Abstract
Acebrophylline is a medication that combines the bronchodilator effects of theophylline-7-acetic acid with the mucolytic effects of ambroxol. It acts via various mechanisms, including relaxation of smooth muscles, increased production of surfactant, and anti-inflammatory action. Accurate quantification of Acebrophylline in pharmaceuticals is crucial for ensuring therapeutic efficacy, patient safety, quality control, and regulatory compliance. A newly developed and validated high-performance liquid chromatography (HPLC) method has been developed to quantify Acebrophylline in pharmaceutical formulations. The HPLC method was validated in accordance with ICH guidelines for accuracy, precision, linearity, specificity, robustness, and limit of detection (LOD) and quantification (LOQ). The method was developed by experimentation and literature survey, and was found to be simple, linear, rapid, accurate, precise, reproducible, and robust. The % RSD was found within the limit, and the method was suitable for accurate, precise, and rapid determination of Acebrophylline in its bulk form and pharmaceutical dosage form. The RP-HPLC method was found to be accurate, precise, linear, robust, and rugged according to the ICH guidelines. The method provides a sharp and proper peak, making it suitable for routine analysis of Acebrophylline estimation in bulk and marketed formulations. This comprehensive overview of developing and validating an analytical method for Acebrophylline is essential for ensuring consistent quality in pharmaceutical products. The developed method is simple, sensitive, accurate, and precise, and has no interference with excipient used in the formulations, making it suitable for routine analysis of Acebrophylline estimation.
Keywords
Developed method, Determination, Bronchi, Acebrofil, Acebrophylline
Introduction
Acebrophylline is a special medication that combines the bronchodilator effects of theophylline-7-acetic acid with the mucolytic effects of ambroxol. It falls into the category of bronchodilator drugs. Acebrophylline acts via the following mechanisms: in bronchodilator action, it relaxes the smooth muscles of the bronchi and bronchioles, allowing for easier breathing; in mucolytic action, it increases the production of surfactant, which lowers the viscosity of mucus, making it easier to expel; in bronchodilator action, it relaxes the smooth muscles of the bronchi and bronchioles; and in anti-inflammatory action, it prevents the production of inflammatory mediators like leukotrienes and cytokines, thereby decreasing inflammation in the airways. It is marketed under the names Duolin, Bronchovent, Acebrofil, and Bronchotone.[1-2]
Accurate quantification of Acebrophylline in pharmaceuticals is critical to ensuring therapeutic efficacy, patient safety, quality control, and regulatory compliance. It promotes clinical research, financial effectiveness, legal compliance, and patient compliance, all of which contribute to improved health outcomes and long-term consumer confidence in pharmaceuticals.[3] Acebrophylline's solubility profile showed that it is soluble in ethanol and only marginally soluble in water and methanol. Analytical techniques are used in the creation and production of drugs to provide information about potency, contaminants, and other characteristics of the drug, such as its crystal shape, release, uniformity, and degradation product.[4]
Acebrophylline in pharmaceutical formulations can now be quantified with ease thanks to a newly developed and validated high-performance liquid chromatography (HPLC) method that is fast, accurate, and precise. In terms of accuracy, precision, linearity, specificity, robustness, and limit of detection (LOD) and quantification (LOQ), the procedure was validated in accordance with ICH guidelines.[5]
MATERIALS AND METHODS:
Materials: As a gift, Ami Lifesciences Pvt. Ltd. kindly supplied a pure sample of acebrophylline. All of the chemicals and solvents were obtained from Merck Pharmaceutical in Mumbai and were of HPLC grade.
Instruments: Chromatographic measurements were obtained using a UV Spectrophotometer created by Shimadzu UV 1800, HPLC made by Analytical Technologies Limited Model no: UV-3000-M. A FTIR examination employing infrared spectroscopy was undertaken (Bruker, Japan). Analytical balance used was Shimadzu Model number AY-220.
Chemicals and Reagents: Chemicals of HPLC grade, such as acetonitrile and methanol, were procured from Merck Specialties Private Limited in Mumbai.
Preparation of standard stock solution for Chromatographic development: [6,7]
A precise weight of 10.0 mg of acebrophylline was added to a 100.0 mL volumetric flask. This was dissolved by adding 50.0 mL of mobile phase, and the drug solution was then diluted with mobile phase until the desired concentration was reached, yielding a stock solution containing 100 µg/ml of acebrophylline. These medications' working standard solutions were created by appropriately diluting the corresponding stock solution with mobile phase.
Preparation of Mobile Phase:
Prepare the mobile phase by varying the proportions of methanol and ACN. Methanol [80: 20% v/v] in ACN. After passing via a 0.45µm membrane filter, the mobile phase was sonicated for 20 minutes to remove any remaining gas.
Chromatographic Conditions:
Column: Cosmosil C18 (4.6mm x 250mm, Particle size: 5µm)
Mobile Phase: ACN: Methanol (80:20)
Flow Rate: 1.0 mL/min
Injection Volume: 20 µL
Detection Wavelength: 275 nm
Column Temperature: Room Temperature
Method Validation: [8-10]
1. Linearity and Range
An analytical process is said to be linear if it can produce test findings that show a clear, consistent relationship between the amount or concentration of the analyte in the sample throughout a certain range. Five different linearity levels, ranging from 10% to 150% of working concentration, were tested.
2. Limit Of Detection (LOD) and Limit Of Quantitation (LOQ)
Different guidelines have proposed a number of methods for calculating a method's LOD and LOQ, including visual examination, the use of the signal to noise ratio, calculations based on the response's standard deviation, and the slope of the calibration curve.
LOD = 3:3 × ? /S LOQ = 10 × ?/s
Where,
? = residual standard deviation of a regression line
S = Slope of regression line
3. Precision
The new method's precision was evaluated using assessments of intermediate precision and repeatability or intra-assay precision. Six replicate injections of the nominal standard solution (50 µg/ml) in volumes of 20 µl each were used to assess repeatability. Both study results were compared (intermediate precision) and presented as a percentage of the measurement's relative standard deviation. mediocre accuracy In order to verify the reproducibility of the results, analysis is carried out on a different day. Six samples were prepared using the same methodology as the Repeatability parameter.
4. Accuracy
The range of accuracy that was measured was between 50% and 150% of working concentration. The answer for each accuracy level was made in triplicate. % Recovery for each sample, Mean% Recovery for every level, and Total Recovery were computed. Additionally, % RSD for every level and % RSD for the total recovery were computed. The percentage RSD should have a maximum value of 2.0%.
5. Robustness
The method's robustness was demonstrated by purposefully varying the temperature by ± 2°C, the flow velocity by ± 0.1 ml, and the detection wavelength by ± 3 nm while estimating the tablet. The method's robustness is demonstrated by the reproducible findings that were produced.
RESULTS AND DISCUSSION:
FTIR spectrum of Acebrophylline:


Development of HPLC method for Acebrophylline
High performance liquid chromatographic method was developed and validated for determination of Acebrophylline in bulk form. Mobile phase consists ACN: Methanol (80:20). Chromatogram obtained was shows the maximum wavelength where the drug shows maximum response was 275 nm

Typical chromatogram of Acebrophylline
Linearity
Drug was found to be linear in the concentration range of 10-50 ?g/ml. Results obtained are shown in Table and calibration plot obtained was shown in Figure.
Table No.: Data of calibration curve of Acebrophylline by HPLC method


Calibration curve for Acebrophylline
Results of LOD and LOQ values of Acebrophylline

Optical characteristics for Acebrophylline

Accuracy
Accuracy was studied by standard addition method and % recovery found was within acceptable limit. Results of recovery study are shown in Table no.16 and statistical validation is shown in Table
Table No.: Data for recovery study of Acebrophylline by HPLC method

Table No.: Statistical validation of Acebrophylline by HPLC method

Average of three determination
Precision
Intraday and interday precision assures the repeatability of test results. The % RSD found was below 2. Result of intraday and interday precision was shown in Table.
Table No.18: Data for intraday precision of Acebrophylline by HPLC method

Table No.: Data for interday precision of Acebrophylline by HPLC method
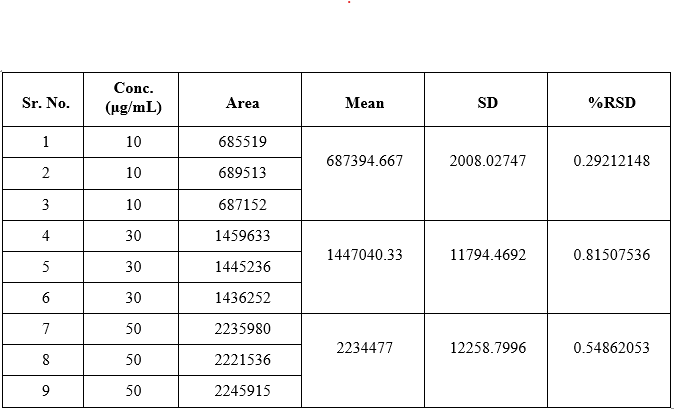
Robustness
Robustness was studied by different deliberate variations in the chromatographic conditions. Results are shown in Table
Table No.21: Data for Robustness study of Acebrophylline by HPLC method

Ruggedness
Ruggedness was studied by different analyst. Results obtained are shown in Table
Table No.22: Data for ruggedness study of Acebrophylline by HPLC method

Specificity
Excipients and impurities were not interacting with the standard drug, hence method is specific. Results of specificity are shown in Table
Table No.23: Data for specificity study of Acebrophylline by HPLC method

Assay:
The % Assay of AB Phylline 200 marketed formulation of SUN Pharma was calculated and given in table
Table No 24: % Assay of Marketed Formulation

System Suitability:
System suitability parameters were measured to verify the system, method and column performance. Standard solution of Acebrophylline was injected into the system for five times and system suitability parameters were checked.
Table No: Data for System suitability study of Acebrophylline by HPLC Method

SUMMARY
A successful attempt was made in the current study to determine the bulk concentration of acebrophylline using high performance liquid chromatography. The method was created by trial and error, drawing from a review of the literature. The goal of this study project is fully achieved by the suggested method's simplicity, speed, reproducibility, and economy. The HPLC method for estimating acebrophylline was created and proven to work. The outcome demonstrated that the suggested approach was appropriate for the quick, accurate, and exact measurement of acebrophylline in both pharmaceutical dose form and bulk form. The ICH guidelines were followed throughout the process of method validation. The approach that was created was inexpensive, selective, accurate, and exact.
The mobile phase was consisting of ACN: Methanol (80:20). Detection was done at 275 nm. The method was found to be simple, linear, rapid, accurate, precise, reproducible and robust. The % RSD was found within limit. The result showed that proposed method was suitable for the accurate, precise and rapid determination of Acebrophylline in its bulk form and pharmaceutical dosage form. Developed method was accurate, precise, robust and rugged, it shows % RSD not more than 2% .The validation of method was caried out using ICH guidelines Acebrophylline in bulk and commercial formulations can be estimated using this method since it produces a crisp and accurate peak.
CONCLUSION
The assay for acebrophylline is determined using the RP-HPLC method, which has been validated for parameters such as accuracy, precision, linearity, robustness, ruggedness, system suitability, limit of detection, limit of quantification, etc. The method has been found to be accurate, precise, linear, robust, and rugged in accordance with ICH guidelines.
This outline gives a thorough overview of the process of creating and validating an Acebrophylline analytical method, which is necessary to guarantee a consistent level of quality in pharmaceutical products. Thus, it was discovered that the devised procedure was straightforward, sensitive, accurate, and exact. They can be utilized for routine examination of acebrophylline estimate because they don't interfere with the excipient used in the formulations.
REFERENCE
- Available from: https://go.drugbank.com/drugs/DB13141
- Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Acebrophylline
- ICH Q2 (R1), Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization, IFPMA, Geneva, 2005.
- Aslam Patel, Rajshree Patil, “Development and validation of UV Spectroscopic method for Estimation of Acebrophylline in Tablet dosage form”, American Journal of Pharmatech Research, Volume:9, Issue:2 Page no. 1-9.
- Sharma Bhavik, Agarwal Sushil Kumar, “RP-HPLC Method Development and Validation for Estimation of Acebrophylline”, Asian Journal of Pharmaceutical Research and Development, Volume:6 Issue:6 Page no. 56-59.
- Sunil R Dhaneshwar, “Development and Validation of Stability Indicating RP- HPLC-PDA Method for Determination of Acebrophylline and Its Application for Formulation Analysis and Dissolution Study”, Journal of Basic and Applied Scientific Research Volume:1 Issue: 11, Page no. 1884-1890
- Chatwal G. R., Anand S. K., Instrumental Methods of Chemical Analysis, Fifth Edition, 2008, Himalaya Publishing House, 2.108-2.124.
- V. Gupta, A.D. K. Jain, N.S. Gill, K. Gupta, Development and validation of HPLC method - a review, Int. Res J Pharm. App Sci., 2(4) (2012) 17-25
- B. Prathap, G.H.S. Rao, G. Devdass, A. Dey, N. Harikrishnan, Review on Stability Indicating HPLC Method Development, International Journal of Innovative Pharmaceutical Research.3(3) (2012) 229237.
- Aslam Patel, Rajshree Patil, “Development and validation of UV Spectroscopic method for Estimation of Acebrophylline in Tablet dosage form”, American Journal of Pharmatech Research, Volume:9, Issue:2 Page no. 1-9.


 Rameshwar P. Tathe* 1
Rameshwar P. Tathe* 1
 Prashant S. Malpure 2
Prashant S. Malpure 2
















 10.5281/zenodo.12738104
10.5281/zenodo.12738104