Abstract
One of the most crucial factors in the creation of novel medications and formulations is the stability studies of pharmaceuticals. Predicting the shelf life plays a crucial part in the creation of pharmaceutical products for all dosage forms. It is also used to provide label instructions and identify specific storage requirements. Before any pharmaceutical product is accepted and approved, stability tests that guarantee the product's quality, safety, and efficacy throughout its shelf life are thought to be necessary. These investigations must be carried out according to predetermined protocols that are followed by the WHO, ICH, and other organizations. To guarantee that pharmaceutical products remain safe, effective, and of high quality for the duration of their shelf lives, stability studies are a standard operating procedure are adhered to when it comes to pharmaceutical items. Evidence of how a drug product's quality varies over time under the impact of different environmental conditions is provided by stability testing. Pharmaceutical Product Stability Studies: Determine how long a pharmaceutical product can stay within specified parameters in a particular packaging system to preserve quality and provide the intended performance during retesting or expiration. This review article includes an introduction to stability studies, lists the many kinds of stability studies, and provides criteria for estimating a pharmaceutical product's shelf life.
Keywords
Pharmaceutical, Stability Studies, WHO, ICH, Shelf Life, Half Life.
Introduction
Pharmaceutical stability studies can be defined as the amount of time that a pharmaceutical product keeps its original physical, chemical, microbiological, and pharmacokinetic features and characteristics during the course of its shelf life. When the chemical falls to 90% of its initial concentration, the product's shelf life is said to have occurred. Expiration date is another way that the technical term "shelf life" is used to indicate how stable a product is. The duration of each medicinal preparation varies. The pharmaceutical dosage form's expiration date is influenced by a number of environmental variables, including temperature, humidity, light, radiation, and others. With a variety of chemical and physical active ingredients in the composition, the kind of container closures utilized, and the storage circumstances. The degradability of active compounds and literature data on their breakdown are often accessible, accompanied by suitable analytical techniques. As a result, dosage form-specific stability studies can be limited. In order to ascertain and guarantee the identity, potency, and purity of ingredients as well as those of the formed products,[1] pharmaceutical analysis and stability studies are among the most crucial phases in the development process. Microbiological alterations, such as the growth of germs in non-sterile items and modifications in the effectiveness of preservatives [2], can also have an impact on the stability of a pharmaceutical product. Furthermore, the data produced by the stability testing is a crucial prerequisite for regulatory approval of any medication or product composition [3].
Importance of stability studies:
- As the active ingredient in the product is unstable, the dose form of the drug may be lowered, resulting in undermedication.
- Toxic byproducts may be produced as a result of the drug or product breaking down.
- The medicine has the ability to modify its physical features while being marketed and transported from one location to another.[4]
Types of stability studies on drug substances:
Physical, chemical, microbiological, therapeutic, and toxicological stability investigations must meet certain standards set out by the United States Pharmacopeial Protocol (USP). [5,6]
Physical stability:
Durability, cracking, uniformity, color, dissolution, palatability, suspend ability, and homogeneity are all considered aspects of physical stability. There's a potential for absorption of moisture, crystal formation, and loss of water and volatile materials. [5,6]
Chemical stability:
It is the propensity to oppose modifications or breakdowns to the drug's actual form. It happens because of the air and temperature, which help the medications create a new chemical structure. Chemical stability is typically less active. Quality and designated strength stay inside the designated bounds. [5,6]
Microbiological stability:
Antimicrobial agents are utilized within certain bounds to preserve the microbiological stability. Manufacturing facilities also use gamma radiation and autoclave sterilization to stop the growth of microorganisms. [5,6]
Therapeutic stability:
The therapeutic impact won’t change. [5,6]
Toxicological stability:
Increased toxicity is essentially non-existent. [5,6]
Different types of stability studies:
The purpose of stability studies is to test the drug product over extended periods of time in different temperature and relative humidity (RH) settings. Long-term stability tests are crucial if the medication is going to be spread across several geographic areas and shipping is needed for transportation. In order to conduct long-term stability studies, the sample is tested at predetermined intervals, and the external parameter conditions are adjusted correspondingly. The primary goal of this investigation is to ascertain the medication product's shelf life. There are four primary types of stability studies: In-use stability studies, Accelerated stability studies, Intermediate stability studies, and long-term stability studies Table 1 displayed the kind of stability studies, their storage circumstances, and the corresponding time period.[7]
Table 1: Types of stability studies
Types of stability studies:
Stability testing is a common practice used on drugs and products at different phases of the goods development process. Initial steps involve conducting accelerated stability tests at elevated temperatures and/or humidity levels to identify potential degradation products that may emerge from extended storage. The shelf life and expiration dates of products are ascertained by testing them under less demanding circumstances, i.e., those advised for long-term shelf storage, at slightly increased temperatures. The primary goal of pharmaceutical stability testing is to offer a reasonable level of assurance that the products will continue to be fit and of acceptable quality for the duration of their availability to patients in the market and that they will continue to be safe for ingestion until the patient uses the last unit of the product. Pharmaceutical stability testing's primary goal is to offer a reasonable assurance that the products will maintain an appropriate level of quality and fitness for as long as they are on the market, available for patient supply, and fit for consumption until the patient uses the last unit of the product.[7]
Stability testing methods:
- Real-time stability testing
- Accelerated stability testing
- Retained sample stability testing
- Cyclic temperature stress testing.
Real-time stability testing:
For real-time stability testing, a longer test period is typically used in order to allow for noticeable product degradation under advised storage circumstances. The test duration is determined by the product's stability, which must last long enough to demonstrate unequivocally that no detectable deterioration takes place and enable separation of degradation from inter-assay variation. Data is gathered during testing at a suitable frequency to enable trend analysis to differentiate between daily ambiguity and instability. The data can be increased by including the durability of the material used as a reference, for which the parameters for stability have already been established. Reference materials for the stability investigation include the reagent stability and instrument performance consistency. It is essential to keep an eye on system performance and to control drift and discontinuity brought on by adjustments to reagents and devices. [8,9]
Accelerated stability testing:
Excessive storage conditions are used in these experiments in order to examine increased rates of chemical and physical breakdown. Formal stability studies include this. These studies' data are used to conduct long-term stability tests, or to calculate the drug product's shelf life.[10]
Retained sample stability testing:
This is standard procedure for any product that is commercialized and needs stability data. Stability samples are chosen for this investigation with the intention of being stored for at least one batch per year. Stability samples from two batches should be obtained if there are more than 50 batches being marketed. Stability samples of each batch may be obtained during the product's initial release onto the market; these samples may eventually be reduced to just 2% to 5% of marketed batches. The stability samples in this study are put to the test at set intervals, such as. It is customary to examine samples at 3, 6, 9, 12, 18, 24, 36, 48, and 60 months if a product has a five-year shelf life. The constant interval method is the name given to this traditional technique for gathering stability data on stored storage samples.12,17 A modified method called "stability testing by assessment of market samples" entails taking samples that are already available in the market and assessing stability qualities. Because it tests the product in both real-world settings and the assumed retained sample storage conditions, this kind of testing is by its very nature more realistic.[7]
Cyclic temperature stress testing:
For marketed items, this is not a standard testing procedure. This method involves designing cyclic temperature stress studies based on product knowledge to replicate possible market storage circumstances. Since the marketed medications are most likely to encounter a 24-hour diurnal rhythm during storage, a 24-hour cycle is the duration of cycle that is most commonly considered. It is advised that the lowest and highest temperatures for the periodic stress testing be chosen product-by-product, taking into account things like the product's suggested storage temperature and unique physical and chemical degradation characteristics. Additionally, it is advised that the test typically consist of 20 cycles. [7,11]
Guidance of stability studies:
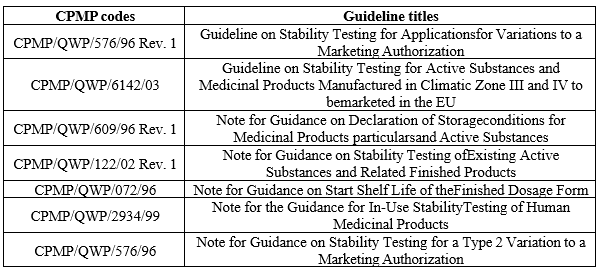
Several nations' regulatory bodies have included clauses in their medication legislation requiring producers to submit stability data in order to ensure that the highest quality molecules and products are produced, distributed, and administered to patients. To introduce consistency in testing among manufacturers was its main goal. These recommendations include fundamental stability-related topics as well as the procedures for carrying out the stability data required for application dossiers. The 1980s [8] saw the first release of these guidelines. Later, in order to get around the obstacle of having to market and register the products in other nations, these were harmonized, or made standardized, by the International Council for Harmonization (ICH). The European Commission, Japan, the United States, and industry all contributed to the formation of the International Council of Harmonization (ICH) in 1991. Through this consortium, several recommendations pertaining to the quality, safety, and efficacy of drug substances and drug products were developed. These recommendations go by the name’s quality, safety, efficacy, and multidisciplinary (also known as Q, S, E, and M) recommendations.[12] In 1996, the World Health Organization (WHO) changed the recommendations since the ICH guidelines did not It only addressed novel drug compounds and products, not the well-known ones that were already in use in the WHO umbrella countries, and it did not address the severe weather conditions present in many nations. A guidance document titled "Expiration dating of solid oral dosage form containing Iron" was also released by the US Food and Drug Administration (US FDA) in June 1997. WHO also published guidelines for stability studies in the global environment in 2004.[13] Later on, the ICH criteria for veterinary goods were also expanded. Also published by the India Drug Manufacturers Association [14] is a technical monograph on stability testing of drug substances and products that are produced in India.8. Additionally, the guideline documents have specified various test conditions and requirements for excipients, medicinal products or formulations, and active pharmaceutical ingredients. the codes and titles that are covered by ICH guidelines. The European Agency for the Evaluation of Medicinal Products (EMEA) has also released a number of stability testing standards through the Committee for Proprietary Medicinal Products (CPMP) to help those requesting marketing clearance for pharmaceuticals in the EU.[8]
Table 2: codes and titles used in ICH Guidelines.

Table 3: CPMP Guidelines for stability studies.
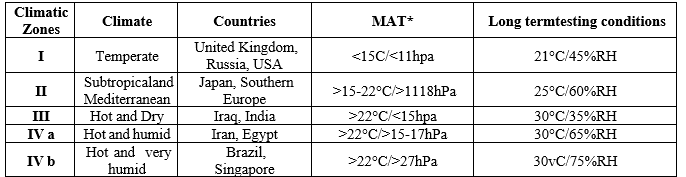
Climatic Zones for stability studies:
Stability studies are carried out globally. The temperature and other variables differ from nation to nation and location to location, making it impossible to conduct these stability tests in one location. For this reason, the globe has been split into four climate zones based on factors such as temperature, allowing for the prediction of product deterioration and shelf life. precisely. Real-time stability testing and expedited stability testing have been developed based on this data. The WHO's standard climate zones for the use of pharmaceutical products along with a breakdown of the environmental conditions resulting from long-term storage settings. [1,3,16,17]
Table 4: Climatic Zones and Long-term stability conditions.
Stability Study for protocol:
Stability testing is an organized method for the process of developing new drugs. The best packaging and storage options for bulk batches of the drug substance are chosen based on stability data for the substance. The purpose of the drug product's stability research is to ascertain its shelf life or expiration date. A documented document outlining the essential elements of a controlled and regulated stability research is called a protocol, and it is a prerequisite for initiating stability testing. The testing procedure is dependent on the kind of drug substance or product since it is dependent on the compound's intrinsic stability, the kind of dosage form, and the suggested container-closure system. Furthermore, whether the medication is novel or already available on the market may have an impact on the protocol.[18,19] The protocol should also take into account the areas in which the product is intended to be sold. For example, if the product is intended for usage in climatic zones I–III, IVa, and IVb, then the stability program should include all of these zones.[19] A well-thought-out stability protocol ought to include the following details:
- Number of Batches
- Containers and closures
- Orientation of storage of containers
- Sampling time points
- Test storage conditions
- Test parameters
- Test methodology
- Acceptance criteria
Number of batches:
In developmental stages, stability studies are typically conducted on a single batch; however, studies meant for registration of new products or unstable established products are conducted on the first three manufacturing batches; two batches are permitted for stable and well-established products. Using the same process as the approved drug application, the first three batches of drug products manufactured after authorization should be placed on long-term studies if the initial results do not come from a full-scale production batch. While they are considered supporting information, data on laboratory-sized batches collected during the development of medications are not recognized as primary stability data. Generally speaking, a random sample of the population of manufacturing batches should be included in the batch selection process.[20]
Containers and closures:
The product is tested using the exact closures and containers that are intended for sale. Aluminum blister packs, Alu-Alu packs, HDPE bottles, and strip packs are some of the packaging materials used. This does not apply to shippers, although it may also apply to supplementary packing. Products in all formats whether distributed or intended for use as promotional and medical samples should undergo independent testing. As long as the prototype container closely resembles the real package, testing in bulk containers is permitted.[20]
Orientation of storage of containers:
It is necessary to maintain upright and place semi-solid drug products, dispersed systems, and sample solutions either on the side or inverted to enable complete product interaction with the container-closure during stability investigations. It is possible to ascertain from this orientation whether chemical substances are extracted from the closure components or product components are adsorbed into the container-closure as a result of interaction between the drug product or vehicle and itself.[18]
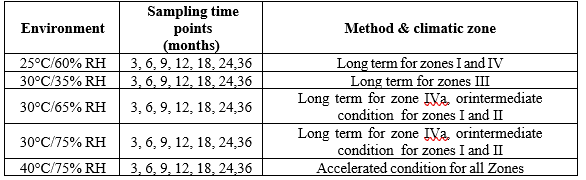
Sampling time points:
To determine the stability profile of the novel pharmacological substance, testing is crucial at specific intervals. The products with a six-month shelf life in the first year, months in the second, and years in the following years throughout the shelf- life projection. Three months, for example, is the minimal number of time points in the case of expedited stability investigations. If the same product is to be evaluated but has variable strengths, sizes, etc. One option is to employ retained stability testing, which requires less points. The statistical designs of bracketing and matrixing serve as the foundation for the reduced testing plans. Only when samples on specific design parameters, including strength and package size, are examined at each of the three time points as in the whole design, is bracketing the design. Strength, batches, container sizes, and interim time points are among the variables that can be manipulated. Table 5 displayed the sample period, environment, and specific climatic zones. [3,21]
Table 5: Test schedule for stability testing of new products
Test storage conditions:
The selection of storage conditions should be based on the climate zones where the product is intended for marketing. Three major bodies have issued recommendations about storage conditions: ICH, CPMP, and WHO. the storage settings for pharmacological items based on the ICH and WHO Stability studies. [13,16,21]
Test parameters:
The parameters under which the stability samples will be assessed should be specified in the stability test methodology. Stability tests are those that keep an eye on characteristics like identity, quality, potency, and purity that could alter throughout storage. Because of this, standard tests on stability test samples include appearance, assay, degradation products, microbiological testing, dissolution, and moisture. When appropriate, such as for liquid injectable formulations, microbiological testing include sterility, preservative efficaciousness, and bacteria count. All testing requirements, such as heavy metals, residue on ignition, residual solvents, etc., must be met by the batches used for the stability research. Certain things must be done when the product is released, but they don't have to be done again when doing stability testing. In ICH guideline Q6A, other tests such as enantiomer purity, particle size, polymorphic form, etc., have also been covered.[19]
Test methodology:
Since the results of the official tests are generally more widely accepted, it is always advised to adhere to the guidelines provided in the official compendia. It is necessary to properly validate any alternative approaches that are used. Nonetheless, a stability-indicating method that was developed by stress testing the medication under conditions of forced decomposition should be used for the experiment. When appropriate, such as for liquid injectable formulations, microbiological testing include sterility, preservative efficaciousness, and bacteria count. All testing requirements, such as heavy metals, residue on ignition, residual solvents, etc., must be met by the batches used for the stability research. While not necessary to repeat throughout stability testing, some of these a necessary at the time of product release. The ICH guidelines Q6A also cover other tests, such as those for enantiomer purity, particle size, polymorphic form, etc. [14,18]
Acceptance criteria:
Prior to starting the stability investigations, it is necessary to validate all analytical procedures. Likewise, it is imperative to predetermine the acceptance criteria for both the analytical data and the existence of degradation products. Every test in the stability study has set acceptance criteria that take the form of numerical limits for results expressed in quantitative terms (moisture pick-up, viscosity, particle size, assay, degradation products, etc.) and pass/fail for tests expressed in qualitative terms (odor, color, appearance, cracking, microbial growth...). Aside from individual and overall upper limits for degradation products, these acceptance criteria should additionally include Regarding impurities in novel drug products, ICH guideline Q3B(R2) discusses degradation products in novel drug formulations. In the event that the suggested thresholds are surpassed, the degradation products of the active or interaction products from the active components and excipients, and/or the active and container component, should be recorded, identified, and qualified. Based on the planned dosage, the impurity reporting threshold is determined. The maximum daily dose is limited to 0.1% if it is less than or equal to 1gm, and to 0.05% if it is more than 1. For a maximum daily dosage of 1 mg to 2 grams, the detection threshold of contaminants falls between 1.0-0.1%. [14,19]
Estimation of shelf life:
Based on information gathered from long-term storage tests, the shelf life is calculated. After linearizing the data, a goodness of fit test is run. After that, the calibrated data is examined to make sure the intercepts and slope match. Table 6 displayed the various scenarios for the concentration-time data pattern of the three batches. The common slope is estimated by pooling the data in this manner.[3] In Statistical methods such as the t-test should be utilized to ascertain the significance of the difference in the case of the slope or the intercept.
Table 6: Pattern of concentration-Time data and Pooling decision.

Evaluation of stability studies:
The parent guidelines' guiding concepts must be followed when planning the stability research. The primary goal of a stability study is to determine the shelf life or retest duration of a drug substance or drug product under particular storage conditions according to the final labelled packaging that has been sold in the market. To establish the data, at least three batches must be used. How certain one may be that a subsequent production batch will remain stable for the remainder of its shelf life or retest period depends on the amount of batch variability.
Current trends in stability studies:
Multinational pharmaceutical corporations are currently leading the trend in stability studies by defining the parameters for stability testing in preparation for international marketing. Companies are adapting their protocols to a single set of circumstances that encompasses harsh environmental conditions in order to achieve this. The accelerated testing period will now last for 12 months instead of just 6, and additional testing will be conducted for three months at 50°C and 75% relative humidity. These are the specific adjustments for the global testing. The idea behind this modification is to save resource usage and prevent the need to repeat stability testing for additional locations because all tests are conducted in a single facility. Additionally, studies conducted under the combined effects of temperature, humidity, and light have been shown to have a greater negative impact on medicinal compounds and goods than those conducted under the conditions of temperature and humidity alone. [3,21,22,23,24]
CONCLUSION
Pharmaceutical stability studies play a crucial procedural role in the development program for novel medications and formulations. These tests have made it simple to forecast the shelf life of pharmaceuticals, including the impact of environmental conditions on product deterioration. Any departure from the specified stability profile may have an impact on the product's efficacy, safety, and quality. In order to ensure that the medication is safe and effective for the duration of its shelf life, stability tests are performed and the suggested storage conditions and shelf life are then mentioned on the label. As a result, the stability tests ought to be conducted in accordance with accepted scientific methods, after being aware of the most recent legal requirements, and in accordance with climate zones.
REFERENCES
- Saranjit Singh and Monika B. Guidance on Conduct of Stress Tests to Determine Inherent Stability of Drugs, Pharmaceutical Technology online, 2000; 24-36.
- Matthews RB. Regulatory Aspects of Stability Testing in Europe, Drug Development and Industrial Pharmacy, 1999; 25(7): 831-856.
- Saranjit Singh. Stability testing during product development in Jain NK Pharmaceutical product development, CBS publisher and distributors, India, 2006; 272-293.
- Thorat Punam, Warad Shubhangi, Solunke Rahul, Ashok Sargar, Anagha Bhujbal, and Asha Shinde. Stability Study of Dosage Form: An Innovative Step. World Journal of Pharmacy and Pharmaceutical Sciences. 2014; 3(2): 1031-1050.
- Z. F, R. R, and S. K, “A REVIEW ON STABILITY TESTING GUIDELINES OF PHARMACEUTICAL PRODUCTS,” Asian Journal of Pharmaceutical and Clinical Research, pp. 3–9, Aug. 2020, doi: 10.22159/ajpcr. 2020.v13i10.38848.
- P. Ghimire, A. Chandra Shrestha, and S. Pandey, “Guidelines on Stability Studies of Pharmaceutical Products and Shelf-Life Estimation,” International Journal of Advances in Pharmacy and Biotechnology, vol. 6, no. 1, pp. 15–23, Mar. 2020, doi: 10.38111/ijapb.20200601004.
- Kommanaboyina B and Rhodes CT. Trends in stability testing, with Emphasis on Stability during Distribution and Storage, Drug Development and Industrial Pharmacy, 1999; 25(7): 857-868
- Bajaj S, Singla D, Sakhuja N. Stability Testing of Pharmaceutical Products. Journal of Applied Pharmaceutical Science. 2012; 2:129-138.
- Anderson G, Scott M. Determination of product shelf life and activation energy for five drugs of abuse. Clinical Chemistry. 1991; 37:398-402.
- Amrita Panda, Sukhadakulkarni, Ravi Tiwari. Stability Studies: An Integral Part of Drug Development Process. International Journal of Pharmaceutical Research and Biosciences. 2013;2(6):69-80.
- Carstensen JT, Rhodes CT. Rational Policies for Stability testing, Clinical Research and. Regulatory Affairs.1993;10:177-185.
- Panda A, Kulkarni S., Tiwari R. Stability Studies: An Integral Part of Drug Development Process. International Journal of Pharmaceutical Research and Bio-Science, 2013;2;69-80.
- WHO. Stability studies in a global environment. Geneva meeting working document QAS/05.146 with comments, 13-14 December 2004.
- Singh S., Bakshi M. Guidance on conduct of stress test to determine inherent stability of drugs. Pharmaceutical Technology Asia, Special Issue, Sep. /Oct. 2000;24-36.
- Kommanaboyina B and Rhodes CT. Trends in stability testing, with Emphasis on Stability during Distribution and Storage, Drug Development and Industrial Pharmacy, 1999; 25(7): 857-868.
- ICH Q1A (R2). Stability testing guidelines: Stability testing of new drug substances and products, ICH Steering Committee, 6th February 2003.
- Grimm Wolfgang. Extension of the international conference on harmonization tripartite guideline for stability testing of new drug substances and products to countries of climatic zones-III and IV, Drug Development and Industrial Pharmacy, 1998; 24(4): 313-325.
- Ali J, Khar RK, Ahuja A. Dosage form and Design. Birla Publications Pvt.Ltd: Delhi,2008;100-123.
- Cha J, Gilmor T, Lane P, Ranweiler JS. Stability studies in Handbook of Modern Pharmaceutical Analysis. Separation Science and Technology. Elsevier, 2001;459-505.
- Singh S., Bakshi M. Guidance on conduct of stress test to determine inherent stability of drugs. Pharmaceutical Technology Asia, Special Issue, Sep. /Oct. 2000;24-36.
- Sanjay Bajaj, Dinesh Singla and Neha Sakhuja, Stability Testing of Pharmaceutical Products, Journal of Applied Pharmaceutical Science. 2012; 02(03): 129-138.
- Singh S, Bhutani H, Mariappan TT, Kaur H, Bajaj M and Pakhale SP. Behaviour of Uptake of Moisture by Drugs and Excipients under Accelerated Conditions of Temperature and Humidity in the Absence and the Presence of light. 1. Pure Anti Tuberculosis Drugs and their Combinations. International Journal of Pharmaceutics. 2002; 245(1-2): 37-
- Saranjit Singh, Hemant Bhutani and Mariappan T.T, Behavior of Uptake of Moisture by Drugs and Excipients under Accelerated Conditions of Temperature and Humidity in the Absence and the Presence of light II. Packaged and Unpackaged anti-Tuberculosis drug products. Pharmaceutical Technology. 2003; 27: 44-52.
- Dhiren P. Shah, Bhavesh patel and Chainesh Shah, Nanosuspension technology: An innovative slant for drug delivery system and permeability enhancer for poorly water-soluble drugs. Journal of Drug Delivery and Therapeutics, 2015; 5(1): 10-23.


 Ramya Teja Medarametla*
Ramya Teja Medarametla*
 K. Venkata Gopaiah
K. Venkata Gopaiah






 10.5281/zenodo.13819980
10.5281/zenodo.13819980