Abstract
The purpose of present study was to develop and evaluate floating drug delivery system of an oral hypoglycemic agent. The floating tablets of Glipizide were prepared by using HPMC K4M, HPMCK100, Xanthan gum and other excipients for various batches of formulations. The pre compression and post compression parameters of various batches of formulations were performed as per pharmacopeial standards. The tablets were prepared by direct compression method. The Dissolution test was carried out in a (USP) dissolution testing apparatus along with the invitro buoyancy studies were performed and Compatibility study was performed by FTIR. The compatibility study of the prepared optimized Glipizide floating tablets confirms that there is no interaction between the drug and polymers used in the given formulation.
Keywords
Glipizide, Floating drug delivery system, FTIR, Compatibility
Introduction
Glipizide is an oral hypoglycemic agent, which is a commonly prescribed drug for the treatment of patients with type II diabetes. It is used adjunct to diet to the management of type II (non-insulin dependent) diabetes mellitus in patients whose hyperglycemias cannot be controlled by diet and exercise alone [1]. Glipizide stimulates insulin secretion from the ? cells of pancreatic islets tissue, increases the concentration of insulin in the pancreatic vein and may increase the number of insulin receptors. Glipizide is a weak acid (pKa = 5.9) which is practically insoluble in water and acidic solutions but as per the Bio-pharmaceutical Classification System (BCS) it has lower solubility and higher permeability (class II). The oral ab-sorption is uniform, rapid and complete with a bioavailability of nearly 100% and an elimination half-life of 2–4 hrs. Glipizide is an effective oral antidiabetic, 100 times more potent than tolbutamide in evoking pancreatic secretion of insulin and have a short biological half-life (3.4 ± 0.7 hrs.) and is rapidly eliminated, so requiring it to be administered in 2 to 3 doses of 2.5 to 10 mg per day. Hence floating drug release formulations are needed for glipizide for better control of blood glucose levels to prevent hypoglycemia and enhance clinical efficiency, to reduce G.I disturbances and to enhance patient compliance [2]. Glipizide is an oral rapid- and short-acting anti-diabetic medication from the sulfonylurea class. It sensitizes the beta cells of pancreatic islets of Langerhans insulin response, meaning that more insulin is released in response to glucose than would be without glipizide ingestion. Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes, which results in the opening of voltage-gated calcium channels [3]. The resulting calcium influx encourages insulin release from beta cells. It may also be used with other diabetes medications. Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual function problems.
MATERIALS AND METHODS
Materials
Glipizide was procured from NATCO pharma private Ltd. Shad Nagar, Hyderabad, India. Hydroxy propyl methyl cellulose (K100 & K4), Xanthan gum, Micro crystalline cellulose, Sodium bicarbonate, Lactose, Magnesium Stearate, Talc were purchased from S.D fine chemicals, Mumbai, Maharashtra, India.
Methods
Preparation of calibration curve of Glipizide in pH buffer
Accurately weighed 10 mg of Glipizide was dissolved in 100 ml of the freshly prepared 1.2 pH buffer to obtain the working standard (stock solution) of 100 mcg/ml. Aliquots of 0.4 ml to 4.0 ml from the above stock solution representing 4 to 40 mcg/ml of drug were prepared and transferred to 10 ml volumetric flask. The volume was adjusted to 10 ml with the same buffer [4]. Absorbance of the all solutions were taken at 276 nm against the blank solution prepared in the same manner without adding the drug. A graph of absorbance vs concentration was plotted.
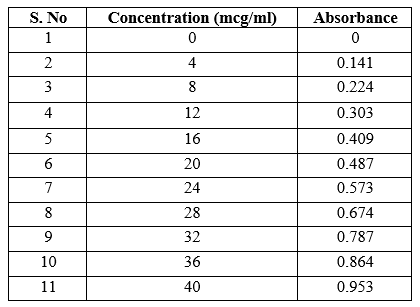
Table 1: Standard Curve of Glipizide
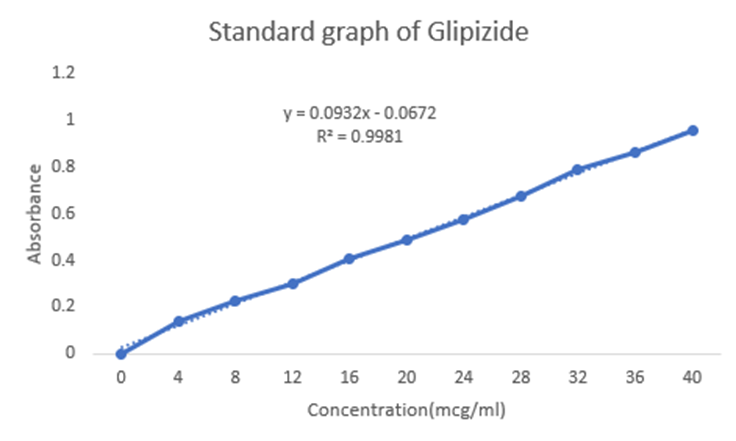
Figure 1: Standard graph of Glipizide
Preparation of Glipizide floating tablets
Preparation of Glipizide floating tablets by direct compression method, using drug and variable concentration of polymers (HPMC K4M, HPMCK100, Xanthan gum), Sodium Bicarbonate, MCC, Lactose, Magnesium stearate and Talc. The respective powders & optional additives were blended thoroughly with a mortar and pestle [5]. The powder blended was then lubricated with Magnesium stearate and purified talc and then compressed on a tablet punching machine. Various formulation batches were selected as per design expert and out of them optimized batch was selected for floating tablet preparation. The formulation was optimized on the basis of buoyancy lag time (BLT), total floating time (TFT) and In-vitro drug release in 1.2 pH buffer.

Table 2: Composition of Glipizide floating tablets.
Drug-Excipient compatibility
Compatibility studies were performed through FTIR spectroscopy. The IR spectrum of pure drug and physical mixture of drug and polymer was studied. The characteristic absorption peaks of Glipizide obtained were obtained at 4000-500cm -1. It has been observed that there is no chemical interaction between Glipizide and polymers used. It was observed that peak obtained in spectra of drug and polymer which show there were no interaction between drug and polymers [6].

Figure 2: FTIR spectra of optimized formulation (F4)
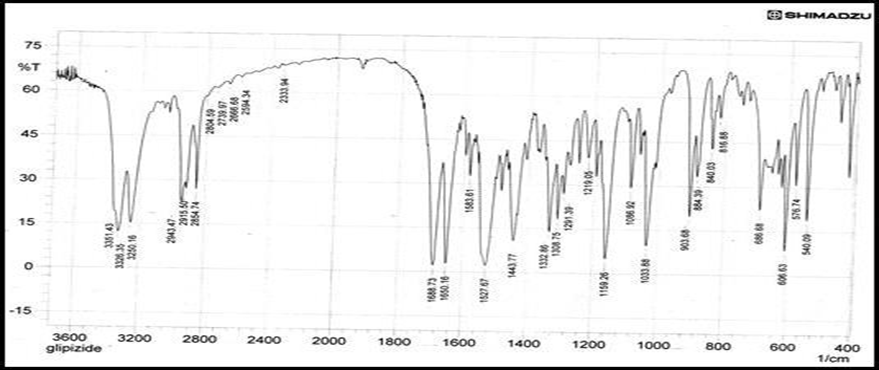
Figure 3: FTIR spectrum of pure Glipizide
Evaluation of pre-compression parameters of Glipizide
1. Angle of Repose (?)
This is the maximum angle possible between the surface of a pile of granules and the horizontal plane.[7] The powders were allowed to flow through the funnel fixed to a stand at definite height (h). The angle of repose was then calculated by measuring the height and radius of the heap of granules formed.
tan ? =h/r and ? = tan-1(h/r)
Where, ? angle of repose, h height of the heap, r radius of the heap.
2. Carr’s Index: The Carr’s index or compressibility index was calculated from the bulk and tapped density value by following equation.[8]
Carr’s index = Tapped density- bulk density x 100
Tapped density
3. Hausner’s Ratio: It is measurement of frictional resistance of tablet blend. The ideal range should be 1.2-1.3. It was determined by the ratio of tapped density and bulk density.[9] Hausner?s ratio=Tapped density/Bulk density
4. Bulk Density: Bulk density is defined as the mass of a powder divided by the bulk volume. The bulk density of a powder depends primarily on particle size distribution, particle shape and the tendency of the particles to adhere to one another. [10,11] Both loose bulk density (LBD) and tapped bulk density (TBD) were determined. A quantity of accurately weighed powder (bulk) from each formula, previously shaken to break any agglomerates formed was introduced into a 25ml measuring cylinder and the initial volume was observed. It is given by the equation as
Bulk density=Mass of the powder/ bulk volume of the powder
5. Tapped density: Weighed quantity of tablet blend was introduced into a graduated cylinder. Volume occupied by the drug was noted down. Then cylinder was subjected to 100, 200 and 300 taps in tap density apparatus. [12,13] According to USP, tapped density was given by Tapped density=Mass of the powder/Tapped volume of the powder
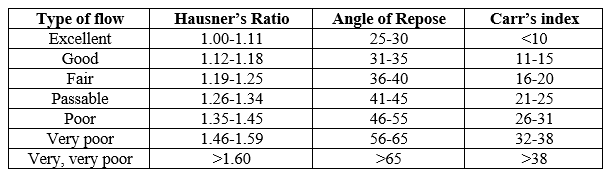
Table 3: Specifications for flow properties.
Evaluation of post compression parameters of Glipizide floating tablets
Weight variation
All formulated Glipizide floating tablets were evaluated for weight variation test. Twenty tablets were weighed collectively and individually using an electronic balance.[14] The average weight was calculated and percent variation of each tablet was calculated. According to USP monograph, the weight variation tolerance limit for the uncoated tablet having average weight 130 mg or less is 10% whereas for average weight between 130-324 mg is 7.5% and for average weight more than 324 mg is 5%. For the tablet to be accepted, the weight of not more than two tablets deviates from the average weight by not more than 7.5%.

Table 4: Specifications for weight variation test of tablets.
Hardness
All the formulations of Glipizide floating tablets hardness were measured by using Monsanto hardness tester [15]. From each formulation the crushing strength of ten floating tablets were recorded in kg/cm2 and average were calculated. According to specifications of USP hardness values of 3-3.5 Kg for floating tablet is considered as acceptable limit.
Friability
Ten floating tablets from each batch were taken in Roche friabilator. After100 revolutions of friabilator tablets were recovered [16]. The tablets were then made free from dust and the total remaining weight was recorded. Friability was calculated from the following formula.
Percent Friability = (W0 – W1)/ W0 × 100
Where W0 and W1 were the initial and final weight of the tablets before and after friability test. The maximum limit up to 1% of the tablet weight are consider acceptable for friability.
In-vitro drug release study
Dissolution study was conducted for all the formulations using USP dissolution rate test apparatus type-II. A total volume of 900 ml of phosphate buffer PH 1.2 was taken as dissolution medium, which was maintain at 37°C ± 0.5°C at 50 rpm. 5ml of aliquots were periodically withdrawn and the sample volume was replaced with an equal volume of fresh dissolution medium.[17] Samples were collected at time interval of 1, 2, 3,4,6,7,8,9,10 ,11 and 12 hours and samples were analyzed spectrophotometrically at 276 nm.
Drug content
The Glipizide floating tablets were tested for their drug content. Five tablets were finely powdered quantities of the powder equivalent to 10 mg of Glipizide were accurately weighed and transferred to a 100 ml of volumetric flask. The flask was filled with 0.1N HCl (pH 1.2 buffers) solution and mixed thoroughly. The solution was made up to volume 100ml and filtered. Dilute 1 ml of the resulting solution to 10 ml with 0.1N HCl. The absorbance of the resulting solution was measured at 276 nm using a Shimadzu UV-visible spectrophotometer [18].
In-vitro buoyancy studies
The in vitro floating behavior of the tablets was studied by placing them in 100 ml beaker with 100 ml of 0.1 N HCl (pH 1.2). The time of tablet required for the emerge on the surface of the fluid is floating lag time (FLT) [19] and the time required for tablet constantly to float on the surface of the medium is called total floating time (TFT).
RESULTS AND DISCUSSION
In the present study, the floating tablets of Glipizide were prepared by using different viscosity grades of hydroxy propyl methyl cellulose HPMC K4M and HPMCK100 and Xanthan gum formulations were found to be compatible with Glipizide and have no physical interaction. The angle of repose of the drug powder was in the range of 25.06 to 28.64, the Carr’s index was found to be in the range of 10.70 to 14.67 indicating compressibility of the tablet. Hausner’s ratio was found in the range of 1.10 to 1.19 is good. Prepared tablets were evaluated for weight variation and percentage deviations from the average weight are reported in table -6 and was found to be within the prescribed official limits.
The friability of the formulations as found to be between 0.71 to 0.95 is reported in table-6 and as that of which was found to be within the official requirement (i.e., not more than 1%). The thickness of the tablet indicates that die fill was uniform. The thickness depends upon the size of the punch and the weight of the tablet (200 mg). The thickness of the batch from F1-F6 was found to be 2.14-2.28 mm and hardness was found to be 4.29-4.89 Kg/cm2 as reported in table-6 which had good mechanical strength. On immersion in 0.1 N HCL solution pH (1.2) at 370C, the tablets floated and remained buoyant without disintegration. From the results it can be concluded that the batch F4 showed good floating lag time (FLT). In-vitro dissolution studies were performed for all the batches of tablets containing Glipizide using USP II dissolution test apparatus at 50rpm, 900ml of 0.1N HCl used as dissolution media. The Formulations F1 to F6 containing drug and polymers carried for invitro drug release studies of 12-hour time period. The drug release percent of each batch ranges from 82.12 to 94.36 in 12 hours respectively. Formulation F4 containing HPMC (K100 & K4) showed complete drug release within 12 hours emerging as optimized formulation that gives better drug release profile.
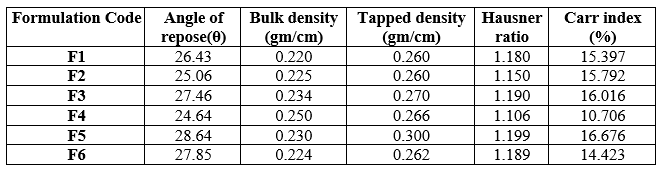
Table 5: Pre-compression parameters of Glipizide floating tablets

Table 6: Post-compression parameters of Glipizide floating tablets

Table 7: In-vitro buoyancy studies
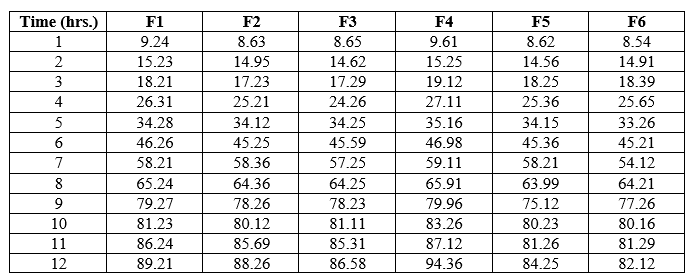
Table 8: In-vitro drug release data of Glipizide floating tablets
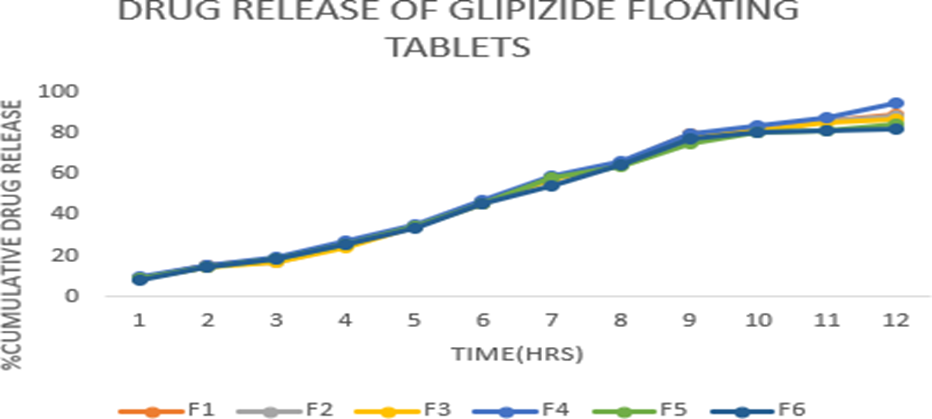
Figure 4: Dissolution profile of Glipizide floating tablets
CONCLUSION
From the compatibility studies, it is concluded that, HPMC K4M, Xanthan gum, HPMCK100, were compatible with drug Glipizide and thus suitable for the formulation of Glipizide floating tablets. Glipizide tablets were fabricated by direct compression method. In-vitro buoyancy studies were performed for all the formulations, F1 to F6 by using 0.1 N HCL solution at 37 0C. The Tablets batch (F4) showed good buoyancy with very short lag time and long floatation time of more than 12 hours in 0.1 N HCL. In-Vitro release study is performed for 12 hours and the optimized formulation (F4) showed better drug release compare to other formulations. From this study, it was concluded that F4 can be used in formulation of Glipizide gastro retentive floating drug delivery system. Overall, this study concludes that viscosity of the polymer is a major factor affecting the drug release and floating properties of the tablets.
ACKNOWLEDGEMENT
The author was Thankful to Dr. Nakka Jyothi, Principal, Marri Laxman Reddy Institute of Pharmacy for her kind support and encouragement. The author was also thankful to the management and faculty members of the institution for completion of the work.
CONFLICT OF INTEREST: NIL
REFERENCE
- Gibert SB, Cristopher IR. Modern pharmaceutics 4 th ed, 2005.
- Ray-Neng C, Hsiu-O H, Chiao-Ya Y, MingThau S, Development of swelling/floating gastro retentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. European Journal of Pharmaceutical Sciences, 2010 Nov; 10(39): 82–89.
- Brahma N. Singh, Kwon H. Kim. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention Drug Delivery Systems. Journal of Controlled Release, 2000; (63): 235–259.
- Gopalakrishnan S. and Chenthil nathan A. Floating Drug Delivery Systems: A Review India. Journal of Pharmaceutical Science and Technology 2011 3(2):548-554.
- Debjit B, Chiranjib. B, Margret Chandira, Jayakar B, Sampath K. Floating Drug Delivery System-A Review. Scholars Research Library Der Pharmacia Lettre, 2009; 1(2): 199-218.
- Shahaa SH, Patelb JK, Pundarikakshudua K, Patelc NV. An overview of a gastro- retentive floating drug delivery system. Asian Journal of Pharmaceutical Sciences, 2009; 4(1): 65-80.
- Pradeep K, Deepika J, Vikas J, Ranjit S. Floating Drug Delivery Systems: An Overview. Journal of Pharmacy Research, 2010; 3(6): 1274- 1279.
- Yiew. W. chien. Noval drug delivery system. 2 nd ed. New York: Marcel Dekker INVC, 2005; 140- 141.
- Shahaa SH, Patelb JK, Pundari kakshudua K, Patel NV. An overview of a gastro- retentive floating drug delivery system. Asian Journal of Pharmaceutical Sciences, 2009; 4(1): 65-80.
- Shah S, Pandya S. A Novel Approach in Gastro Retentive Drug Delivery System: Floating Drug Delivery System. International Journal of Pharmaceutical Sciences and Research, 2010; 1(6): 7-18.
- Pahwa R, Gupta N. A Review: Super disintegrants in the development of orally disintegrating tablets. Int J Pharm Sci Res. 2011;2(11):2767-2780.
- Patel U, Patel K, Shah D, Shah R. A review on immediate release drug delivery system. Int. j.pharm. Res. Bio-Science. 2012;1(5):37-66.
- Chugh I, Seth N, Rana A.C. Oral sustained release drug delivery system. International Research Journal of Pharmacy 2012;3(5):57-62.
- Gennaro AR. (Ed.) Remington’s. pharmaceutical science. 20thEdn. Lippincott Williams and Wilkin publishing co, New York. 2000; 1:905-06.
- Kumar KPS, Bhowmik D, Chiranjib, Chandira M, Tripathi KK. Innovations in Sustained Release Drug Delivery System and Its Market Opportunities. J Chem Pharma Res. 2010;2(1):349-360.
- Singh A, Sharma R, Jamil F. Sustained Release Drug Delivery System: a Review. Int Res J Pharma. 2012;3(9):21-4.
- Patel H, Panchal DR, Patel U, Brahmbhatt T, Suthar M. Matrix type drug delivery system: A Review. Journal of Pharmaceutics Science and Bio-scientific Research (JPSBR) 2011;1(3):143-51.
- Reddy P, Rao D, Kumar RK. Bi-layer Technology-An Emerging Trend: A Review. Int J Res Devl Pharma Life Sci. 2013;2(3):404-11.
- Gopinath C, Bindu VH, Nischala M. An Overview on Bi-layered Tablet Technology. J Glob Trends Pharma Sci. 2013;4(2):1077-85.


 M. Maheshwar*
M. Maheshwar*
 Madhulapally Swathi
Madhulapally Swathi












 10.5281/zenodo.13144109
10.5281/zenodo.13144109