Abstract
Etoricoxib is a selective COX-2 inhibitor. The way non-steroidal anti-inflammatory drugs (NSAIDs) function is by blocking the COX enzyme, which is an inflammatory mediator. Osteoarthritis, rheumatoid arthritis, and primary dysmenorrhea are all treated with etoricoxib. Therefore, the main objective of this work is to examine etoricoxib in pharmaceutical and biological formulations using both qualitative and quantitative methods. In this review paper, we have compiled the methodologies for estimating etoricoxib based on liquid chromatography-mass spectroscopy (LC-MS), high-performance liquid chromatography (HPLC), and UV/Vis spectroscopy. Furthermore, we have discussed the bioanalytical methods for ETX analysis. To sum up, this review article will assist researchers in developing new methods for estimating drug concentrations in biological fluids and pharmaceutical dose forms.
Keywords
Etoricoxib, Analytical method, High-performance liquid chromatography, liquid chromatography-mass spectroscopy, Bioanalytical methods.
Introduction
Rheumatoid arthritis pain and inflammation are frequently managed using non-steroidal anti-inflammatory medications (NSAIDs). Their suppression of cyclooxygenase (COX) enzymes, which convert arachidonic acid into prostaglandins, is responsible for their analgesic and anti-inflammatory properties as well as part of their chemopreventive actions.(1) Etoricoxib, 5-chloro-3-(4-methanesulfonylphenyl)-6-methyl-[2,3]-bipyridinyl is a highly selective cyclooxygenase–2 (COX-2) inhibitor.(2) The two types of the enzyme are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 regulates normal physiological prostaglandin-mediated functions such gastric cytoprotection and platelet aggregation. Nonselective NSAIDs' suppression of COX-1 has been connected to both platelet inhibition and gastric injury. It is well known that prostanoid mediators of pain and inflammation are produced in large part by COX-2.(2) Etoricoxib is used to treat osteoarthritis, rheumatoid arthritis, chronic low back pain, acute pain, dysmenorrheal, acute gouty arthritis and ankylosing spondylitis.(3)


Figure 1: Chemical Structure of Etoricoxib
Mechanism of Action :
One possible explanation for the anti-inflammatory, analgesic, and antipyretic effects of NSAIDs is the inhibition of prostaglandin synthesis. The exact mechanism of action is yet unknown, but it appears that these effects are produced by blocking the COX-2 isoenzyme at the sites of inflammation, which in turn causes a reduction in the production of several prostaglandins from their precursors, arachidonic acid. Etoricoxib particularly inhibits the COX-2 enzyme, which is essential for the control of pain and inflammation. In contrast to non-selective NSAIDs, it does not stop platelet aggregation. Moreover, affinity for COX-1 is minimal to nonexistent.(4)
Pharmacokinetics :
Absorption :
Bioavailability is 100% following oral administration.(4)
Protein binding :
92%(4)
Metabolism :
Hepatic, primarily via CYP3A4. (4)
Pharmacodynamics :
Etoricoxib is a COX-2 selective inhibitor (approximately 106 times more selective for COX-2 inhibition over COX-1). (4)
Analytical Account of ETX
An extensive literature search revealed a variety of analytical methods, including UV/Visible Spectrophotometry, HPLC, HPTLC, UPLC, LC-MS/MS, and bioanalytical approaches, for the determination of RFX in bulk and pharmaceutical formulations. Celecoxib (CXB), Paracetamol (PCT), Salicylic acid (SCA), Ketoprofen (KPF), Nimesulide (NMS), Riluzole (RLZ), Drotraverine (DRT), Thiocholchicoside (THC), Pregabalin (PGBN), Tolperisone (TOP) are all evaluated alone as well as in conjunction with ETX.

Figure 2 shows different analytical methods implemented for the estimation of ETX
Bio-analytical method for ETX
A branch of analytical chemistry known as "bio-analysis" deals with the quantitative measurement of biotics (macromolecules, proteins, DNA, large-molecule drugs, metabolites) and xenobiotics (drugs and their metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 1
Table 1: Bioanalytical determination of ETX

*** Not Provided
UV-Visible spectroscopy method for ETX
The spectrophotometric methods have been accounted for the determination of ETX. The details of Spectrophotometry determination of basic principle, sample matrix, lambda max, solvent linearity range and the correlation coefficient are summarized in Table 2.
Table 2: Spectrophotometric methods used for determination of ETX

*** Not Provided
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for ETX:
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction. In this section, we have discussed the LC-MS methods for the determination of ETX in a dosage form Table 3.
Table 3. Summary of LC-MS methods for the determination of ETX in a dosage form

HPLC method for ETX
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The summary of the reported HPLC methods is shown in Table 4.
Table 4: Summary of HPLC methods for the determination of ETX in a single and combined dosage form

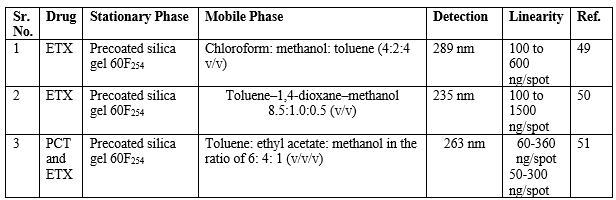
HPTLC method for ETX
Thin-layer chromatography is a popular technique for the analysis of a wide variety of organic and inorganic materials, because of its distinctive advantages such as minimal sample clean-up, a wide choice of mobile phases, flexibility in sample distinction, high sample loading capacity and low cost. The summary of the reported HPTLC methods is shown in Table 5.
Table 5: Summary of HPTLC methods for the determination of ETX in a single and combined dosage form
UPLC methods for ETX
Ultra-performance liquid chromatography (UPLC) is a new category of separation based on well-established principles of liquid chromatography, which utilizes sub-2-mm particles for the stationary phase. The developed UPLC method is validated and therefore could be further used for quantitative analysis of Etoricoxib. Sanjay Shesha Shetgar, Ramadevi Dharmasoth, Bandlamudi Mallikarjuna Rao, Basavaiah Keloth established UPLC method development and validation for simultaneous estimation of Etoricoxib and Thiocolchicoside in tablets. UPLC was carried out in Hibar, C18 column of dimension 100 × 2.1 mm, 1.8 ?m,at 30°C, by using mobile phase 0.1% orthophosphoric acid (pH 2.5) and acetonitrile in a ratio of 90:10 (v/v). The column effluents were monitored at 256 nm using a Acquity Tunable UV detector at a flow rate of 0.3 ml/minute. The linearity of the calibration curve ranged from 1–6 ?g/ml of Thiocolchicoside and 15–90?g/ml of Etoricoxib and the regression coefficient (r2) was 0.999 for both Etoricoxib and Thiocolchicoside drugs.(53)
CONCLUSION
The current review article offers a thorough understanding of the many analytical and bioanalytical techniques both singular and combined developed for etoricoxib. Numerous novel analytical techniques, including as UV spectroscopy, UPLC, HPLC, and HPTLC, have been published for analysis purposes. The method has been tabulated and includes information regarding the mobile phase, stationary phase, retention time, etc. for the researchers' convenience. Future analytical techniques for the bio-analysis of etoricoxib in pharmacological and biological formulations can be developed using the information acquired. In conclusion, it offers an opportunity to gain additional insight into past achievements and prospective future initiatives and modifications aimed at expanding our understanding of etoricoxib.
CONFLICT OF INTEREST
The authors declare that no conflict of interest
ABBREVIATIONS
- UV/VIS – Ultra violet/visible spectroscopy
- HPLC – High-performance liquid chromatography
- HPTLC – High-performance thin layer chromatography
- LC-MS/MS – Liquid chromatography-mass spectroscopy-mass spectroscopy
- UPLC – Ultra performance liquid chromatograpy
- RP – Reverse phase
- nm – Nanometer
- ?g/mL – Micro gram per Milliliter
- PDA - Photo diode array
- VDX – Valdecoxib
- SCA – Salicylic acid
- KPF – Ketoprofen
- NMS – Nimesulide
- CXB – Celecoxib
- RLZ – Riluzole
- DRT – Drotraverine
- THC – Thiocholchicoside
- PCT – Paracetamol
- PGBN – Pregabalin
- TOP – Tolperisone
REFERENCE:
- Niederberger, E., Manderscheid, C., Grösch, S., Schmidt, H., Ehnert, C. and Geisslinger, G., 2004. Effects of the selective COX-2 inhibitors celecoxib and rofecoxib on human vascular cells. Biochemical pharmacology, 68(2), pp.341-350.
- Starek, M., Krzek, J. and Dechnik, J., 2009. Separation and determination of rofecoxib and its degradation products by TLC chromatography. Journal of Analytical Chemistry, 64, pp.623-631.
- Ramakrishna, N.V.S., Vishwottam, K.N., Wishu, S. and Koteshwara, M., 2005. Validated liquid chromatographic ultraviolet method for the quantitation of etoricoxib in human plasma using liquid–liquid extraction. Journal of Chromatography B, 816(1-2), pp.215-221.
- https://go.drugbank.com/drugs/DB01628
- Rajan, D.S., Bose, A., Gowda, K.V., Ghosh, A. and Pal, T.K., 2006. Development and validation of an HPLC method for analysis of etoricoxib in human plasma. Indian journal of pharmaceutical sciences, 68(4).
- Shakya, A.K. and Khalaf, N.A., 2007. High performance liquid chromatographic determination of Etoricoxib in human plasma. Asian Journal of Chemistry, 19(7), p.5241.
- Radwan, M.A., Zaghloul, I.Y. and Abd Elbaky, N.A., 2009. Stability indicating high performance liquid chromatographic assay for the pharmacokinetics of cyclooxygenase (COX-2) inhibitor etoricoxib in rats. African Journal of Pharmacy and Pharmacology, 3(7), pp.339-346.
- Dalmora, S.L., Brum Junior, L., Ferretto, R.M., Oliveira, P.R.D., Barth, T. and Sangoi, M.D.S., 2008. Determination of etoricoxib in human plasma using automated on-line solid-phase extraction coupled with LC-APCI/MS/MS. Química Nova, 31, pp.574-578.
- Bräutigam, L., Nefflen, J.U. and Geisslinger, G., 2003. Determination of etoricoxib in human plasma by liquid chromatography–tandem mass spectrometry with electrospray ionisation. Journal of Chromatography B, 788(2), pp.309-315.
- Jalakam, S.P., Waghmode, J., Pawar, P. and Mane, G., 2016. Development of Simple and Rapid LC-MS/MS Method for Determination of Etoricoxib in Human Plasma and its Application to Bioequivalence Study. Biomirror, 7.
- Brum Junior, L., Cátia Ceni, D., Fronza, M., Renato de Oliveira, P. and Luiz Dalmora, S., 2006. Validation of an LC?tandem MS/MS method for the determination of etoricoxib in human plasma and pharmaceutical formulations. Journal of liquid chromatography & related technologies, 29(1), pp.123-135.
- Werner, U., Werner, D., Hinz, B., Lambrecht, C. and Brune, K., 2005. A liquid chromatography–mass spectrometry method for the quanti?cation of both etoricoxib and valdecoxib in human plasma. Biomedical Chromatography, 19(2), pp.113-118.
- Pavan Kumar, V.V., Vinu, M.C., Ramani, A.V., Mullangi, R. and Srinivas, N.R., 2006. Simultaneous quantitation of etoricoxib, salicylic acid, valdecoxib, ketoprofen, nimesulide and celecoxib in plasma by high?performance liquid chromatography with UV detection. Biomedical Chromatography, 20(1), pp.125-132.
- Zhang, X., Guo, N., Ji, W. and Wen, Q., 2019. Rapid quantitative analysis of etoricoxib in human plasma by UPLC?MS/MS and application to a pharmacokinetic study in Chinese healthy volunteers. Biomedical chromatography, 33(2), p.e4414.
- Eure, W.D., Grossman, R.G., Horner, P.J. and Chow, D.S.L., 2021. LC-MS/MS assay of riluzole and etoricoxib in rat plasma and brain tissue with applications for sampling and evaluation in pre-clinical rat model of traumatic brain injury. Talanta Open, 4, p.100052.
- Shahi, S.R., Agrawal, G.R., Rathi, P.B., Shinde, N.V., Somani, V.G., Mahamuni, S.B. and Padalkar, A.N., 2008. Development and validation of UV spectrophotometric method for the determination of etoricoxib in bulk and tablet formulation. Rasayan J Chem, 1(2), pp.390-394.
- Chaple, D.R. and Bhusari, K.P., 2009. Spectrophotometric Methods for the Determination of Etoricoxib in Pharmaceutical Formulations. Research Journal of Pharmacy and Technology, 2(3), pp.597-8.
- Manideep, G., Shane, N.L.J., Pai, G. and Sathyanarayana, M.B., 2018. Development and validation of a UV spectroscopic method to estimate Etoricoxib in bulk and tablet formulation. Research Journal of pharmacy and Technology, 11(2), pp.758-760.
- Singh, S., Mishra, A., Verma, A., Ghosh, A.K. and Mishra, A.K., 2012. A simple Ultraviolet spectrophotometric method for the determination of etoricoxib in dosage formulations. Journal of advanced pharmaceutical technology & research, 3(4), p.237.
- Choudhari, V.P., Parekar, S.R., Chate, S.G., Bharande, P.D. and Kuchekar, B.S., 2011. Development and validation of UV-Visible spectrophotometric baseline manipulation methodology for simultaneous analysis of drotraverine and etoricoxib in pharmaceutical dosage forms. Pharmaceutical methods, 2(4), pp.247-252.
- Acharjya, S.K., Rajesh, Y., Panda, P., Mallick, P. and Annapurna, M.M., 2010. Spectrophotometric methods for simultaneous estimation of etoricoxib and thiocolchicoside in bulk and combined pharmaceutical dosage form. Journal of Pharmaceutical Education and Research, 1(1), p.75.
- Brum Jr, L., Fronza, M., Ceni, D.C., Barth, T. and Dalmora, S.L., 2006. Validation of liquid chromatography and liquid chromatography/tandem mass spectrometry methods for the determination of etoricoxib in pharmaceutical formulations. Journal of AOAC International, 89(5), pp.1268-1275.
- Gangane, P.S., Bagde, S.M., Mujbaile, S.G., Niranjane, K.D. and Gangane, P., 2014. Development and Validation of HPLC assay method for etoricoxib in bulk drug and tablet formulation. Indian J Nat Sci, 4(24), pp.1565-1625.
- Haque, M., Nasrin, S., Monir, M.M., Rahman, M.M. and Chowdhury, S., 2012. Method development and validation of RP-HPLC method of etoricoxib in bulk and its tablet dosage forms. American Journal of PharmTech Research, 2(6), pp.275-283.
- Singh, B., Santhakumar, R., Bala, I., Prasad, S.B. and Verma, S., 2014. Development and validation of RP-HPLC method for the dissolution and assay of etoricoxib in pharmaceutical dosage forms. International Journal of Pharmaceutical Quality Assurance, 6(1), pp.1-7.
- Bhattacharyya, I., Bhattacharyya, S.P., Sen, S. and Laha, T.K., 2009. Reverse Phase High Performance Liquid Chromatographic Method for the Analysis of Etoricoxib in Pharmaceutical Dosage Form. Asian Journal of Research in Chemistry, 2(3), pp.297-299.
- Patel, H.M., Suhagia, B.N., Shah, S.A. and Rathod, I.S., 2007. Determination of etoricoxib in pharmaceutical formulations by HPLC method. Indian Journal of Pharmaceutical Sciences, 69(5), p.703.
- Rao, B.S. and Nagaraju, K.S., development, validation & stress degradation studies of etoricoxib using diclofenac as an internal standard by hplc.
- Shakya, A.K. and Khalaf, N.A., 2007. High Performance Liquid Chromatographic and Ultra Violet Spectroscopic Determination of Etoricoxib in Pharmaceutical Formulations. Asian Journal of Chemistry, 19(3), p.2059.
- Bagade, S.B., Meshram, D.B. and Tajne, M.R., 2011. Estimation of Etoricoxib in tablet Dosage form by RP-HPLC using Internal Standard with Emphasize on Specificity parameter Method. Oriental Journal of Chemistry, 27(2), p.697.
- Topalli, S., Chandrashekhar, T.G. and Annapurna, M.M., 2012. Validated RP-HPLC method for the assay of etoricoxib (a non-steroidal anti-inflammatory drug) in pharmaceutical dosage forms. E-Journal of chemistry, 9(2), pp.832-838.
- Venugopal, S., Tripathi, U.M. and Devanna, N., 2011. Validated Reverse Phase HPLC Method for the Determination of Impurities in Etoricoxib. E-Journal of Chemistry, 8(S1), pp.S119-S126.
- Palte, D. and Kondalkar, A., 2015. Stability studies in combine dosage form of Etoricoxib and Thiocolchicoside using RP-HPLC. Int J Res Stud Biosci, 3(9), pp.163-70.
- Singh, B., Chaudhary, A. and Sharma, A., 2022. RP HPLC Method Development for Simultaneous Estimation of Etoricoxib and Thiocolchicoside. Journal of Pharmaceutical Research International, pp.39-44.
- Zaveri, M. and Khandhar, A., 2010. Quantitative determination of Etoricoxib and Paracetamol in pharmaceutical dosage form and in-vitro comparison by reversed-phase high performance liquid chromatography (RP-HPLC). Asian Journal of Pharmaceutical Research and Health Care, 2(4).
- Kumar, S., Joshi, A., Thakur, R.S., Pathak, A.K. and Shah, K., 2011. Simultaneous estimation of etoricoxib and thiocolchicoside by RP-HPLC method in combined dosage forms. Acta Poloniae Pharmaceutica, 68(6), pp.839-843.
- Narajji, C. and Karvekar, M.D., 2011. Method development and validation for simultaneous estimation of Paracetamol and Etoricoxib in pharmaceutical dosage form by RP-HPLC method. Der Pharma Chem, 3(4), pp.7-12.
- Yeluri, R.R., Reddy, B.S. and Kumari, R.R., 2022. Quantification of pregabalin and etoricoxib combo in tablets and bulk with developed rp-hplc method: stability indicating feature assessment. Journal of Advanced Scientific Research, 13(04), pp.31-36.
- Padmavathi, K. and Rao, M.S., 2016. Development and validation of a new stability indicating liquid chromatographic method for the simultaneous determination of thiocholchicoside and etoricoxib in combined dosage form. World Journal of Pharmaceutical Sciences, pp.76-84.
- Chaudhary, A. and Singh, B.K., 2021. Simultaneous Estimation of Pregabalin and Etoricoxib using Novel HPLC Method: An Application in Quantitative Analysis of Pharmaceutical Dosage Forms. Indian Journal Of Pharmaceutical Education And Research, 55(3), pp.S837-S843.
- Rao, K.P. and Ramana, G.V., 2014. Cost effective isocratic RP-HPLC method for simultaneous determination of Etoricoxib and Paracetamol in pure and in tablet formulation. J Advan Stud Agric Biol Environ Sci, 1(2), pp.201-209.
- Andraws, G. and Trefi, S., 2020. Ionisable substances chromatography: A new approach for the determination of Ketoprofen, Etoricoxib, and Diclofenac sodium in pharmaceuticals using ion–pair HPLC. Heliyon, 6(8), p.e04613.
- Pattan, S.R., Jamdar, S.G., Godge, R.K., Dighe, N.S., Daithankar, A.V., Nirmal, S.A. and Pai, M.G., 2009. RP-HPLC method for simultaneous estimation of paracetamol and etoricoxib from bulk and tablets. Journal of Chemical and Pharmaceutical Research, 1(1), pp.329-335.
- Rani, K.S. and Parameshwar, P., 2021. Method development for simultaneous estimation of etoricoxib and thiocolchicoside in tablet formulation by rp-hplc.
- Goudar, N., Tejas, B., Badamane, M., Sathyanarayana, A.P. and Pai, V., 2022. Quantitative determination and validation of etoricoxib and paracetamol combined tablet dosage form by reverse phase-hplc. Rasayan Journal of Chemistry, 15(3), pp.1702-1708.
- Gupta, K.R., Likhar, A. and Wadodkar, S.G., 2010. Application of stability indicating HPLC Method for quantitative determination of etoricoxib and paracetamol in pharmaceutical dosage form. Eurasian J. Anal. Chem, 5(3), pp.218-226.
- Solanki, R.V., Patel, R.B., Patel, R.K. and Sheth, R.A., 2022. Development and Validation of Fast and Robust Stability Indicating RP-HPLC Method for Simultaneous Estimation of Tolperisone Hydrochloride and Etoricoxib in Pharmaceutical Dosage Form. International Journal of Pharmaceutical Investigation, 12(1), pp.56-61.
- Sumithranandan, E.S.N. and Ajitha, M., 2022. A new validated method for the estimation of pregabalin and etoricoxib an using high performance liquid chromatography and of its degradation: https://doi. org/10.54037/WJPS. 2022.101001. World Journal of Pharmaceutical Sciences, pp.1-11.
- Shah, N.J., Shah, S.J., Patel, D.M. and Patel, N.M., 2006. Development and validation of HPTLC method for the estimation of etoricoxib. Indian journal of pharmaceutical sciences, 68(6), p.788.
- Maheshwari, G., Subramanian, G.S., Karthik, A., Ranjithkumar, A., Ginjupalli, P.M.K. and Udupa, N., 2007. High-performance thin-layer chromatographic determination of etoricoxib in the bulk drug and in pharmaceutical dosage form. JPC–Journal of Planar Chromatography–Modern TLC, 20(5), pp.335-339.
- Dhaneshwar, S.R., Raut, K.O. and Bhusari, V.K., 2011. Validated HPTLC Method for Simultaneous Estimation of Paracetamol and Etoricoxib in Bulk Drug and Formulation. Asian Journal of Pharmaceutical & Biological Research (AJPBR), 1(2).
- Rajmane, V.S., Gandhi, S.V., Patil, U.P. and Sengar, M.R., 2010. High-performance thin-layer chromatographic determination of etoricoxib and thiocolchicoside in combined tablet dosage form. Journal of AOAC International, 93(3), pp.783-786.
- Shetgar, S.S., Dharmasoth, R., Rao, B.M. and Keloth, B., 2022. RP-UPLC method development and validation for simultaneous estimation of Etoricoxib and Thiocolchicoside in tablets. Journal of Applied Pharmaceutical Science, 12(2), pp.144-151.


 Shweta Vinayak Rane* 1
Shweta Vinayak Rane* 1








 10.5281/zenodo.11203913
10.5281/zenodo.11203913