Abstract
Topical drug delivery system is good drug delivery system, in which drug directly contact with skin produces its pharmacological effect to targeted site to cure and treat various disorder. Emulgels is an emerging topical drug delivery system that will prove boon to skin care and cosmetology. Emulgels are prepared by the incorporation of the emulsion into the gel with constant stirring at a moderate speed. Emulsions are either available in an oil in water or water in oil type. Incorporation of emulsion into a gel makes it a dual control release system, increasing its stability and provides better drug releases. Due to presence of gel phase which enhances patient compliance. Emulgels contains permeation enhancers to overcome permeation problems and improve transmission of drug into skin. Emulgels are non-greasy, improves bioavailability, patient compliance and stability over than other semi solid dosage forms like lotions, creams, gels, emulsions and ointments. Emulgels is used to treat aches and pains caused by colds, headaches, muscle, aches, backaches, arthritis and other conditions. The prepared emulgels are evaluated by various tests - PH, viscosity, particle size, zeta potential, spread ability, swelling index, drug content, stability study, skin irritation test, Extrudability test, ex vivo permeation test. This review gives knowledge about emulgels including its properties, advantages, formulation components, evaluation tests and applications.
Keywords
Emulgels, Emulsion, Gelling agent, Topical drug delivery.
Introduction
Over the last decade, scientist and industrial researchers have become more interested in pharmaceutical semisolid dosage forms, particularly emugel. The skin is a key site for systemic and local drug administration [1] .Many kind of drugs are employed in medical practice for their action upon the skin and mucous membrane[2]..Topical drug delivery as the application of a drug, containing formulation to the skin to treat cutaneous disorder directly. The topical drug delivery system is generally used where other routes [like oral, sublingual, rectal, parenteral] of drugs administration fails or in local skin infection [3] .These are applied as a wide spectrum of preparations in case of both cosmetic and dermatological to the healthy or diseased sink [4] .Topical formulations are prepared in different consistency such as solid, semisolid and liquid [5].
Advantages:
Topical drug delivery system several advantages such as ability to deliver drug more selectively to a specific site, avoidance of gastro intestinal incompatibility and metabolic degradation associated with oral administration more over topical deliveries provide an increased bio-availability by avoiding first pass metabolism by liver and a consistent delivery for extended period[6] .The main advantage of the topical drug delivery system is to bypass, first pass metabolism avoidance of the risks and inconveniences of intravenous therapy and of the varied conditions of absorption, like PH changes, the presence of enzymes, gastric emptying time are another advantage of the topical drug delivery system is generally used where the others system of drug administration fails [7] .Topical drug delivery system will be used considerable to improve patient compliance[8].The topical drug delivery system is failed in the administration of hydro phobic drugs .In each formulation with the active ingredients many excipients are used. Some times more than one formulation can be combined to enhance the drug delivery; emulgels is such type of combination. Emulgel is combination of emulsion and gel [9] .
Gels:
According to USP defines gels as the semisolid system consisting of dispersion of made up of either small inorganic particle or large organic molecule enclosing and interpenetrated by liquid [10].Gels have many favourable properties like spread ability, non-staining ,greaseless and thixotropic but a major drawback in delivering hydrophobic drugs to the skin. Active ingredients with a hydrophobic nature exhibits improper drug release .In gels due to lack of solubility in the aqueous phase, they are not suitable to be added into the gel base. Therefore to residue there drawback, emulsion-gel based drug delivery system are being used [11].
Emulsion:
Emulsions are thermodynamically unstable biphasic dosage forms consisting of two liquids ,one of the uniformly dispersed as globules [phase-1]throughout the second phase [external phase].Emulsion allow the incorporation of hydrophobic medicinal agents into the oil phase which facilitates the dispersion of oil globules in the aqueous phase and produces an oil-in-water [o/w] emulsion. These are capable of acting as drug delivery system where the Medicinal agent to be delivered is stored inside the oil phase. This internal oil phase of an emulsion will function as a drug reservoir and the drug will be released to the skin in a controlled manner. The major disadvantage is there reduced contact time on the skin surface both gel and emulsion individually possess many advantages but due to inherent drawbacks. Mother dosage form superior to each preparations was identified and thus the discovery of emulgel was made [12].
Emulgel:
1. Emulgel is a preparation that comprises both emulsion and gel.
2. The emulsion type used can be oil-in water [o/w] or water –in –oil [w/o], which is eventually mixed with a gelling agents to formulate an emulgel.
3. This type of novel formulation has been developed for topical drug delivery and has proven its suitability carrying hydrophobic drugs.
4. Emulgel possesses the characteristics of both gel and emulsion it operation controlled drug release system.
5. Emulgel generally used where the other systems of drug administration fails to directly treat cutaneous disorders such as fungal infections, acne, psoriasis etc..
6. Emulgels have been of growing importance in the field of pharmaceutical semi solid dosage forms.
7. Combination of gel & emulsion, both oil-in-water & water-in oil type of emulsion used as a vehicle to deliver various drugs to the skin [13].

Figure-1-Structure of emulgel
Types Of Emulgels:
Based on the type of API:
A] Natural / poly-herbal combination:
Ex: Anti Inflammatory, anti-fungal, Levorag emulgels, curcuactin emulgel
B] Allopathic:
EX: voltaren ,miconaz-H , penox emulgels
Based on type of emulsion:
A] Macro emulgel: size of dispersed phase droplets more than 400nm and prepared by high energy and low energy method.
B] Micro emulgel: droplet size between 1nm to 100 nm. Prepared by phase inversion and phase separation
C] Nano emulgel: droplet size less than 1nm.
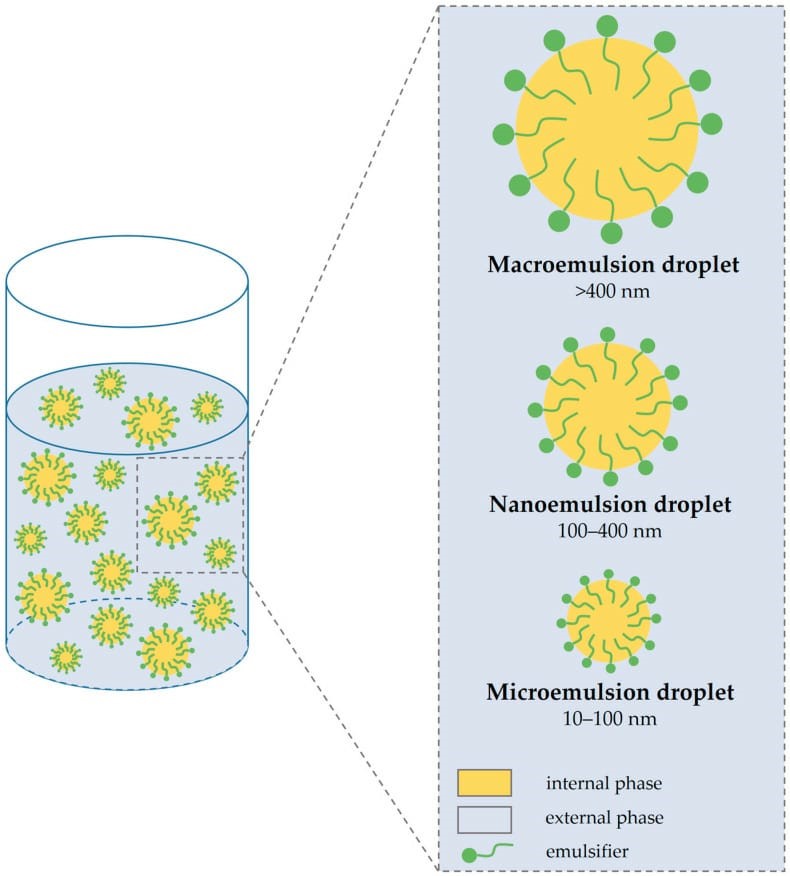
Figure-2- Types of emulgels
Advantages:
- Avoidance of first pass metabolism.
- Avoidance of gastrointestinal incompatibility.
- More selective to a specific site.
- Improve patient compliance.
- Ability to easily terminate medication when needed.
- Better stability.
- Produce feasibility and low preparation cost.
- Controlled release
- Suitability for self- medication.
- Providing utilization of drug with short biological half –life and narrow therapeutic window
- Convenient and easy to apply
- Incorporation of hydrophobic drugs
- No intensive sonication [14].
Dis Advantages:
- Skin irritation on contact dermatitis
- The possibility of allergenic reactions
- The poor permeability of some drug through the skin
- Drug of large particle size not easy to absorb through the skin
- The occurrence of the bubble during formation of emulgels [15].
Formulation Of Emulgel:
Drug:
Drug substances Mainly NSAID’s agent, antibacterial agent, antifungal agent etc can be used for delivery of drug across the skin. The reasonable choice of the drug play an important role in successful development of a topical drug delivery products. Some of desirable properties of drug that effect its diffusion through the device as well as through skin are as follow:
- Molecular weight of drug should be less than 500 Daltons.
- Drug should have better affinity for both hydrophilic and hydrophobic phases.
- Drug should have a low melting point. Drug should not be highly acidic nor alkaline in solution.
- pH of saturated aqueous solution of drug should be in range of 5 - 9.
- he drug should be potent enough.
- Half-life of drug should be short.
- Drug should not induce any allergic reactions or trauma.
- The drug should not be immunogenic.
- Drugs, which degrade in gastrointestinal tract or are inactivated by hepatic first pass effect, are suitable for topical delivery.
- Tolerance to the drug must not develop under the near zero order release profile of topical delivery.
- Drugs which have to be administered for a long time or which cause adverse effects to nontargeted tissue can also be formulated for topical delivery
Vehicles:
In emulgel preparation efficiently deposit the drug on the skin with even distribution. Release the drug so it can migrate freely to the site of action. Sustain a therapeutic drug level in the target tissue, for a sufficient duration provides pharmacological effect. Rate and extent absorption vary depending on characteristics of the vehicle, influenced the active itself.
EX:Oily and aqueous vehicles are used [16].
Aqueous Material:
This forms the aqueous phase of emulsion. The commonly used agents are distilled water and Alcohols may be used.
Eg: Distilled water, Alcohol
Oils:
These agents from the oily phase of the oily phase of the emulsion for externally applied emulsions, mineral oil, either alone or combined with soft or hard paraffin, are widely used both as the vehicle for the drug and for their occlusive and sensory characteristics.
Ex: vegetable oils, arachis oil, emu oil, fish liver oil, clove oil etc..[17].
Emulsifiers:
Emulsifying agents are used both to promote emulsification at the time of manufacture and to emulsions to months or years for commercial preparations.
EX; polyethylene glycol 40 stearate , sorbitan mono- oleate [span80], polyoxy ethylene sorbitol [tween80], steric acid ,sodium stearate etc[18]
Gelling Agents:
These are agents used to increase the consistency of any dosage form can also be as thickening agent. Gelation use full in the formulation of stable systems by reducing interfacial and surficial tension there by increasing the viscosity of the liquid phase .
Eg:HPMC, sodium CMC, Aloe Vera gel, xanthium gum . Gelatin, carboxymethyl cellulose, Carbopol-934, carbopol-940[19].
Penetration Enhancers:
In order to promote a absorption of drugs, vehicles often include penetration Enhancing Ingredients that temporarily disrupts the skin barrier, fluidize the lipid channels between corneocytes, alter the of the drugs in to the skin structures, or otherwise Enhance delivery in to skin.
EG: menthol, pyrrolidones, alcohols, sulfoxides, glycols, azoles, laurocapram, propylene glycol, surfactants, terpenes [20].
Preparation Of Emulgel:
- Step- 1: Formulation of emulsion either o/w or w/o.
- Step-2: Formulation of gel base
- Step – 3: Incorporation of emulsion into gel base with continuous stirring [21].
Formulation Of O/W [Or] W/O Emulsions:
The initial step of emulsion formulation involves the dissolution of the oil soluble substance in the oil vehicle. T he emulsification of industrial manufacturing is generally performed using mechanical stirrers, ultra sonifers , homogenizers , or colloidal mills [22] .
Formulation Of Gel Base:
To begin the water-soluble substances or excipients are dissolved in the aqueous vehicle using mechanical stirring in a mixing vessel. To avoid aggregation, the hydro phyllic polymer is slowly added to the stirred mixture , and stirring is continued until the polymer has dissolved while the PH remains within the desired range . superfluous stirring of pharmaceutical gels may result in the entrapment air ,so the mixing rate must be at a moderate phase[23-24] .
Incorporation Of Emulsion In To Gel Base With Continous Stirring :
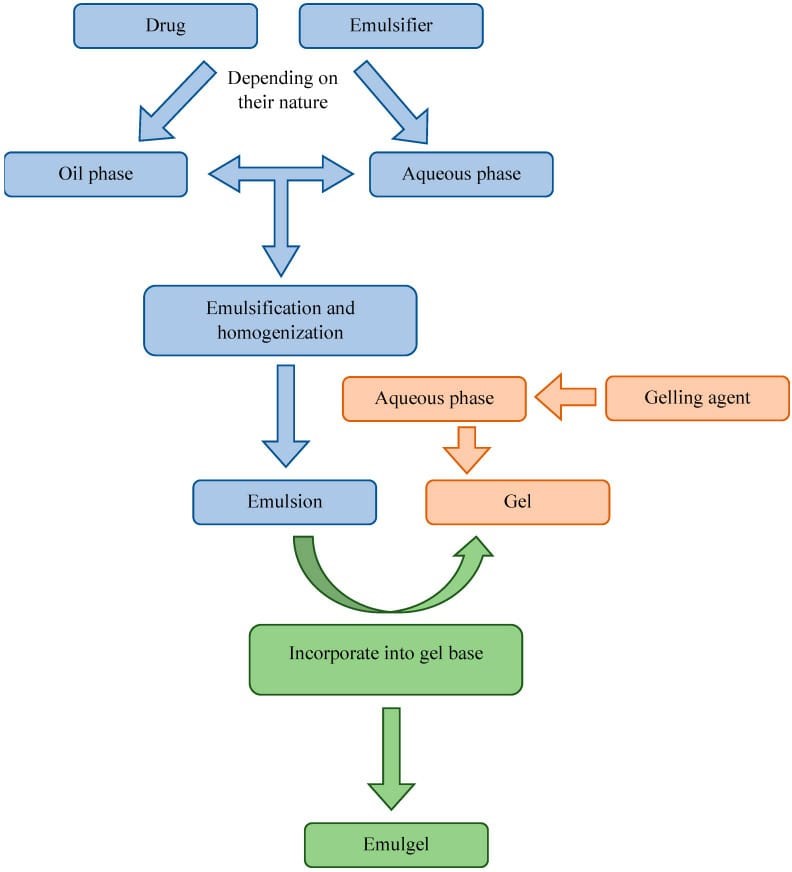
Figure-3- Steps of emulgel formation
Applications Of Emulgel:
1] Topical Applications:
Skin Care:
Emulgels are used as moisturizers , emollients and for treating conditions like roughness, dullness , irritation , ingredients like glycerin ,acetyl alcohol ,chamomile extract, aloe Vera etc. are incorporated.
Hair Care:
Emulgels containing proteins, oils, keratins, etc. are used as conditioners, styling agents, treatment for dandruff etc. coconut oil and glycerin are commonly used.
Cosmetics:
Emulgels are used as foundations, lip balms, sunscreen, etc. Ingredients include pigments, waxes, silicon, emulsifiers, etc. for desired effects. Sun protection factor depends on the emulgent and sunscreen used [25].
2] Parenteral Applications:
Emulgels provide sustained release of drugs through injection. They release drugs over prolonged periods, reducing dosing frequency and maintaining adequate drug levels. Water soluble corticosteroids and antibiotics are commonly incorporated.
Ex: sustained release injectable, prolonged anesthetics, anti-cancer emulgels, vitamin emulgels, cosmeceuticals, improved nutrition [26] .
3] Oral Applications:
Emulsion –based oral drug delivery system include;
Emulgels:
Emulgels contain both emulsions and gels for controlled release of drugs .oil –in-water or water-in –oil emulsions are used based on solubility of drug.
Liquid Filled Gelatin Capsule [Lfgc]:
Emulsions contained within gelatin capsules. LFGC s provide floating, sinking remained buoyant to release drug at specific sites. Used for site –specific release.
Emulsifying Drug Delivery Systems [Sedds]:
Contain emulsifiers and solvents to produce fine o/w or w/o emulsion upon dispersion in aqueous media with low energy. Improve solubility, absorption and bio availability of drug.
Microemulsion:
Thermodynamically stable, isotropic and have a droplet size below 100 nm. Micro emulsions provide maximum surface area for absorption and enhance solubility. They are suitable for lipophilic, amphiphilic and hydrophobic drugs [27] .
Others:
- Emulgel possesses many promising properties for dermatological use such as being greaseless, easily removable, and easily spreadable.
- Emulgels are emollient ,non – staining ,
- These have longer shelf – life , transparent
- These are also having elegant appearance and thixotropic
- Having less potential to cause serious side effects
- Anti-inflammatory activity
- Analgesic activity
- To impart better patient compliance
- This novel drugs delivery become more popular
- Helps to delivery of hydrophobic drugs via skin
- Show dual release control system.
Evaluation Of Emulgels:
Physical appearance:
he color, consistency and homogeneity of the prepared formulation are visually inspected for observations of physical properties [28].
Measurement of PH:
The PH values of solutions and prepared gels were measured by a digital PH meter. Calibration of the pH meter is performed before using a standard buffer solution. 1 gm of the formulation is dissolved in distilled water until a uniform suspension is formed and is kept aside for 2 hours. After 2 hours the glass electrode is dipped in the suspension and the pH is measured [29].
Globule Size Disribution:
Globule size distribution was determined by Malvern zeta-sizer [30].
Drug content determination:
Drug concentration in gel based emulsion was determined by spectrophotometer .Drug content in gel based emulsion was measured by dissolving known quantity of gel based emulsion in solvent [methanol] by sonication [31].
Swelling index:
For determination of swelling index:
[sw % ] = [(wt-w0)/w0. 100]
Where,
W0= initial weight of emulgel at zero time
Wt.=weight of swollen emulgel after time(t)
[Sw]%= percent swelling index [32]
Spread ability:
Spread ability is measured on the basis of ‘slip’ and ‘drag’ characteristics of emulgel.spread ability was calculated by using the formula
S=m.l/t
Where,
S = spread ability
M =weight tied to upper slide
L = length of glass slide
T = time taken to separate the slides completely from each other [33].
Extrubability study of emulgel [tube test] :
Extrubability test is based upon the extrude 0.5cm ribbon of emulgel in 10 sec from lacquered collapsible aluminum tube. The test was performed in triplicate and the average values were calculated.
Extrubability = applied to extrude emulgel from weight tube [gm.] /Areas [cm2] [34].
Rheological study:
The viscosity of the different emulgel formulations is determined at 25 degrees C using a cone and plate viscometer OR Brooke field viscometer. [35].
Skin irritation test:
The preparation is applied on the properly shaven skin of rat or mice or goat and its adverse effects like change in colour, change in skin morphology should be checked up to 24 hours. If the skin irritation symptoms occur in more than 2 rats the study should be repeated.The vitro release study of the formulated emulgels was carried out by using franz diffusion cell with dialysis membrane.In which the collected samples are analyzed by U.V spectrophotomer [36].
in – vitro drug release study:
The in vitro release study of the formulated emulgels was carried out by using franz diffusion cell with dialysis membrane.In which the collected samples are analyzed by U.V spectrophotometer [37].
EX –vivo permeation studies:
Male rats weighing 105 -120 gm. free from any visible sign of disease were selected using a depilatory preparation hair was removed from the skin and the cleared area was washed thoroughly with distilled H20 .Abdominal skin of full thickness was excised from the rat .This was mounted on the donor compartment .The emulgel was placed over it and the permeation study was carried out in the similar manner as that with artificial]. membrane [38]
Stability studies:
The optimized emulgel formulation was selected for stability study .Sufficient quantity of emulgel formulation was selected to stability studies at 5,25,60%RH ,30 65%RH &40/75% RH for a period of 3 months .The samples were analyses at pre-determined time intervals for PH .Physical appearance , Rheological properties and drug content [40].
Kinetics modelling:
Data obtained from ex-vivo permeation studies were fitted in to zero order, first order Higuchi and mathematical models for evaluation of drug release kinetics [41].
Zeta potential
The Zeta potential of the emulgel preparation is determined by zetasizer (Malvern Zetasizer) The formulation is placed in a clear, disposable zeta cell, and the result is determined. Before experimenting, cuvettes are washed with methanol and then the sample is placed[42].
Particle size and polydispersity index (PDI)
The globule size of emulgel is measured at 250 C by using a zetasizer (Malvern zetasizer instrument, ZS90). The sample is diluted before the experiment [43].
Microbiological assay:
For this method Ditch plate technique is used. Through this method the bacteriostastic or fungistastic activity is evaluated.
Packaging Of Emulgels [44]
Packaging of emulgels are usually done in membrane sealed lacquered aluminium tube with inner coating of a phenoxy-epoxy based lacquer closed with propylene screw cap or an aluminium laminated tubes closed by a moulded seal, with a propylene screw cap.
Material for laminates tubes
1. Foil laminates It provides light, air and moisture barrier.
2. All plastic laminates
Marketed Products:
|
S. No
|
Active Ingredient
|
Marketed Products
|
Thrapeutic Uses
|
|
1
|
Diclofenac –di ethyl –ammonia
|
Voltaren emulgels
|
Muscolo skeletal disorder strain, sprain, arthritis, continuation post traumatic pain, low back pain.
|
|
2
|
Miconazole nitrate, hydrocortisone
|
Miconaz-H- emulgels
|
Eczema and dermatitis
|
|
3
|
Clindamycin, adapalene
|
Exec gel
|
To treat acne [pimples].
|
|
4
|
Benzoyl peroxide
|
Pernox gel
|
To treat acne , anti septic gel or face wash containing 5% BP
|
|
5
|
Metronidazole, clindamycin
|
Lupigyl gel
|
To treat skin lesions caused by rosacea.
|
|
6
|
Clindamycin phosphate, Allantion
|
Clinagel
|
To treat inflamed acne, minimize the formation of excessive natural oil like sebum, reduce swelling due to acne.
|
|
7
|
Clobestasole propionate
|
Topinate gel
|
To help relieve redness, itching, swelling or other discomfort caused by certain skin condition.
|
|
8
|
Koji acid, dipalmitate
|
Kojivit gel
|
It is used as a skin –lightening agent to lightening the darkened skin due to sun damage, freckles, and age spots. It also reduces the formation of wrinkles and fine lines . It further improves skin tone and texture by preventing skin discoloration.
|
|
9
|
Aceclofenac
|
Acentgel
|
Treatment of rheumatoid arthritis and osteoarthritis.
|
|
10
|
Azithromycin
|
Avindo gel
|
Azithromycin is used to treat bacterial infections, that is amicrolide type of anti biotic, it works by stopping the growth of bacteria .This medication will not work for viral infections [such as flu, cold.
|
|
11
|
Clotrimatozle , Beclomethasone
|
Clobengel
|
To treat fungus infections help to relieve redness, swelling, itching and other discomfort of fungus infections.
|
|
12
|
Nadifloxacin
|
Nadicin cream
|
The treatment of bacterial infections ,treat stuff like acne and infection on the skin ,inhibiting the activity of DNA gyrase ,a bacterial enzyme ,prohibition bacterial cells from dividing and repairing ,resulting in their death.
|
|
13
|
Tezarotene
|
Zorotene gel
|
Treat psoriasis, plague psoriasis, [making the skin less red and reducing the number and size of lesions of the skin.
|
|
14
|
Hibiscus ,liqourise and natural extracts
|
Levorag emulgels
|
Applying a topical gel containing liquorise root extract significantly improved eczema, also used acne research on its effectiveness is mixed and quite limited.
|
|
15
|
Diclofenac sodium
|
Penn said
|
To treat pain and other symptoms of arthritis of the joints ex: osteo arthritis such as inflammation, swelling, stiffness and joint pain.
|
|
16
|
Ethylhexyl methoxy cinnamate
|
Byland’s emulgels
|
Sunscreen ,UV+IR protection
|
|
17
|
Curcumin and boswellia serrata extract
|
Curcuactin emulgels
|
Increase the efficacy of OA treatment
|
CONCLUSION:
Emulgel is a novel approach that has been proven to be the most convenient, superior and efficient delivery system. Because of its non-greasy nature and lack of oil bases it gives excellent drug release when compared to conventional topical drug delivery system. Emulgel has a high drug loading capacity and is effective in drug delivery at the target site .These formulation possessed many advantages with very few undesired side effects .They offer sustained release ,improved stability ,enhanced cosmetic properties and versatile applications in drug and cosmetics delivery emulgels .Mainly the hydrophobic drug formulation can be developed using emulgel technique because it contain both oil and aqueous phase while hydrogels are not suitable for hydrophobic drugs. In the future, there physical and physico chemical properties will be utilized to deliver a greater number of topical, oral and parenteral medications, such as emulgel .Topical drug delivery system will be used extensively due to better patient compliance.
ACKNOWLEDGEMENT:
Authors are thankful to Adarsa college of pharmacy, G. kothapalli.
REFERENCES
- Akshara k, soniwala M, Shah K. Emulgel: a novel drug delivery system .J Pakistan ASSOC Dermatologist. 2016; 26[3]; 244-9 .
- Krantz c. jhon: Pharmacological principles of medical practice. 7ted. The William and Wilkins Company, 1969, 751.
- Kullar R, Saini S, Steth N, and Rana AC. Emulgel a surrogate approach for topical used hydrophobic drugs. Int J Pharm Biol Sci 2011; 1:117-28.
- Single V, Saini S, Joshi B, and Rana AC. Emulgel: a new platform for topical drug delivery. Int J Pharm Biol Sci 2012; 3:485-98.
- Sreevidya VS an Overview on Emulgel. Int J Pharm Phytopharmacological Res. 2019; 9(1) 92-97.
- Ajazuddin. Alexenderamit, KhichariyaAjita, Gupta Saurabh Patel Ravish.J, Giri Kumar Tapanand Tripathi Krishna Dulal: Recent expansion in an novel drug delivery emergent novel technology: Emulgel, J Control Release, 2013,171, 122-132.
- Single V, Saini S, Joshi B, and Rana AC. Emulgel: a new platform for topical drug delivery. Int J Pharm Biol Sci 2012; 3:485-98
- Aggarwal G,Nagpal M, Pharmaceutical polymer gels in Single V, Saini S, Joshi B, Rana AC. Emulgel: a new platform for topical drug delivery . Int J Pharm Biol Sci 2012; 3:485-98
- Sreevidya VS an Overview on Emulgel. Int J Pharm Phytopharmacological Res. 2019; 9(1) 92-97
- Bhoyar N, Giri T.K, Tripathi D.K. Alexender.A and Ajazuddin: Recent advances in novel drug delivery system through gels: review, J. Pharm. allied health Sci, 2012, 2(2), 21-39.
- Ceve G, Mazgareanu S. Rother M. Preclinical characterization of NSAIDs in ultradeformable carriers’ orconventional topical gels. Int J Pharm. 2008; 360(1-2): 29-39.
- Panwar AS, Upadhyay N. Batrag M. Cater G, EMULGEL A REVIEW Asian J Pharm Life SC 2011;1[3]:333-43.
- Phad AR, Dilip NT, Ganapathy RS. Emulgel: A comprehensive review for topical delivery of hydrophobic drugs. Asian J pharma . 2018:12[2]:382-93.
- Mishra AM. Controlled and novel drug delivery. 4 ed. CBS Publisher and Distributers, Delhi, 1997 . p. 107-9.
- Mishra AM. Controlled and novel drug delivery. 4 ed. CBS Publisher and Distributers, Delhi, 1997 . p. 107-9..
- Bonacucina G. Cespi M, Palmieri GF Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS Pharm sci tech, 2009; 10(2): 368-375.
- Vyas, S.P: Knar, R.K. Controlled Drug Delivery. 1st Ed. Vallabh Prakashan, 2002;416-417 .
- Vilasau J, Solans C, Gomez M, Dabrio J and Esquena J. Influence of a mixed ionic/nonionic surfactant system and the emulsification process on the properties of paraffic emulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011: 384(1-3): 473-481. Eugine L, Soosai PR, Sangameswaran B. Short Review- Emulgel. Journal on Comprehensive Pharmacy, 2016; 3(1): 34-37.
- Zhang XL, Zhao R, Qian W. Preparation of an emulgel for treatment of aphthous ulcer on the basis of carbomers. Chin Pharm J. 1995; (30); 417-418.
- Singla V, Saini S, Joshi B. Rana A. Emulgel: A new platform for topical drug delivery. Int J Pharma and Bio Sci, 2012; 3(1):485-498.
- Singla V, Saini S, Joshi B. Rana A. Emulgel: A new platform for topical drug delivery. Int J Pharma and Bio Sci, 2012; 3(1):485-498.
- Hardenia A. Jayronia S, Jain S. Emulgel: An Emergent Tool In Topical Drug Delivery. Int J Pharm Sci Res. 2014;5(5): 1653-60.
- Latha SM, Sridevi G. Role of Polymers as Gelling Agents in the Formulation of Emulgels. Polym Sci. 2016; 2(1): 1- 0. .
- Latha SM, Sridevi G. Role of Polymers as Gelling Agents in the Formulation of Emulgels. Polym Sci. 2016; 2(1): 1- 0. 1
- Y. de Lafuente, A. Ochoa-Andrade, M.E. Parente, M.C. Palena, A.F. Jimenez-Kairuz, Preparation and evaluation of
- BG. Marti-Mestres, F. Nielloud, Emulsions in health care applications-an overview, Journal of Dispersion Science and Technology. 23 (2002) 419-439.
- S.K. Yadav, M.K. Mishra, A. Tiwari, A. Shukla, Emulgel: a new approach for enhanced topical drug delivery, Int J Curr Pharm Res. 9 (2016) 15-19 https://doi.org/10.22159/jcpr 2017v91.16628..
- Prasad B, Tyagi Y, Raghavendra NG. A Review on Emulgel: The Topical Drug Delivery System, World J Pharm and Life Sci, 2020; 6(6): 47-55.
- El-Bary AA, Shalaby S, Elaal AS. Formulation and stability of chloramphenicol gel and emulgel . Bulletin Fac Pharm, 2001: (39): 89-99.
- Nandgude T, Thube R, Jaiswal N, Deshmukh P. Chatap V. Hire N. Formulation and evaluation of pH induced in-situ nasal gel of salbutamol sulphate. International Journal of Pharmaceutical Sciences and Nanotechnology, 2018; 1: 177-82.
- Chaudhari P. Ajab A, Malpure P. Kolsure P. Sanap D. Development and i evaluation of thermorevesible in-situ nasal gel formulations of Rizatriptan ben Indian J. Pharma Edu Res, 2009; 43(1): 55-62.
- Kumar N, Saxena C. A Novel Approach for Topical Drug Delivery System-Emulgel . Trends in Pharmaceuticals and Nanotechnology, 2019; 1(2): 27-32.
- Kasliwal N, Derle D. Negi J, Gohil J. Effect of Permeation Enhancers on the release and permeation kinetics of Meloxicam Gel formulations through rat skin. Asian Jof Pharm Sci, 2008; 3(5): 193-199.
- Joshi B. Singh G, Rana A, Saini S, Singla V. Emulgel: A Comprehensive Review on the recent advances in Topical Drug Delivery, Int Res J Pharm, 2011; 2(11): 66-70.
- Masmoudi H, Piccerelle P. Le Dreau Y. Kister J. A rheological method to evaluate the physical stability of highly viscous pharmaceutical oil-in-water emulsions . Pharm Res. 2006; 23(8): 1937-1947.
- Guntupalli G, Gudelli MR, Nori LP. Manikiran SS. Studies on the Development of Promising Herbal Emulgel of Coccinia Grandis Leaf Extract for Dermatological Complications . Journal of Pharmaceutical Sciences and Research, 2019; 11(8): 2915- 2920.
- Shah M, Modi D. Shah D. A New Future Approach in Novel Drug Delivery System through Micro-emulgel: review. World J Pharmacy Pharm sci, 2016; 5(5): 243-259.
- Bhanu P vijaya, Shanmugum V and Lakshmi P K:Development and optimization of novel diclofenacemulgelfor topical drug delivery, UCP, 2011, 9(10), 1-4.
- Jain SK, Bajapi P. Modi SK, Gupta P. A Review on Emulgel, as a Novel Trend in Topical Drug Delivery System . Recent Trends Pharm Sci Res, 2019, 1(2): 30-39.
- Guideline IHT. Stability testing of new drug substances and products . QIA (R2), current step ,2023 ;4;1-24
- Singh J. Gupta S, Kaur H. Prediction of in vitro drug release mechanisms from extended release matrix tablets using SSR/R2 technique Trends Appl Sei Res. 2011; 4)400-409
- Suman D, Sangeeta, Beena K, Emulgel for topical drug delivery: A novel approach, GSC Biological, and Pharmaceutical Sciences, 2020; 11(03): 104- 114.
- Chaitali J, Vaishali K, Santosh P, “Formulation and Evaluation of Antifungal Non-aqueous Microemulsion for Topical Drug Delivery of Griseofulvin, Inventi Impact: Pharm Tech, 2015; 1: 38
- Sreevidya V.S (2019). “An overview on emulgel”, International Journal of Pharmaceutical and Phytopharmacological Research, 9 (1), pp.92-97.


 Kancharla Kameswararao*
Kancharla Kameswararao*
 Dr Md Abdul Sattar
Dr Md Abdul Sattar
 Chintakayala Sairam
Chintakayala Sairam
 Inakoti Siva Sankara Vara Prasad
Inakoti Siva Sankara Vara Prasad
 N M S Narayana Murthy
N M S Narayana Murthy



 10.5281/zenodo.14244530
10.5281/zenodo.14244530