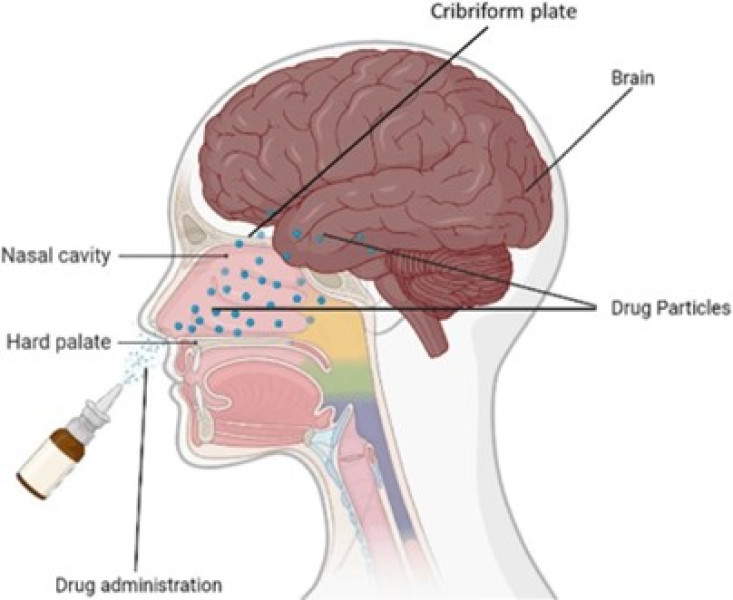
Targeted drug delivery is a way to administer medication to a patient so that more of the drug is present in some areas of the body than others. Targeted drug delivery seeks to concentrate the drug in the tissues of interest while reducing the relative concentration of the drug in the remaining tissues. The brain is a fragile organ, and nature has very efficiently protected it. However, most of the current therapeutic drugs can only relieve the symptoms of brain diseases, and it is difficult to achieve satisfactory therapeutic effect of brain diseases are mainly affected by factors like the protection conservation of blood brain barrier (BBB), blood cerebrospinal fluid barrier (BCSFB), blood tumour barrier (BTB) and the complexity of the brain microenvironment. It is important to consider not only the net delivery of the agent to the cerebrospinal fluid but also ability of agent to access the relevant target site of the CNS. Extreme research studies have recently concentrated on the development of new idea for more successfully delivery medications to the brain. In this review, we deeply describe the physiological characteristics of barriers in the brain targeted drug delivery system, mechanism of transfer of medication via BBB, difficulties faced in brain targeted drug delivery, different approaches of brain targeted drug delivery system and its application to cure the various CNS malignancies.
Brain targeted, blood brain barrier, Drug delivery system, liposomes, Nanoparticles.
Effective delivery of medication across the blood brain barrier (BBB) is major limiting factor in successful treatment of brain diseases. The BBB prevent more than 98% of small molecule drug and almost 100% of macromolecule therapeutics from entering the brain. The brain is protected against potentially toxic substances by the presence of two major barrier systems: blood brain barrier (BBB) and the blood cerebrospinal fluid barrier (BCSFB). Unfortunately, the same mechanism that protect it against intrusive chemical can also foil therapeutic intervention. The current strategies involve in field of drug delivery include development of targeted delivery in which the drug is only active in the target area of the body. Targeted drug delivery system has been developed to optimize reproductive techniques. We will try to give a thorough summary of the medication delivery methods that have been researched for the treatment of brain illnesses in this article. We will go over the BBB's alterations in brain disorders and the BBB-penetration targeting techniques. The review will also highlight the relevant delivery methods and tactics used to alter the microenvironment in order to treat brain disorders. Finally, we want to understand the design concepts behind these delivery systems so that we can use them as a guide for creating new drugs to treat brain illnesses [1].
The parameters considered optimum for a compound to transport across the BBB are as follows:
- Compound should be unionized.
- Approximately log P value must be 2.
- Its molecular weight must be less than 400 Da.
- Cumulative number of hydrogen bonds must not go beyond 8 to 10.
- It is estimated only 2% of small molecular weight drug will across BBB.[1]
Barriers to the delivery of drugs to the brain
A number of barriers that prevent drug transport to the CNS can be taken into account in order to explain why systemically administered medications are unable to successfully treat many CNS illnesses. The blood and the extracellular fluid of the brain are separated by physical barriers.
- Blood–Brain Barrier
- Blood-cerebrospinal Fluid Barrier
- Blood-Tumour Barrier
- The Blood-Brain Barrier
The blood-brain barrier (BBB), which separates circulating blood from brain extracellular fluid in the central nervous system, is a highly selective permeability barrier (Fig.1). The capillary endothelial cells that make up the blood brain barrier are joined by tight junctions that have an extraordinarily high electrical resistance of at least 0.1 m. The blood-brain barrier permits the selective movement of molecules like glucose and amino acids, which are essential for neuronal function, as well as the passage of water, certain gases, and lipid soluble compounds by passive diffusion. On the other hand, the blood-brain barrier may work as an active transport mechanism through P-glycoprotein to block the entry of lipophilic potential neurotoxins. It takes astrocytes to build the blood-brain barrier. The BBB is thought to prevent more than 98% of small-molecule medications from passing through and nearly 100% of large-molecule therapies (mostly peptides and proteins) produced for CNS disorders. Endothelial cells facilitate the passage of small hydrophobic molecules, such as O2, CO2, hormones, etc., but inhibit the diffusion of microscopic items (such as bacteria) and big or hydrophilic molecules into the cerebrospinal fluid (CSF) [2].

Fig. 1: Blood-Brain Barrier
Functions of BBB
The blood-brain barrier shields the brain from numerous common bacterial illnesses quite well. Thus, brain infections are quite uncommon. When they do occur, brain infections are frequently severe and challenging to cure. Only some antibiotics can pass across the blood-brain barrier because antibodies are too big to do so. In some circumstances, the medication needs to be injected right into the cerebrospinal fluid. The convoluted nature of the brain's interstitial space may be the reason why medications administered directly to the CSF do not successfully reach the brain tissue. Inflammation makes the blood-brain barrier more permeable. Due of this, phagocytes and certain antibiotics can pass across the BBB. However, this also makes it possible for viruses and bacteria to enter the BBB [2-4].
2. Blood-cerebrospinal fluid barrier, or BCSFB
The blood-cerebrospinal fluid barrier (BCSFB), which divides blood from cerebrospinal fluid (CSF), is another barrier preventing blood from reaching the brain (Fig.2). Since its surface area is 5000 times smaller than the BBB's, this barrier is not regarded as a primary pathway for drug absorption. The interstitial fluid of the brain parenchyma and CSF can exchange molecules, and the BCB carefully controls the entry of blood-borne molecules into the CSF. The choroids plexus epithelium, which is structured in a way that restricts the entry of molecules and cells into the CSF, is where the BCB is physiologically located. At the barriers separating the blood and CSF, the choroid plexus and the arachnoid membrane collaborate. The blood-CSF barrier is formed by the arachnoid membrane, which is typically impermeable to hydrophilic substances and plays a largely passive role in this process. The choroid plexus creates the CSF and actively controls its molecular concentration [1,4].

Fig.2: Blood-Cerebrospinal Fluid Barrier
Blood- Tumor Barrier
Delivering drugs intracranially when a CNS tumor is the target is significantly more difficult. For instance, brain metastases frequently continue to develop even when primary and secondary systemic cancers respond to chemotherapeutic drugs administered via the circulatory system. A number of physiological barriers common to all solid tumors prevent drug delivery via the circulatory system in CNS malignancies if the BBB is severely damaged. Additionally, as a tumor enlarges, the vascular surface area diminishes, which inhibits the exchange of blood-borne chemicals between the vessels (Fig.3). A greater diffusional requirement for drug delivery to neoplastic cells results from an increase in intracapillary distance at the same time as a rise in hydrostatic pressure in the healthy brain parenchyma next to the tumor due to the high interstitial tumor pressure and associated peritumoral edema. Because of this, the brain may be less drug-permeable than the typical brain endothelium [5].

Fig.3: Comparison between Blood-Brain Barrier and Blood-Tumor Barrier
Approaches for delivering drugs specifically to the brain:
The neuroprotective BBB can be opened or disrupted by osmotic imbalance, ultrasonography, or vasoactive substances (such as bradykinin or P-glycoprotein inhibitors) to actively improve medication transport to the brain following systemic administration. While the first method has the drawback of certain neurons perhaps suffering semi-permanent harm as a result of unwelcome blood components entering the brain, Positive charge has also been added to compounds or drug carriers as a third option (combining elements of both approaches) to successfully improve absorptive-mediated transport across the BBB. But to date, it hasn't been determined whether this essentially hazardous transport mechanism has a useful therapeutic window. multiple medication delivery techniques have been created in order to get over the multiple obstacles the administration of possible therapeutic substances into the CNS. These methods typically come under one or more of the following headings: invasive, non-invasive, or other procedures. The CNS drug delivery tree encompassing the various possible strategies is shown below in the Fig.4 [5,6].
Invasive Approaches
Drilling a hole in the skull can be used to transfer drugs to the brain, where they can subsequently be implanted or infused intracerebroventricularly (ICV). This approach has the benefit of allowing a large variety of compounds and formulations to be taken into account for ICV or IC administration. In order to ensure prolonged release, both big and tiny compounds can be supplied, either by themselves or in different polymer compositions. Various intrusive methods include [7,8]:
- Intra-cerebro-ventricular infusion:
It has been claimed that at merely 1-2 mm from the surface, a drug's concentration in the brain is just 1% to 2% of the CSF concentration. Via intraventricular drug infusion, drugs might be administered to the surface of the brain with ease, but not effectively to the brain parenchyma. After ICV delivery, pharmacologic effects may be observed if the drug's target receptors are found close to the brain's ependymal surface.
Limitations:
There is relatively little drug diffusion in the parenchyma of the brain. It is not a reliable way to administer drugs unless the target is close to the ventricles. Example: Glycopeptide and an aminoglycoside
antibiotics used in meningitis.
- Convection enhance delivery:
A small-calibre catheter is stereotactic inserted into the brain parenchyma as the basic idea behind CED. Infuscate is actively pushed into the brain parenchyma and permeates the interstitial space through this catheter. After many days of infusion, the catheters are withdrawn at the patient's bedside. After as little as 2 hours of continuous infusion, CED has been demonstrated in laboratory studies to deliver high molecular weight proteins 2 cm from the injection site in the brain parenchyma.
Limitations:
Some parts of the brain, especially infiltrated tissues surrounding cavities, are challenging to completely saturate with infuscate. Catheters must be positioned correctly for effective drug administration.
- Intra cerebral injections or use as an implant:
Delivery of drugs directly into the brain parenchymal space, the drugs can be administered by: Direct injection via intrathecal catheter. Microencapsulated chemicals. Control release matrices. The main mechanism is diffusion. Useful in the treatment of different brain diseases e.g., brain tumor, Parkinson’s Disease etc.
Limitations:
With increasing distance, diffusion-based distribution in the brain reduces rapidly. For effectiveness and to avoid the issue with drug diffusion in the brain parenchyma, the injection location must be mapped with extreme precision.
- Disruption of the BBB:
This method, which disrupts the BBB, is commonly employed for CNS medication delivery. BBB disruption may result from solvent injections such as ethanol and dimethyl sulfoxide as well as exposure to X-rays. by creating pathological circumstances including ischemia, hypoxia, or hypertension. BBB may also be interfered with. Alcoholic and hypoglycaemic comas have differing impacts on the BBB permeability. The process of energy metabolism determines the outcome. The following are some crucial methods for preventing BBB:
- Osmotic disruption
- MRI guided focused ultrasound BBB disruption technique
Limitations of invasive Approaches:
All of these procedures are rather expensive, call for aesthesia, and involve hospitalization. After the BBB has been successfully disrupted, these methods might facilitate the spread of the tumor. Unwanted blood components that enter the brain have the potential to irreversibly harm neurons.
Non-invasive Approaches
The distribution of drugs through the brain's blood vessel network has been studied as a means of non-invasive brain drug delivery. Non-invasive procedures typically rely on medication modifications, which can involve changes like [9-12]:
- Chemical technique
Prodrug
Prodrug that can penetrate the BBB and is lipid soluble. The parent drug is created by the brain's metabolism of the prodrug. In addition to morphine being diacetylated to become heroin. Prodrugs are substances that lack pharmacological activity. Most chemical changes aim to enhance a particular physical attribute, like membrane permeability or solubility. A covalently joined drug and an inert chemical moiety make up a prodrug. When the connected moiety in the prodrug is split by hydrolytic or enzymatic activities, the active drug is created. The attaching chemical moieties of prodrugs should be chosen so as to increase the drug's lipoidal character.
Examples: Niflumic acid, valproate, levodopa, GABA.
Limitations of Prodrug:
The negative pharmacokinetics are the approach.
The drug's increased molecular weight as a result of lipidation.
- Colloidal techniques:
When certain amphiphilic building blocks are exposed to water, vesicular systems—highly organized assemblies of one or more concentric lipid bilayers—are created. It is possible to create drug carriers that are site-specific, slowly degrade, and respond to stimuli. The ultimate goal is to reduce drug loss and degradation, avoid negative side effects, and maximize drug availability at the site of the disease. Some benefits of vesicular drug delivery systems include:
- If selective absorption can be achieved as a result of the treatment being delivered directly to the infection site, the drug's time in the systemic circulation will be prolonged, and it may even have fewer harmful effects.
- Enhances bioavailability, particularly for medicines that are difficult to dissolve.
- Drugs that are both lipophilic and hydrophilic can be included.
Nanoparticles
Nanoparticles (NPs) are solid colloidal particles with a size between 1 and 1000 nm that are composed of polymeric materials. This concept covers both nanospheres and nano capsules with a core-shell structure (a reservoir system and a matrix system, respectively). The medicine is dispersed, trapped, encapsulated, adsorbed, or chemically attached to the surface using NPs as the carrier system. In contrast to conventional therapies, CNS-targeted medication therapy using nanoparticle systems offers improved therapeutic and diagnostic agent penetration with lower risk. Nanotechnology enables regulated drug release, avoidance of degradation processes, and medication delivery to the targeted region across the BBB. With these approaches, it is possible to decrease biodegradability and toxicity to peripheral organs.
Advantages of using nanoparticles for CNS targeted drug delivery
-
- Drugs are shielded from enzymatic and chemical breakdown by nanoparticles.
- The prolonged drug release at the intended site after injection over a period of days or even weeks is made possible by the use of biodegradable ingredients in the creation of nanoparticles.
- They can help lessen the negative effects of some active medications.
Examples
Radiolabelled polyethylene glycol coated hexadecyl cyanoacrylate nanospheres targeted and
accumulated in a rat gliosarcoma. However, this method is not yet ready for clinical trials due to the Conglomeration of the nanospheres in peripheral healthy tissues.
Liposomes
Liposomes, also known as lipid-based vesicles, are microscopic (multi- or unilamellar) vesicles that develop when phospholipids self-assemble in an aqueous medium to form closed bilayered structures. Both water and lipid soluble medications can be efficiently entrapped into liposomes because they have an aqueous core enclosed by a lipid bilayered membrane. Water soluble or hydrophilic medications become trapped in the watery core of the vesicles, whereas lipid soluble or lipophilic drugs become trapped within the bilayered membrane. Liposomal delivery of nucleic acid sequences for gene and antisense therapy, antigen distribution for immunization, and Parkinson's disease treatment are all possible uses for liposomes as drug delivery systems.
Advantages
Liposomes can deliver hydrophobic, amphipathic, and hydrophilic medicines and agents because they combine an aqueous "milieu interne" with a lipophilic environment.
In addition to tiny molecules, liposomes are capable of encapsulating macromolecules such as superoxide dismutase, haemoglobin, erythropoietin, interleukin-2, and interferon-g.
Liposomes help to decrease exposure of sensitive tissues to toxic drugs [13,14].
RECENT ADVANCES IN BRAIN TARGETING
- Dendrimers:
Dendrimers are polymers with branches that resemble a tree in structure. When sufficiently expanded, a dendrimer, which is normally symmetric around the core, frequently takes on a spheroidal three-dimensional form in water. They have a central core with at least two chemical functions that are the same; from these groups, repetitive units of other molecules concentric layers with increased crowding are produced as a result of the formation of chains and branching. As a result, the structure is densely packed in the perimeter and sparsely packed in the center, leaving gaps that are crucial to dendrimers' ability to entrap drugs. Perhaps the most well-known molecule for the manufacture of dendrimers is poly(amidoamine), or PAMAM. The basic component of PAMAM is a diamine (usually ethylene diamine), which is converted into methyl acrylate and then PAMAM by further reactions with ethylene diamines. Higher generations are produced by subsequent reactions. After intraparenchymal or intraventricular injections showed that systemically administered polyamidoamine dendrimers localize in activated microglia and astrocytes in the brain of newborn rabbits with cerebral palsy, Albertazzietal demonstrated that functionalization of PAMAM dendrimers has a dramatic impact on their capacity to diffuse in the CNS tissue in vivo and penetrate living neurons, opening up possibilities for clinical translation in the treatment [9].
- Scaffolds:
Scaffolds are implantable and can be used to deliver medications to treat neurological diseases like Parkinson's disease and Alzheimer's disease, as well as a number of conditions related to brain injury and diseases. Therapeutic agent delivery through scaffolds may aid in preventing neuronal damage while maintaining their functionality.
Although neural tissue engineering has a wide range of possible applications for scaffolds, building scaffolds for the brain has similar challenges. Among the factors are:
-
- Minimizing cell death and inflammation after implantation of scaffolds, by choosing biocompatible materials.
- Controlling drug release over an appropriate time period to prevent multiple surgeries or injections.
- Making the full process minimally invasive to preserve the integrity of the BBB.
- Scaffolds should be small and minimally invasive.
Scaffolds for brain drug delivery
In a fimbria-fornix lesion cavity, Woerly S. et al. investigated the effectiveness of poly (hydroxyl phenyl methacrylate) [PHPMA] and PHEMA scaffolds containing glucosamine or N-acetyl glucosamine groups. It was discovered that PHEMA scaffolds displayed significantly less connective tissue invasion than PHPMA.[7]
- Lipoplexes and Polyplexes:
The DNA must be shielded from harm, and its entry into the cell must be encouraged in order to increase the transport of the new DNA into the cell. This is accomplished through lipoplexes and polyplexes. Both have the capacity to shield DNA during the transfection process against unwanted deterioration. Lipids can wrap plasmid DNA in an organized form, like a micelle or a liposome. A lipoplex is what is created when DNA complexes with the ordered structure. Lipids come in three different varieties.
- Anionic (negatively charged)
- Neutral
- Cationic (positively charged)
In the beginning, neutral and anionic lipids were utilized to make lipoplexes for synthetic vectors. The toxicity of lipoplexes is extremely low; they may be modified to be tissue-specific, and they are compatible with bodily fluids. The only drawback is that they take a lot of effort and time to manufacture. The cationic variants were thus the focus of attention. Because of their positive charge, cationic lipids can easily form complexes with DNA, which has a negative charge. The positive charge also interacts with the cell membrane, causing endocytosis to take place and the DNA to be released into the cytoplasm. The cationic lipids help guard against cell-mediated DNA deterioration. Dioleoyl phosphoethanolamine (DOPE) and dioleyloxytrimethylammonium chloride (DOTMA) are examples of cationic lipids. Polyplexes are complexes of polymers and DNA. Ionic interactions produce the majority of polyplexes, which are made up of cationic polymers. One significant distinction between lipoplexes and polyplexes' modes of action is that polyplexes are unable to release the associated DNA into the cytoplasm. Transfection with endosome-lytic agents, which lyse the endosome, is required for release, and the polyplex must enter the cell. This isn't always the case, though; some polymers, such polyethyleneimine (PEI), chitosan, and trimethyl chitosan, have unique ways of disrupting endosomes. By electrostatic interactions, PEI creates dense nanoscale particulates and complexes with negatively charged DNA. The PEI/DNA combination adopts a global positive charge, interacts with negatively charged cell membrane components, and enters cells via endocytosis. Through non-specific adsorption-mediated endocytosis, the PEI/DNA complex penetrates the cells. PEI continues to protonate when the endosomal compartment becomes more acidic after endocytosis. By using a device known as a proton sponge, PEI is subjected to protonation. Osmotic edema and endosomal disruption are the results of this. Only one nucleus localization signal (NLS) peptide covalently attached to DNA was necessary to improve the transfection efficiency when the plasmid was injected intracellularly, as shown by Zhanta et al [7,14].
- Polyanhydrides in brain tumor:
The use of nanoparticles as a vehicle for drug delivery to the brain provides the following benefits:
- Outstanding engineerability
- No toxicity.
- Controlled loading and release of active substances (drugs, contrast materials)
- Targeted nanoparticles are capable of delivering massive doses of imaging or therapeutic substances.
- The nanoparticles with improved surface qualities (targeting and/or hydrophilic coating) might be able to deliver large amounts of medications or contrast agents specifically to tumor areas, enhancing the effectiveness of current imaging and cancer treatment in general [7].
- Modified nanoparticles:
Multifunctional nanoparticles:
Nanoparticles with a diameter of 20–200 nm are considered multifunctional. Probes Encapsulated by Biologically Localized Embedding, or PEBBLEs, is another name for them.
-
- A targeting agent that directs the particle to cancer cells.
- A protective coating (PEG) that helps it cross the BBB.
- Photodynamic molecules that catalyse the conversion of oxygen to highly reactive oxygen singlets.
- Magnetically dense metals for MRI contrast imaging.
- Fluorescent “beacon” to visually pinpoint the nanoparticles’ location.
- The major potential advantage of using PEBBLES to treat cancer is their multifunctionality.
- One target molecule immobilized on the surface could guide the PEBBLE to a tumor.
- Another agent could be used to help visualize the target using (MRI).
- MRI contrast element: Gadolinium.
- Third agent attached to the PEBBLE could deliver a destructive dose of drug or toxin to nearby cancer cell.
One tiny polymer sphere can combine all three functionalities to create a strong cancer treatment device. The nanoparticles move through the bloodstream after being introduced into it (Fig.5). The PEBBLEs accumulate in the brain tumor due to their ability to cross the BBB and the targeted chemical they carry, enabling a clear MRI image in just a few hours. A photocatalyst is carried by each pebble. The photocatalyst transforms oxygen into the singlet state, which efficiently bleaches and kills adjacent cells when activated by a light source through a fibre optic probe that is less than a micro meter and introduced into the skull. The pebbles are innocuous until the light is turned on and then become active. Cancer cells can be eliminated when used in conjunction with MRI imaging, and imaging can also be used to monitor the treatment's efficacy. When comparing targeted therapies using PEBBLEs to conventional chemotherapy, there are several benefits. Very little harm is done to nearby healthy tissue since PEBBLEs are highly confined to the cancer target. The harmful oxygen given by PEBBLES works on the outside of the cell and does so fast, depriving cancer cells of the time to survive and acquire resistance. They successfully combat drug resistance by doing this. They circumvent multiple drug resistances in this way. Surgery has limited success in treating glioblastoma, the most prevalent primary brain cancer, because it is difficult to visually distinguish malignant cells from normal brain tissue, and any cancer cells that are left behind are likely to multiply and generate new tumors. Fluorophores, or glowing molecules, were created as a result, marking the boundaries of tumors to make them easier to find and remove [15,16].

Fig. 5: Generalized structure of Nano emulsion
Magnetic nanoparticles for MRI:
Typically, short-lived gadolinium-based contrast agents are used for CNS MRI scans. MRI contrast agents based on iron oxide nanoparticles have excellent potential for CNS imaging. Depending on the size distribution of the nanoparticles, the iron oxide contrast agents are referred to as super-paramagnetic iron oxide (SPIO) or extremely tiny super-paramagnetic iron oxide (USPIO) agents. Some SPIOs and USPIOs have already received clinical approval or are undergoing preclinical testing.
Two generic types of magnetic nanoparticles have been used.
Iron oxide core surface-coated with polymer
The effectiveness of dextran-coated USPIO as MRI contrast agents in the brain has been studied in vitro in cellular studies, in vivo in animal studies, and in human studies. The USPIO is composed of a dextran coating covering a 5–6 nm iron oxide core with a hydrodynamic diameter of roughly 20–30 nm.
- Using fluorescently or 125I-labeled dextran-coated iron oxide nanoparticles, in vitro cellular uptake experiments were conducted in tumor cells. It was discovered that the cellular absorption was non-saturable, widespread, and varied throughout tumor cells. This implied that fluid-phase endocytosis was their mode of transport.
- Rats implanted with gliosarcoma were used in the in vivo animal studies. An MRI was performed 14 days after tumor implantation. Pictures were taken with a 1.5-T superconducting magnet both before and 24 hours after the injection of dextran-coated iron oxide nanoparticles containing 19 mg/kg iron. The total amount of nanoparticles taken up by the glioma was found to be sufficient to change the MRI signal intensity at tumors in comparison to surrounding brain tissues.
- Using 125I-labeled dextran-coated iron oxide nanoparticles, in vivo bio-distribution research was carried out. The results revealed that, 24 hours after IV treatment, the amount of iron oxide nanoparticles accumulated in brain tumors was 10-fold higher than in the healthy brain tissue next to the tumor, although it was only 0.11% of the administered dose. An analysis of the iron oxide nanoparticle distribution pattern within the tumor revealed that the nanoparticles accumulated in a heterogeneous manner across the remaining portion of the tumor and preferentially around it.
These compounds have a lengthy plasma half-life (24–30 hours), making them suitable for both operational and postoperative imaging of residual malignancy. USPIO nanoparticles cause MRI enhancement when they seep through the broken blood-brain barrier and become trapped inside cells by macrophages and astrocytes in and around the tumor [17,18].
- Receptor-mediated transport (RMT):
For the transportation of endogenous peptides like insulin or transferrin, the BBB expresses RMT mechanisms. The traditional carrier-mediated transporters (CMT), which move specific small-molecule vitamins, hormones, and nutrients, work in tandem with the RMT systems. The RMT systems serve as entry points for large-molecule medications that are bound to endogenous RMT ligands, just as the CMT systems do for small-molecule pharmaceuticals with chemical structures that mimic those of an endogenous CMT substrate [19].
Monoclonal antibody (MAb) molecular Trojan horses (MTH)
Monoclonal antibodies can be produced by genetic engineering in chimeric or humanized forms. Monoclonal antibodies against the human insulin receptor are the strongest antibody-based MTH that have been identified to date. Recently, this antibody was humanized, and non-human primates were used to demonstrate in vivo BBB crossing. The RMT systems bind to specific peptidomimetic MAbs. These antibodies that are specific for BBB RMT bind receptor epitopes that are physically distant from the endogenous ligand binding site. The peptidomimetic MAbs function as MTH to transport a medication, protein, antisense agent, or non-viral plasmid DNA over the BBB. Histone, receptor-associated protein (RAP), the tat transduction domain peptide, and other cationic peptides or polymers are some of the non-antibody delivery techniques that have been studied. While the transport of ligands like RAP is thought to be receptor-mediated, it is thought that the transport of cationic peptides is mediated by charge-based absorption-mediated endocytosis processes. There have been recent reports of the delivery of biopharmaceuticals through the BBB using a related RMT method. In human vaccinations and more recently in anti-cancer trials, a carrier protein known as CRM197 was employed as a secure and efficient carrier protein. CRM197 uses the diphtheria toxin receptor (DTR), also known as the membrane-bound precursor of heparin-binding epidermal growth factor (HB-EGF), as its transport receptor. CRM197 is actually a benign mutation of the diphtheria toxin. Blood-brain barrier endothelial cells and a variety of other cells express membrane-bound HB-EGF on a constitutively active basis. This means that the brain and other important cellular reservoirs like T lymphocytes, monocytes, and macrophages can be accessed. Additionally, the substantial upregulation of HB-EGF expression under inflammatory illness conditions will greatly improve targeted delivery. By using this technique, CRM197 can transfer siRNA across the blood-brain barrier. Other applications could be in connection with other brain-related illnesses, including Parkinson's, Alzheimer's, or multiple sclerosis, as well as neurotropic infections like the West Nile virus and poliovirus [20].
Trojan horse liposomes for CNS gene therapy
Due to the quick breakdown of extracellular nucleic acids and the pro-inflammatory effects of naked DNA, gene transfer across the BBB may be futile. Plasmid DNA can be enclosed inside pEGylated liposomes to remove its nuclease sensitivity and pro-inflammatory effects. PEGylated liposomes, by themselves, cannot cross the BBB. The PEGylated immunoliposomes, also known as Trojan horse liposomes, are transported across the BBB when an MTH is attached to the ends of the polyethylene glycol strands, enabling the liposome to engage the BBB RMT system. 24–48 hours after this new technology is given to mice, rats, or monkeys, the non-viral transgene begins to express itself throughout the brain. In a model of experimental Parkinson's disease, the BBB administration of immunoliposomes with an expression plasmid encoding tyrosine hydroxylase resulted in a complete recovery of striatal tyrosine hydroxylase enzyme activity. Mice with intracranial human brain tumors lived 90% longer after receiving an intravenous injection of immunoliposomes with an expression plasmid expressing a short hairpin RNA directed against the human epidermal growth factor. Intravenous RNA interference (RNAi) in the brain is made possible by PEGylated Immunoliposome Gene Transfer [21].
In vivo brain imaging of gene expression
Nuclear medicine imaging methods like PET or SPECT may be used to image gene expression in the brain using antisense radiopharmaceuticals. To be effective as brain gene imaging agents, antisense radiopharmaceuticals must be altered because they cannot cross the BBB on their own. It is possible to biotinylate and radiolabel peptide nucleic acids with indium-111. A conjugation or fusion protein of avidin and a molecular booby trap for the BBB can be created concurrently. The avidin-biotin bridge is subsequently used to bind the peptide nucleic acid to the MTH. Such targeted antisense radiopharmaceuticals pass through the blood-brain barrier and the membrane of brain cells, allowing for in vivo imaging of gene expression in the brain [22].
- Transporter-independent mechanisms to circumvent the BBB:
Convection-enhanced drug delivery (CED)
CED is a technique for direct local or regional micro infusion into brain tissue. Therapeutic drugs are distributed into the interstitial space over the course of hours or days by a continuous infusion pressure gradient. The CED approach is generally employed for large molecular weight agents, including viruses, oligonucleotides, nanoparticles, liposomes, and targeted immunotoxins, that exhibit limited BBB leakage and/or have considerable systemic toxicity. The infusion parameters (rate, volume, duration, cannula size), infuscate characteristics (molecular weight, surface properties, tissue affinity), and tissue properties (tissue density, extracellular space, vascularity, and interstitial fluid pressure) all have an impact on the CED volume of distribution. Animal experiments have shown that by incorporating contrast ants into the infusate, it is possible to image the volume of distribution produced by CED using magnetic resonance in real time. Targeted therapy for glioblastoma will be the primary clinical use of CED. Interleukin 13/pseudomonas exotoxin alone or in combination with radiation/temozolomide, as well as radio immunotherapy using MBAs targeting tenascin or tumor necrosis factor, have all been the subject of recent investigations. Despite encouraging initial findings, two industry-sponsored phase III trials of CED immunotoxins seem to have ended in failure. Distribution heterogeneity, high interstitial fluid pressure, and quick drug efflux from the injection site are some of the mechanisms causing CED therapy failure. Increased residence time is required to improve targeted toxin receptor binding and absorption by the malignant cells in order to address these difficulties. The CED method (Fig.6), which primarily targets brain tumors, may also be useful for localized neurodegenerative diseases. For example, CED has been used to infuse 6 glucocerebrosidase into the frontal lobe and brainstem of a patient with neuronopathic the Gauche disease. Infusion of adenovirus vectors or neurotrophic factor has been assessed in the Parkinson disease [11,23].

Fig.6: Illustration Convection-enhanced drug delivery
Bradykinin receptor-mediated BBB opening:
An endogenous peptide called bradykinin that mediates the inflammatory response has the ability to cause brief changes in blood vessel permeability that are extremely selective to tumor vasculature. RMP-7 (lobadimil) is a mouse-specific synthetic 7-brodykinin analog that is 100 times more powerful than bradykinin and is specific for the B2 receptor. Although gains in delivery may only be minor and dependent on the type or model of tumor being treated, pharmacological modulation of the BTB offers the option of very selective opening and targeted drug delivery to the tumor. For both adults and children with gliomas, clinical investigations conducted in the last five years have shown that concurrent administration of RMP-7 with carboplatin, with or without radiation therapy, is safe. RMP-7 had no impact on the pharmacokinetics or toxicity of carboplatin, and two studies found no evidence of any interaction between RMP-7 and carboplatin in the treatment of high-grade or brain stem gliomas. Increased carboplatin transport to the tumor may necessitate higher dosages of RMP-7, but this could potentially enhance toxicity in the healthy brain [24].
Ultrasound (US)-mediated BBBD strategy:
Pressure waves with a frequency of at least 20 kHz make up the US. Ultrasonic waves can be focused, reflected, and refracted through a medium, much like optical and audio waves can. Poor US penetration through the skull has been a major barrier to its use for BBBD, and for many years, it was assumed that the skull bone needed to be removed in order to administer US treatments to the brain. However, practical and theoretical studies have demonstrated that it is possible to use large surface area phased arrays to provide focal, trans-skull focused US (FUS) exposure of brain tissue. Therapeutics can now be delivered to the targeted brain regions via the intact skull thanks to newly developed image-guided (e.g., MRI-guided) FUS clinical systems, and both animal investigations and clinical trials have yielded promising results. Ultrasonic microbubbles and FUS can be employed as medication carriers for targeted delivery, as shown in Fig.7. In order to provide a repeatable cavitation environment with a managed supply of cavitation nuclei, preformed microbubbles with a narrow size distribution have been used. The oscillation of bubbles in an acoustic field is known as cavitation. Cavitation can cause significant cell stressors in order to create a variety of "bioeffects." For instance, it could result in physical shearing of the cell membrane to enhance direct entry of medicines into the cytosol or boost drug interaction by upregulating pathways of various forms of stress response. The amount of acoustic energy needed by cavitation will be significantly reduced if ultrasonic microbubbles are present in the blood vessels. With this method, the dangers of overheating the skull are greatly diminished, making the treatment more practicable for administration through an unbroken skull. Additionally, by using these medications, the contact of the US with the endothelial cells can be restricted, reducing the possibility of harm to other brain regions [13,25].

Fig. 7: Illustration of ultrasonic microbubbles for drug targeted delivery
Miscellaneous Techniques
Iontophoretic can be defined as the permeation of ionized drug molecules across biological membranes under the influence of electric current. The physical medication delivery method known as iontophoresis was first developed at the turn of the previous century. For many years, iontophoresis research was limited to a few therapeutic uses (such as the treatment of keratitis, psoriasis, and myringotomy) and concentrated on understanding delivery-enhancing mechanisms. However, in recent years, the range of target tissues and organs for iontophoresis has expanded to encompass new tissues and organs like the suprachoroidal area, nibbles, and intestinal mucosa. To further optimize medication delivery, iontophoresis has also been extensively researched in combination with other techniques like microneedles, nanocarriers, and chemical enhancers. In addition, smart iontophoretic device design is quickly developing to enable routine iontophoresis applications without medical help. In order to forecast iontophoretic drug transport across various tissues, considerable progress has been made in mathematical and computer simulations in tandem with this technological improvement. As a result, this study covers the most recent developments in iontophoresis technology in terms of therapeutic applications, pairings with other drug delivery methods, smart device design, and mathematical modelling [28].
Malignant brain tumor sufferers are faced with a poor outlook. The blood-brain barrier is regarded as the main barrier to the transport of therapeutic drugs to the brain. Since years, attempts have been made to deliver therapeutic drugs intravenously (IA) after osmotic BBB opening, but their broad use has been constrained by considerable variability. Recent animal investigations have demonstrated that MRI is more effective than X-rays for guiding IA infusions because it provides direct sight of the brain parenchyma being perfused and predictable drug targeting. Furthermore, bevacizumab delivered intraarterial (IA) rather than intravenous (IV) causes brain buildup, which provides significant support for using the IA route [29].
In the current situation, many integrated blood-brain barrier centers have been identified. This will bring together all the researchers, scientists, and novices to create fresh targeting tactics that will enable brain targeting. These BBB facilities have lead generation tools for pharmaceutical formulations based on both large and small compounds. It primarily refers to medications with low or no blood-brain barrier permeability. These centers will be based on technology and applied sciences. Here, the development of novel drug delivery systems is the main goal. Utilizing such technology causes the pharmaceuticals to be reformulated in a way that allows them to cross the BBB [30]. New BBB transporters would be discovered for brain targeting, targeting systems would be validated through in vivo models, in vivo model pharmacokinetics would be optimized, and targeting processes would be developed through which recombinant proteins and neurotherapeutics could be effectively delivered. Better brain targeting can be accomplished by acquiring a variety of tactics and tools [31].
Lack of precise and effective procedures affects how well medications are delivered for treating CNS-related diseases. Despite these challenges, the techniques for brain targeting have advanced significantly. However, none have been shown to be successful. This review leads to the conclusion that the medicine can be effectively given across the BBB using the methods indicated above. Recent advancements in BBB-wide medication delivery have proven effective in removing obstacles to brain drug delivery. Thus, brain targeting in these ways can offer better therapeutic effectiveness, but nonetheless, the most trustworthy techniques or strategies with significant clinical impact are needed.


 Dnyaneshwar D. Deshmane* 1
Dnyaneshwar D. Deshmane* 1








 10.5281/zenodo.10881325
10.5281/zenodo.10881325