreadable, readily removable, emollient, non-staining, water-soluble, prolonged shelf life, bio-friendly, clear, and aesthetically beautiful emulgels are ideal for use in dermatology. Because emulgels contain a variety of permeation enhancers that might intensify the effect, they can be used as more effective topical drug delivery systems. The emulgels that penetrate skin to deliver antifungal medications. Two grades of acrylic acid copolymers and jojoba oil were employed in the formulation of clotrimazole, which demonstrated good stability and high drug releases.(32, 33)
Types of Emulgel:
Macroemulgels: These are the most prevalent kind of emulgels, with emulsion droplets larger than 400 nm in size. Although they are not visible to the naked eye, the individual droplets are plainly visible under a microscope.
Microemulgel: Micro-emulsions do not coalesce and have droplet sizes ranging from 100 to 400 nm. They are translucent and thermodynamically stable. In certain ratios, oil, surfactant, co-surfactant, and water make up microemulsions.
Nanoemulgel: A gel that incorporates nano-emulsion is referred to as nano-emulgel. Transparent oil and water dispersions that are thermodynamically stable and sustained by an interfacial film of surfactant and cosurfactant molecules with globule sizes less than 100 nm are known as nanoemulsion. Both in vitro and in vivo, nano-emulsion formulations have developed transdermal and dermal distribution capabilities. Comparing nano-emulsion to traditional topical formulations like emulsion and gels, the transdermal penetration of the medicine is enhanced.
Ideal Properties of Emulgel
- Being greaseless.
- Easily spreadable.
- Easily removable.
- Emollient.
- Non-staining.
- Longer self-life, bio friendly.
- Pleasing appearance. (34)
Advantages of emulgel
- Avoidance of first-pass metabolism.
- Avoidance of gastrointestinal incompatibility.
- More selective to a specific site.
- Improve patient compliance.
- Suitability for self-medication.
- Providing utilization of drugs with short biological half-life and narrow therapeutic window.
- Ability to easily terminate medication when needed.
- Convenient and easy to apply.
Disadvantages of emulgel
- Skin irritation on contact dermatitis.
- The possibility of allergenic reactions.
- The poor permeability of some drugs through the skin.
- Drugs of large particle size are not easy to absorb through the skin.
- The occurrence of the bubble during the formation of emulgel.
Rationale of Emulgel as a Topical Drug Delivery System:
There are numerous drawbacks to many commonly used topical medications, including ointments, creams, and lotions. When administered, they are quite sticky and make the patient uneasy. They also require rubbing in application and have a lower spreading coefficient. Additionally, they display the stability issue as well. The usage of transparent gels in pharmaceutical and cosmetic preparations has increased as a result of all these elements falling within the major group of semisolid preparation.
Numerous medicinal products that either improve or restore a basic skin function or pharmacologically change a tissue's response are applied to the skin or mucous membrane. These goods are known as dermatological or topical goods. There are numerous drawbacks to many commonly used topical medications, including ointments, creams, and lotions. They are sticky by nature, making the patient feel uneasy when applied, they have a low spreading coefficient, making them difficult to apply by rubbing, and they also have a stability issue. The usage of translucent gels in pharmaceutical and cosmetic preparations has increased as a result of all these aspects falling under the larger category of semisolid preparations. Despite the many benefits of gels, one significant drawback is their inability to administer hydrophobic medications. so that gels can effectively incorporate and administer even a hydrophobic medicinal molecule.(35)
Important Consistuent Used in Formulation of Emulgel:
Aqueous material:
It is the emulsion's aqueous phase. As an aqueous phase for Emulgel, water and alcohol are frequently utilized.(36)
Oils:
These are used in the emulsion development oil phase. The Emugel formulation uses a variety of oil phases, such as non-biodegradable oil and mineral oil either alone or in conjunction with paraffine. Depending on the medication and formulation, mineral oil is frequently used either by itself or in conjunction with hard and light paraffin. For example, emulsions and mineral oils are frequently utilized as drug vehicles due to their occlusive and sensory qualities, as well as their use alone or in combination with soft or hard paraffin. Non-biodegradable mineral and castor oils, which have a local laxative effect, as well as fish liver oils and various fixed oils derived from vegetables (such as Arachis, cottonseed, and maize oils) are commonly used in oral formulations as nutritional supplements.
Gelling agent:
These are the thickening agents that can also be employed to improve the consistency of any dosage form. These are mostly of two types: synthetic and natural. The primary goal of the gelling agent is to create a thixotropic formulation. These two gelling agents—carbopol and HPMC—are frequently used with Emulgel. Unlike HPMC, carbopol absorbs water instantly. HPMC and Carbopol exhibit far superior control release properties. Emulgel-containing HPMC exhibits improved control release. (37)
Emulsifiers:
Emulsifying compounds are used to control stability over a shelf life that can range from days for spontaneously generated emulsions to months or years for commercial preparations, as well as to encourage emulsification during the manufacturing process. When preparing an emulsion, various types of emulsifying agents are used.(38)
Preservatives:
Preservatives are added to emulgel to prevent microorganisms from spoiling the formulation and are used to suppress the growth of microorganisms. For instance, benzoic acid, propyl alcohol, methyl paraben, benzoalkonium chloride, etc. (39)
Antioxidants
Antioxidants are added to emulgels in order to improve the stability of medicinal ingredients. For example, BHA, BHT, etc.
Humectants
In emulgel formulations, the humectant is employed to keep the mixture moist. The two most often used humectants are glycerine and propylene glycol.
Permeation enhancer:
These substances cause a transient and reversible increase in skin permeability by partitioning into and interacting with skin components. Drug delivery vehicles frequently contain penetration-improving ingredients that temporarily disturb the stratum corneum skin barrier's highly ordered structure, fluidize the lipid channels between corneocytes, alter how the drug is partitioned into skin structures, and enhance skin delivery in order to promote drug absorption through the skin barrier. For instance, lecithin, urea, eucalyptus oil, chenopodium oil, pyrrolidone, lanocapran, dimethyl sulfoxide, lutein, menthol, and so on.(40)
Applications of Emulgel
- Emulgel facilitates the easy joining of hydrophobic solutions into the oil stage. Soon after, smooth globules are spread in the liquid stage to create an O/W emulsion. This emulsion can be successfully blended into a gel base to improve the prescription's entry and reliability.
- Emulgels offer greater security and consistency compared to other transdermal regimens.
- Improved stacking limit is a result of the massive structure Emulgels offer superior stacking capacity.
- Differentiated itself from other cutting-edge concepts such as liposomes and noisomes.
- The emulgel preparation process requires basic and few steps, which reduces the cost of generation and makes production achievable. These are new, conservative measurement frameworks.
- Elevated sonication is not necessary; hence item debasement is prevented.
- Emulgels with controlled release can be used to delay the effects of medications with short half-lives.
- lengthens the medication's mean live course of action and contact time.
- Emulgels can serve a variety of useful functions.
- Less smooth and simple to use, they increase patient consistency (41).
CONCLUSION
The article addresses the drawbacks of traditional topical antifungal formulations and explores the creation and advantages of Emulgel as a cutting-edge approach to topical medication administration. It looks into cutting-edge methods to improve the effectiveness of topical antifungal medication, such vesicular systems and nanoparticulate carriers. The mechanics of drug penetration through the skin are also covered, along with the architecture and physiology of the skin. The report also discusses the categorization and features of fungus, prevalent types of fungal infections, treatment choices, and issues with standard dosing forms. It also highlights the rising global frequency of superficial fungal infections.
List of Abbreviation
BHA: Butyrated hydroxy anisole
BHT: Butyrated hydroxy toluene
HPMC: Hydroxypropyl methyl cellulose
TDDS: Transdermal drug delivery system
NSCs: Nanostructured lipid carriers
SLNs: Solid lipid nanoparticles
ACKNOWLEDGMENT
All the authors are thankful for providing the necessary contributions to execute this manuscript.
Conflicts of interest/Competing interests
The authors declare no competing interest
Code availability: NA
REFERENCE
- Güng S. New formulation strategies in topical antifungal therapy. 2013.
- de Pauw BE. What are fungal infections? Mediterranean journal of hematology and infectious diseases. 2011;3(1).
- Hay R. Superficial fungal infections. Medicine. 2013;41(12):716-8.
- de Melo W, Scorzoni L, Rossi S, Costa-Orlandi C, Yonashiro M. Update on Fungal Disease: From Establish Infection to Clinical Manifestation. J Biotechnol Biomater. 2017;7(273):2.
- Ramos-e-Silva M, Lima CMO, Schechtman RC, Trope BM, Carneiro S. Systemic mycoses in immunodepressed patients (AIDS). Clinics in Dermatology. 2012;30(6):616-27.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2016;62(4):e1-e50.
- Kwizera R, Bongomin F, Lukande R. Deep fungal infections diagnosed by histology in Uganda: a 70-year retrospective study. Medical mycology. 2020;58(8):1044-52.
- Puebla LEJ. Fungal infections in immunosuppressed patients. immunodeficiency. 2012.
- Chandler FW, Watts JC. Fungal diseases. Journal of Histotechnology. 1995;18(3):247-52.
- Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs in context. 2020;9.
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186-94.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4(165):165rv13-rv13.
- Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Frontiers in microbiology. 2013;4:337.
- Pfaller MA, Diekema D. Epidemiology of invasive candidiasis: a persistent public health problem. Clinical microbiology reviews. 2007;20(1):133-63.
- Burns T, Breathnach SM, Cox N, Griffiths C. Rook's textbook of dermatology: John Wiley & Sons; 2008.
- Hall L, Wohlfiel S, Roberts GD. Experience with the MicroSeq D2 large-subunit ribosomal DNA sequencing kit for identification of filamentous fungi encountered in the clinical laboratory. Journal of clinical microbiology. 2004;42(2):622-6.
- Meis JF, Verweij PE. Current management of fungal infections. Drugs. 2001;61:13-25.
- Bianchi J, Cameron J. Management of skin conditions in the older population 2. British Journal of Community Nursing. 2008;13(Sup4):S6-S14.
- AM K. Hydration injury to human skin: a view from the horny layer. Handbook of occupational dermatology. 2000:76-80.
- Vyas SP, Khar RK. Controlled drug delivery concepts and advances. vallabh prakashan. 2002;1:411-47.
- Dubey A, Prabhu P, Kamath J. Nano Structured lipid carriers: A Novel Topical drug delivery system. International Journal of PharmTech Research. 2012;4(2):705-14.
- Nida Akhtar NA, Anurag Verma AV, Kamla Pathak KP. Topical delivery of drugs for the effective treatment of fungal infections of skin. 2015.
- Garg BJ, Saraswat A, Bhatia A, Katare OP. Topical treatment in vitiligo and the potential uses of new drug delivery systems. Indian Journal of dermatology, venereology and Leprology. 2010;76:231.
- Devi M, Kumar MS, Mahadevan N. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Rec Adv Pharm Res. 2011;4:37-46.
- Kumar L, Verma S, Bhardwaj A, Vaidya S, Vaidya B. Eradication of superficial fungal infections by conventional and novel approaches: a comprehensive review. Artificial cells, nanomedicine, and biotechnology. 2014;42(1):32-46.
- Akhtar N, Verma A, Pathak K. Topical delivery of drugs for the effective treatment of fungal infections of skin. Current pharmaceutical design. 2015;21(20):2892-913.
- Souto E, Müller R. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. Journal of microencapsulation. 2006;23(4):377-88.
- Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, et al. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. Journal of liposome research. 2010;20(4):341-50.
- Lalit SK, Panwar AS, Darwhekar G, Jain DK. Formulation and evaluation of fluconazole amphiphilogel. pharmaceuticals. 2011;6:7.
- Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG. Effect of formulation components on the in vitro permeation of microemulsion drug delivery system of fluconazole. Aaps Pharmscitech. 2009;10:917-23.
- Thakur N, Garg G, Sharma P, Kumar N. Nanoemulsions: a review on various pharmaceutical application. Global Journal of Pharmacology. 2012;6(3):222-5.
- Glujoy M, Salerno C, Bregni C, Carlucci AM. Percutaneous drug delivery systems for improving antifungal therapy effectiveness: A review. Int J Pharm Pharm Sci. 2014;6:8-16.
- Singla V, Saini S, Joshi B, Rana A. Emulgel: A new platform for topical drug delivery. International Journal of Pharma and Bio Sciences. 2012;3(1):485-98.
- Yadav SK, Mishra MK, Tiwari A, Shukla A. Emulgel: a new approach for enhanced topical drug delivery. Int J Curr Pharm Res. 2016;9(1):15-9.
- Panwar A, Upadhyay N, Bairagi M, Gujar S, Darwhekar G, Jain D. Emulgel: A review. Asian J Pharm Life Sci. 2011;2231:4423.
- Prasad B, Tyagi Y, Rao N. A Review on Emulgel: The Topical Drug Delivery System. World Journal of Pharmaceutical and Life Sciences. 2020:47-55.
- Kasliwal N, Derle D, Negi J, Gohil J. Effect of permeation enhancers on the release and permeation kinetics of meloxicam gel formulations through rat skin. Asian Journal of Pharmaceutical Sciences. 2008;3(5):193-9.
- Mohite SV, Salunkhe AK, Sudke SG. Emulgel: A novel approach for hydrophobic drugs. American journal of pharmtech research. 2019;9(2):209-24.
- Baibhav J, Gurpreet S, Rana A, Seema S, Vikas S. Emulgel: a comprehensive review on the recent advances in topical drug delivery. International Research journal of pharmacy. 2011;2(11):66-70.
- Maitri S, Modi D, Shah H. A new future approach in novel drug delivery system through micro-emulgel: a review. World J Pharm Pharm Sci. 2016;5(05):243-59.
- latha Samala M, Sridevi G. Role of polymers as gelling agents in the formulation of emulgels. Polymer science. 2016;2:1-8


 Yogend Chaurasia*
Yogend Chaurasia*
 Deepak Kumar
Deepak Kumar
 Abhishek Kumar Singh
Abhishek Kumar Singh



 10.5281/zenodo.12801025
10.5281/zenodo.12801025