Abstract
Pneumonia is a serious lung infection that can affect children of all ages ,but it's particularly dangerous for young children and infants Viral pneumonia is a lung infection caused by a virus. It occurs when a virus, such as influenza, RSV, infects the air sacs in the lungs. These air sacs become inflamed and fill with fluid, making it difficult to breathe. While less severe than bacterial pneumonia in many cases, viral pneumonia can still cause significant illness, especially in young children, the elderly, and people with weakened immune system Pneumonia in infants occurs when the tiny air sacs in their lungs become inflamed and fill with fluid. This can happen due to various germs, including bacteria, viruses, and fungi. Infants are particularly vulnerable due to their developing immune systems, smaller airways, and limited ability to clear infections effectively. Common causes in infants include respiratory syncytial virus (RSV), influenza, and certain types of bacteria.ems. In these review article I have mentioned certain parameter of which mentioned bellowed through which we can say that pneumonia treatment aims to combat the infection and provide supportive care. Antibiotic therapy is crucial for bacterial pneumonia, while antiviral medications may be helpful for certain viral infections. Supportive care, including rest, hydration, and fever management, is essential for recovery. Hospitalization may be necessary for severe cases to provide intensive care and monitor the patient closely.
Keywords
Respiratory infection, Inflammation, Syncytial Virus, Bronchiolitis , Pneumococcal, Episodescausing.
Introduction
Although the most common cause of infection, Deaths in the United States and Most Western Countries In some countries [1] (Metlay, 2019), there is still no simple clinical definition of pneumonia. clinical symptoms Findings - Cough, fever, shortness of breath, crackling (or crackling) symptoms upon examination and infiltration Breast imaging - non-specific, there are some. Alternate diagnosis. because we're just watching It could be said that the symptoms, that is, we do not actually see pneumonia, but only indirectly through the perspectives we provide diagnostic tool. A changing perspective Widespread use of chest computed tomography (CT) and advances in ultrasound technology. Chest CT provides a more accurate view of lung parenchyma than X-rays, which have demonstrated both low sensitivity and positive predictive value when compared to chest CT [2(Self, 2013),3(Claessens, 2015)]. The use of CT has dramatically increased in the past two decades,[4] (Smith-Bindman, 2019) which raises the question as to whether previous studies that employed chest radiographs as a gold standard carry the same meaning forward, or whether patients diagnosed by CT maybe sufficiently different to indicate different treatment. One surveillance study of hospitalized patients with a pneumonia diagnosis compared 66hospitalizations with CT-confirmed, radiograph negative pneumonia to 2185 radiograph-positive cases, and found similar rates of pathogen detection, Intensive Care Unit (ICU) admission, and length of stay,[5] (Upchurch, 2018) suggesting that we may be able to apply similar diagnosis and treatment approaches to newer populations. However, larger studies could examine these populations more thoroughly. Ultrasound is also an emerging technology that has expanded in its availability of point-of-care tools with increasingly high quality. Several studies have suggested that lung ultrasound demonstrating airspace consolidation or focal distribution of B lines may have closer alignment with the clinician diagnosis of pneumonia [6] (Bourcier, 2014) or CT findings [7] (Cortellaro, 2012) than chest radiographs but the quality of a breast ultrasound depends on both factors Technician Skills and Interpretation Images (neither of which is very standardized) A more recent practice than radiological imaging. Furthermore, no studies have examined: Lung ultrasound improves diagnosis and outcome. Perceived Relative Superiority, Level of Acceptance, and Consistency for Chest Radiographs Roles are affected when using this technique Future new technology.
- Advances In Knowledge: What Causes Pneumonia?
Our perspective on the pathogenesis of pneumonia is also changing due to advances in microbial detection and clinical epidemiology (Figure 1). Previously thought to be a sterile space, the lung is now recognized as a complex ecosystem of microbes, with equally complex relationships to their host and each other, analogous to an 'adaptive island' with dynamic interactions that drive changes in species prevalence within a host.[8] (Dickson, 2014) Within this model, the theory of lung infection has changed from pathogen invasion of a sterile space to disruption of balance in existing microbes, with or without the introduction of a new pathogen. The characteristics of the host, and the interaction between the host and pathogen, are additional factors that influence the ultimate consequences of this disruption.[9] (Dela Cruz, 2018)The widespread availability of rapid molecular diagnostic testing has provided a new insight into etiologies of pneumonia that challenges paradigms set by earlier studies from microbiology cultures. One example is a large population-based prospective observational study of adults hospitalized with radiographically confirmed pneumonia,[10] (Jain, 2015) which employed aggressive diagnostic testing including rapid molecular diagnostic testing and found a predominance of viruses, with rhinovirus and influenza overshadowing Streptococcus pneumoniae as the most commonly detected pathogens. Human metapneumovirus, respiratory syncytial virus, parainfluenza, coronaviruses, and adenoviruses were also identified, and multiple pathogens were detected in 13% of all cases in which a cause was identified. These findings were similar to other smaller studies,[11] (Holter, 2015),[12] (Ça?layan Serin, 2014) with viruses being commonly identified with S. pneumoniae. Uncertainties remain as to the meaning of these findings: whether pathogens detected in the nasopharynx or sputa represent infection versus colonization versus co-infections with other undetected microbes remains unclear, and the lack of a gold standard against which to measure the accuracy of new testing technologies continues to obscure our understanding. Further, only 38–63% of patients studied yielded a pathogen at all, which raises the question as to whether failure to detect pathogens in pneumonia reflects continued shortcomings of our diagnostic capabilities, novel pathogens yet to be discovered, or misclassification of non-infectious syndromes that mimic lung infection. Biomarkers, including C-reactive peptide, procalcitonin, and newer diagnostic technologies that combine microbial detection with inflammatory patterns [12] (Ça?layan Serin, 2014) are a promising path to elucidate our understanding further and refine our paradigm of lung infection, but to date have yet to deliver meaningful clinical interventions. Growing evidence of the presence of multiple potential pathogens in patients with community-acquired pneumonia (CAP) also challenges the classical model of pulmonary infection, although these cases are not true co-infections. It is unclear whether it is an acute infection, a continuous infection, or an acute infection with the pathogen. It is not yet known whether chronic infection with one or more other pathogens that do not cause pneumonia is involved. Only serial quantitative testing on samples from the lower respiratory tract can answer this question.
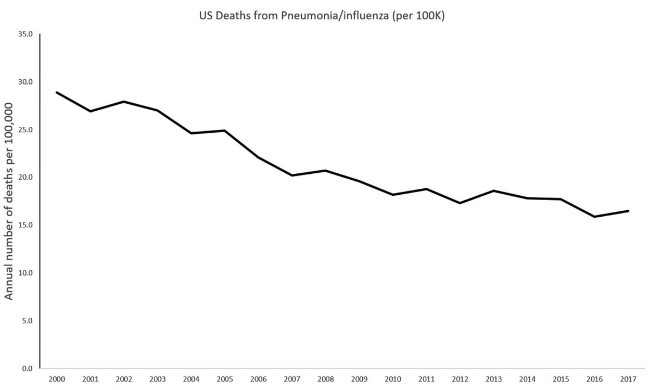
Figure 1. Timeline of annual number of US deaths from pneumonia by year, 2000–2017. Markers indicate [13](Jones B, 2020)
Pneumonia, a type of pneumococcal disease, kills nearly 1 in 5 children under the age of 5 worldwide and, based on 2000 data, kills more than 1.6 million children annually[14] (Hoffman, 2009). Reducing mortality associated with pneumococcal disease is critical for the international community to achieve the MDGs, especially MDG4 (reducing global child mortality). WHO's official position is that national immunization programs should prioritize the implementation of pneumococcal vaccines, especially in countries with high infant mortality rates. In children, pneumococci are the major cause of many clinical manifestations, including pneumonia, meningitis, bacteremia, sepsis, and acute otitis media (AOM), but are not the sole cause of these conditions. The WHO estimates of the contribution of pneumonia to childhood mortality published in the Global Action Plan for Pneumonia Prevention (GAPP) are shown in Figure 1. Pneumococcal disease is relatively common and can be severe, making it an important cause of morbidity, mortality, and healthcare costs [15] (Ray GT, 2006). In 2010, the United Nations (UN) published his latest report on MDG4, and in 1990 he showed that significant progress had been made in reducing child mortality since the introduction of the MDGs [16] (Hoffman, Millennium Development Goal 4: reduce child mortality, 2018). According to United Nations estimates, in 2009 he had 12,000 fewer children dying per day than in 1990. More importantly, progress has accelerated since 2000. While North Africa and East Asia have seen the greatest progress, estimates for Latin America and the Caribbean show that the under-five mortality rate has declined from 52 to 23 per 1,000 live births (a 55?crease). is shown to exist. Infant mortality remains unacceptably high, but with continued efforts, his MDG4 target of reducing mortality by two-thirds appears achievable.
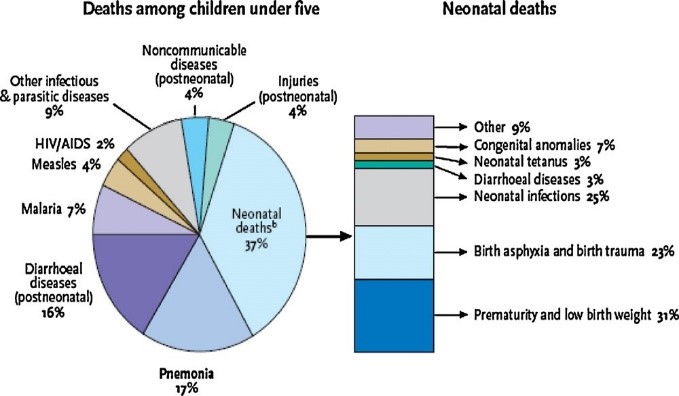
Fig.1.Causesofmortalityinchildren<5year>
- Why Pneumonia Occurs In Children?
Community-acquired pneumonia (CAP) is an acute infection of the lung parenchyma in non-hospitalized patients. It is characterized by the development of fever and/or respiratory symptoms, and the presence of lung infiltrates and consolidation on chest radiographs [18](K, 2002). Ideally, the definition should include isolation of the causative organism. However, in many cases the pathogen is not identified and therefore does not meet the clinical definition. CAP is classically classified into three syndromes. Typical or bacterial CAP, atypical (due to viral or atypical bacteria) and indeterminate (cases that do not meet the necessary criteria for inclusion in the first two groups). Because it is often difficult to unambiguously distinguish between types of CAP, diagnostic algorithms have been developed to serve as guides for diagnosis, based on a combination of clinical, analytical, and radiological criteria [19](Manejo racional de la neumonía aguda de la comunidad, 1999). Bacterial CAP in infants and young children is most often caused by a previous viral infection, causing a sudden high fever and general deterioration of the patient. It may also present as fever of unknown. origin, the "silent" pneumonia characteristic of pneumococcal CAP. (Table 2).
|
Synopsis of main recommendations
|
|
Etiology and epidemiology
|
Table 2: Synopsis of main recommendations.[20](García-Elorriaga G1, 2015)
|
Streptococcus pneumoniae (S. pneumoniae) is the most frequent cause of bacterial pneumonia in childhood.
Age is a good predictor of probable pathogens:
Viruses are a more common cause in small children.
In older children, if a bacterial agent is found, it is more commonly S. pneumoniae followed by Mycoplasma and chlamydial pneumonia.
An important proportion of CAP (8-40%) is the result of a mixed infection.
|
|
Clinical characteristics
Bacterial pneumonia should be considered in children up to 3 years of age if fever is >38.5°C, retractions are present and respiratory rate is >50/min. In older children, a history of respiratory distress is more useful than clinical signs.
If wheezing is present in a toddler, primary bacterial pneumonia is improbable.
|
|
Radiographic findings
Chest X-Rays should not be routinely obtained in children with acute, uncomplicated lower respiratory tract infections.
Radiological findings are not good indicators of the etiology.
• Chest X-Ray follow-up is only useful after lobar collapse, apparent pneumonia or persistence of symptoms.
|
|
General testing
Pulse oximetry must be obtained in all children with pneumonia admitted to the hospital.
Acute phase reactants do not differentiate between bacterial and viral infections in children.
|
|
Microbiological testing
Specific microbiologic tests.
Blood cultures should be obtained in all children suspected of harboring bacterial pneumonia.
Obtain paired serum samples.
Severity evaluation
Indicators of hospital admission in babies:
oxygen saturation <92>70 breaths/min; respiratory distress.
Indicators of hospital admission in older children:
oxygen saturation <92>50 breaths/min; respiratory distress.
Management of antibiotics
Small children with mild symptoms of lower respiratory tract infection do not require treatment with antibiotics.
Oral amoxicillin is the first-choice antibiotic in children under the age of 5 since it is effective against most pathogens causing CAP in this group, it is well tolerated and low-cost.
Since mycoplasma pneumonia is more frequent in older children, macrolide antibiotics may be used as empiric first-line therapy in children above age 5.
Complications
If a child remains febrile and in bad overall health 48 hours after admission, he should be re-evaluated while considering possible complications.
|
Pneumonia is responsible for 15% of all deaths in children under the age of 5, killing 922,000 children in 2015. 3 CAP is one of the most common childhood infections, with a reported prevalence of 1,000-4,000/100,000 children/year [21(Salud, 2015),22(Montejo M, 2005)]. In the United States, CAP is the leading cause of infection-related death and the sixth leading cause of death overall. There are approximately 4.2 million outpatient visits annually and more than 60,000 deaths [23](Chen K, 2015). Managing CAP is not easy. Establishing an etiologic diagnosis and initiating appropriate antibiotic therapy is often a complex task. Testing for CAP etiology is complicated by the low yield of blood cultures [24](Juven T, 2000), the difficulty in obtaining adequate sputum samples, and reluctance to perform lung aspiration and bronchoalveolar lavage in children. It is difficult to quantify the proportion of CAP by bacteria. Streptococcus pneumoniae is thought to be the most common bacterial causative agent of CAP, but this organism is rarely identified in blood cultures. The Pneumonia Etiology Research for Children's Health (PERCH) project refers to a complete, standardized, multinational assessment of the pathogens that cause severe and very severe pneumonia in children in developing countries. PERCH will be the largest and most comprehensive study of childhood pneumonia etiology to date [25](Levine OS, 2012).
Acute Respiratory Infection (ARI) in Children Under 5 Years of Age is the leading cause of infant mortality in the world. Most of these deaths are due to pneumonia bronchiolitis. According to WHO estimates, the number of infected people per year is Her ARI-related deaths (excluding those due to) in this age group. Measles, whooping cough, and neonatal deaths) accounted for 2.1 million (26)(Murray CJL, 2001). About 20% of childhood deaths are due to them. This was primarily based on mortality estimates published by Williams. Other. (27)(Williams BG, 2002). The main purpose of this work is to estimate Incidence of pneumonia and bronchiolitis world. This can be taken as a good approximation The global incidence of these diseases is nearly 90%. Children under the age of 5 living in developing countries (27)(Williams BG, 2002), and the incidence of these diseases is much higher for children in developing countries (28(Jokinen C, 1993), 29(Weigl JA, 2003)). This study was commissioned and supervised by the Child Health Epidemiology Reference Group (CHERG). WHO Child and Adolescent Health Service, And the resulting estimates received critical reviews such as: this group. Acute lower respiratory tract infection (ALRI) is defined as: International Statistical Classification of Diseases and Related Disorders Health issues, 10th revision, infectious diseases of concern, etc. Airway below the epiglottis. These include laryngitis, tracheitis, bronchitis, bronchiolitis, acute pulmonary manifestations.an infection, a combination thereof, or a combination thereof Upper respiratory tract infections, including influenza. The focus of the global burden of disease programme has been on conditions accounting for a substantial loss of disability-adjusted life years. Within CHERG, a decision was made to concentrate on pneumonia and bronchiolitis because these are considered to be the major components of ALRI that account for the global burden of disease from acute respiratory infections among young children. This approach is consistent with the WHO approach to the case management of ARI, which was introduced first by the programme for the control of ARI in order to standardize and facilitate clinical decision-making in places with limited resources and which has now been incorporated into the global Integrated Management of Childhood Illness, or IMCI, programmes. IMCI programmes train health workers to identify fast breathing, lower chest wall indrawing or selected danger signs in children with respiratory symptoms (such as cyanosis or an inability to drink). For the programme's purposes, these are labelled “pneumonia”, although it is recognized that children identified in this way include those with pneumonia, bronchiolitis and a proportion of those with reactive airways disease associated with a respiratory infection. The study described in this article has taken this approach, and we have taken it as well. The symptoms identified were termed "clinical pneumonia" of Therefore, in this review, pneumonia, bronchiolitis, and Reactive airway disease associated with respiratory infections in some studies. No cases of acute laryngitis have been reported bronchitis or tracheitis.
Treatment Of Pneumonia
- Diagnosis of pneumococcal diseases in children
A definitive diagnosis of pneumococcal disease requires laboratory detection of Streptococcus pneumoniae. difficult to estimate Exposure to pneumococcal disease (which is often not the case) Received for clinical examination. In this case, the sensitivity of standard diagnostics is low. H. A positive reaction was obtained in a laboratory test Only a minority of children actually have pneumococcal disease. Therefore, observational studies and surveillance efforts It systematically underestimates the burden of pneumococcal disease [30](Constenla D, 2011). Potentially more accurate estimates of actual pneumococcal disease burden as part of vaccine trials Determine the actual rate of pneumonia cases to S lung infection. However, such research results can still be falsified by differences in technical implementations in laboratories test [30](Constenla D, 2011). Conventionally, pneumonia is not included Diseases notifiable by public systems worldwide. Therefore, laboratory monitoring systems play an important role in this matter. Determination of bacterial pneumonia burden; this is achieved by assessing serotype distribution and patterns antimicrobial resistance of selected isolates in hospitalized patients forpneumonia [31](Andrade AL, 2004). In 1993, the Pan American Health Organization (PAHO) initiated a coordinated surveillance program for pneumococcal disease. Supporting the Americas through vaccine programs, Coordinating vaccination with local systems vaccine (SIREVA) [30(Constenla D, 2011),32(Garcia S, 2006)]. SIREVA collects serotype data samples Distribution and antimicrobial resistance associated with pneumonia and pneumonia Bacterial meningitis occurs in this area. The program started Only 6 countries: Argentina, Brazil, Chile, Colombia and Mexico Uruguay – but at the same time he expanded his presence in 22 countries [30](Constenla D, 2011). In addition to monitoring pneumococci, PAHO also monitors Haemophilus influenzae and meningococci of the initial goal of a surveillance system involving children under 6 years of age was to examine prevalence. This article describes the types of capsules that cause invasive pneumococcal disease (IPD). Strengthen local laboratory and epidemiological capacity Monitoring of pneumococcal serotypes and antimicrobial resistance, database creation and support of isolates and samples Pneumococcal vaccine development program [33(Hoffman, Millennium Development Goals (MDGs), 2018),34(Nervi, 1993)]. of the latter goal has been successfully achieved, but new vaccines with extended coverage continue to be developed. Much work remains to be done to ensure that immunization programs are implemented in the United States. Develop the most profitable areas. the system will continue to work further developed and expanded at the same time by several collections Incidence data [30](Constenla D, 2011). Coordinated efforts are being made to systematically collect laboratory data, with the support of international organizations for pneumonia. As in Andrade et al., [31](Andrade AL, 2004), this surveillance approach is based on examination of samples Hospitalized children tend to be reported as more severe A case where the course is unfavourable. Similarly, this approach underestimates cases of mild and moderate illness that may occur as well public health impact due to high incidence. As with most surveillance programs, the denominator is (number of children at risk). Not known; therefore, efforts are needed to estimate pneumococcal burden Child illnesses at the local level may be inaccurate, they lack external validity [31(Andrade AL, 2004),35(Dagan, 2010)]. Population-based epidemiological data are difficult to obtain Pediatric Pneumococcal Infection and Latin Pediatric Pneumonia America has several reasons. This includes the low sensitivity of bacteriological diagnostics available in the regionand the fact that many cases of pneumonia are not bacteremia makes isolation and testing of pathogens difficult [36](Hortal M, 2007)of An Attempt to More Accurately Estimate the Pneumonic Disease BurdenAn assessment of the impact of immunization programs is determined by the WHO Immunizations, Vaccines and Biologicals AgencyWorking Group to Standardize Classification of Radiologylung infection. The presence of significant alveolar consolidation Itis considered by most experts to be the most specific radiographCurrently available predictors of bacterial pneumonia [37](Cherian T, 2005). Three Latin American countries (Argentina, Chile and Uruguay) are foundedPopulation-based surveillance for radiographically confirmed pneumonia andData from some of these surveillance systems have recently been published [30(Constenla D, 2011), 38(Lagos R, 2003), 39(Gentile ABJ, 2008)]. This recently published data should tell the story.Benchmarks for assessing the impact of inclusions. Pneumococcal vaccines included in national immunization programs LatinAmerica.
- Strategies for treatment, prevention and protection from pneumonia
Key Strategies to Protect Children from Infectious Diseases Prevention of pneumococcal infections, causative infections Medicine and treatment for children suffering from pneumoniaEstablished by WHO/United Nations Children's Fund (UNICEF).[14]((??UNICEF), 2009) is shown in Figure 2. Her three pillars of these strategies are considered to be promotion of breastfeeding (protection) and vaccination. (prevention) and case management (treatment). WHO/UNICEFEstimates of the impact of the 2015 MDG target year on childhoodMortality rates for these key measures are based on levels of coverage.Each intervention at the global level is shown in Figure 3.
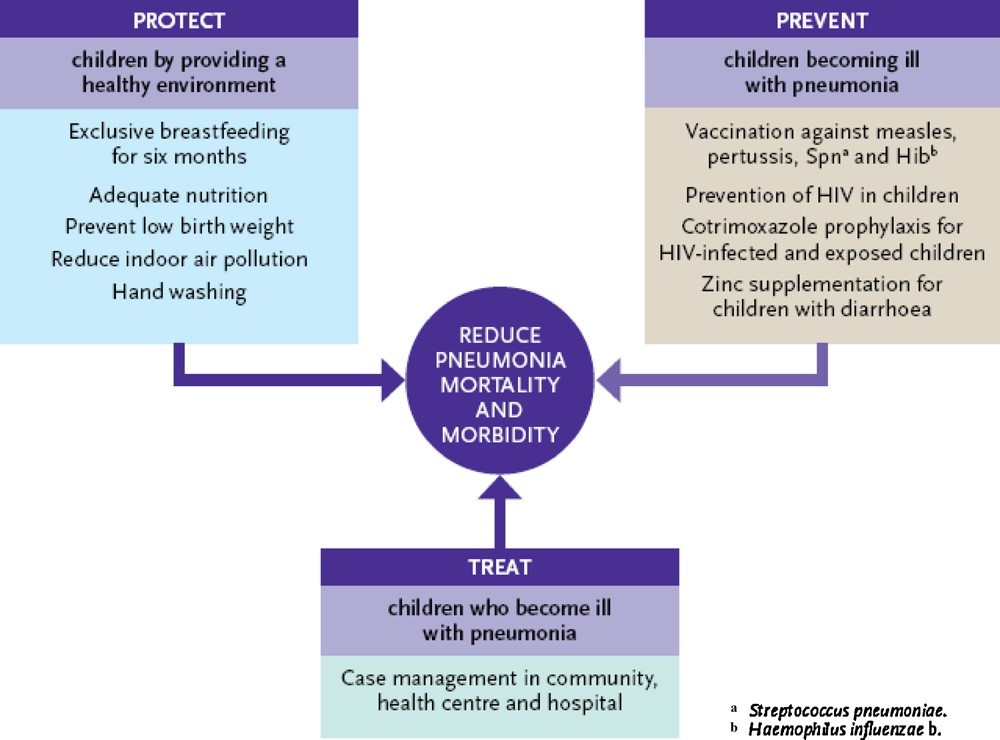
Fig. 2. Framework for pneumonia control outlined by WHO/UNICEF based on protection from exposure to agents that cause pneumococcal disease, prevention of illness in exposed children and treatment of children who fall ill with pneumonia. Reproduced with permission from WHO/UNICEF Global Action Plan for Prevention and Control of Pneumonia (GAPP) [40](Gentile & Bazán, 2011).
- Case management at all levels
Increased access to case management in the community and within health care facilities is a crucial component of pneumonia control and reduction of childhood mortality.
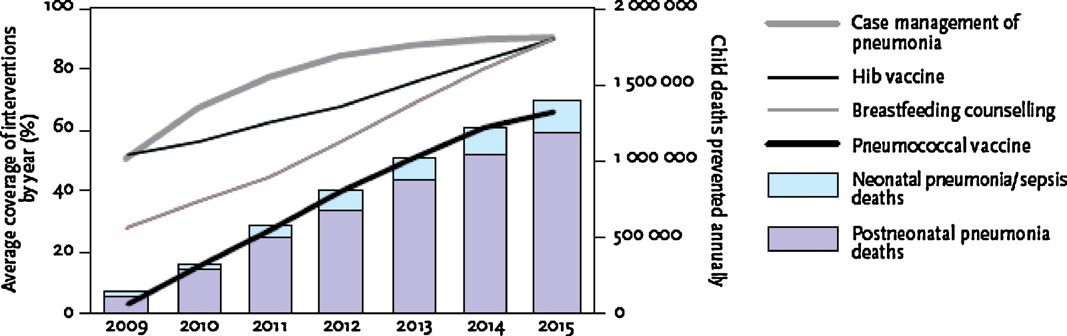
Fig. 3. WHO/UNICEF estimates of childhood pneumonia deaths that could be prevented in the 68 “Countdown” countries by case management, H. influenzae b vaccination, breastfeeding counselling, and pneumococcal vaccination. The left axis shows average coverage for each of these interventions; the right axis shows estimated childhood deaths averted. Reproduced with permission from WHO/UNICEF Global Action Plan for Prevention and Control of Pneumonia (GAPP) [41](Gentile & Bazán, WHO/UNICEF estimates of childhood pneumonia deaths that could be prevented in the 68 “Countdown” countries by case management, H. influenzae b vaccination,, 2011).
Pneumococcal disease is preventable through vaccination.Pneumonia and other diseases are caused by pneumococci, Haemophilus influenzae can be treated with antibiotics.Bacterial resistance to commonly used antibiotics is a problem worldwide, which is why we need to promote diseasePrevention through the use of an effective pneumococcal vaccine.Unfortunately, delays of up to 15 hours are commonYears to vaccine availability in developed countriesIt is widely available in developing countries [42](Nayar , 2011). It is unacceptable from the MDGs point of view. Vaccination is one of the most importantan efficient and cost-effective way to reduce morbidity and mortality from infectious diseases [43](Levyt, 12 JUNE 2009 – 31 MARCH 2010). For pneumococcithe pneumococcal conjugate vaccine (PCV) is for S. Pneumonia in infants, can occur indirectlyProtection of unvaccinated community members, including: (unvaccinated children) due to the dependent "group effect "Immunization reduces transmission of pathogens Individual. Pneumococcus is the world's biggest vaccine-preventable killer, so widespread use of PCVcan have a significant impact on overall reductions Infant mortality [43](Levyt, 12 JUNE 2009 – 31 MARCH 2010). This is especially true for developmentSince more than 98% of deaths are due to pneumococci,The disease occurs in developing countries [43](Levyt, 12 JUNE 2009 – 31 MARCH 2010). GAPP's vision is"All children are protected from pneumonia by a healthy diet.”” [44(World Health Organization. Global immunization data, 2008)(Jones G, 2003)(Sazawal S, 2003)(M., 2009)(Niessen LW, 2009)-48]. Specific numerical goals related to pneumococcaldisease are to:
• Reduce mortality from pneumonia in children less than 5 years ofage by 65% by 2015 compared to 2000 levels;
•Reduce the incidence of severe pneumonia by 25% in children lessthan 5 years of age by 2015 compared to 2000 levels.
The following targets have been established for the end of 2015:
•90%coverage of eachrelevant vaccine (with80%coverage ineverydistrict);
• 90?cess to appropriate pneumonia case management;
• 90% coverage of exclusive breastfeeding during the first sixmonths.
The WHO and UNICEF in the GAPP have outlined the additionalspecific recommendations, outlined in the following sections, thatcan contribute to reducing the incidence of pneumonia infectionand improving outcomes in infected children [14](M., Global health: some neglected diseases are more neglected than others., 2009). Because of thevalue of widespread implementation of pneumococcal vaccinationin achieving the MDG goal of reducing childhood mortality, theGAVI Alliance, with its partners, launched a new financial mechanism called the Advance Market Commitment (AMC) [42(Nayar , 2011),49( Advance Market Commitment for Pneumococcal Vaccines, 2008)], whichis committed to facilitating global uptake of pneumococcal vaccination by (50)(WHO. Millennium Development Goals (MDGs)., n.d.) accelerating the development of pneumococcal vaccinesthat meet the needs of developing countries, (51)(United Nations Development Program, n.d.) bringing forwardvaccine availability in developing countries by guaranteeing aninitial purchase price, (52)(Millennium Development Goals Indicators, n.d.) accelerating vaccine uptake by ensuring predictable vaccine pricing, and (53)(www.whqlibdoc.who.int, 2009) by pilot testing the AMCmechanism as an incentive for implementation of needed vaccineprograms and to learn lessons for the future [49]( Advance Market Commitment for Pneumococcal Vaccines, 2008).
- Prevention and management of HIV infection
The GAPP has specifically identified the need to develop strategies to prevent mother-to-child transmission of HIVand to improve themanagement of HIVinfectionand P. jiroveci pneumonia prophylaxis in children, countries where HIV is prevalent.
- Improvement of nutrition and reduction of low birth weight
Promoting exclusive breastfeeding to allow transmission of passive immunity from mother to child through contagionThe concentration of antibodies in breast milk is an important factor in pneumonia prevention. Zinc supplements that help a lot It can also improve outcomes in children with diarrhoea.Benefits for children suffering from pneumonia. Strategies for price reductionLow birth weight and malnutrition prevent pneumonia,improve outcomes for sick children and should be encouraged
- Control of indoor air pollution
Indoor air pollution increases the risk of pneumonia strategy Reducing indoor air pollution can and should prevent pneumonia get promoted. Specific measures described in GAPP include. The use of liquid fuel stoves or modified solid fuel stoves is increasing. GAPP also mentions other prevention strategies such asB. Handwashing should be encouraged finally, Pneumonia is a common and serious consequence of influenza. Preparing for an influenza pandemic must also include prevention Increase control of pneumonia and urgency of community-acquired infections management.
CONCLUSION
This overview shows the magnitude of the clinical burden Pneumonia in developing countries is often considered only for mortality. Definition of clinical pneumonia in this studyClinical manifestations include reactive airway disease associated with pneumonia, bronchiolitis, and respiratory disease, Infectious disease (however, the proportion of episodes due to the latter was low among the populations discussed). these episodescausing or causing acute distress or short-term complications adversely affect or may adversely affect a child's nutritional status It influences the risk of other childhood diseases. moreover,many studies show the presence of clinical pneumonia Early childhood can have long-term effects on respiratory function and health. This review shows that there is a large variation in annual incidence among young children.in different settings. This indicates the possibility of interventionPreventing risk factors to prevent clinical pneumonia and significantly reduce the resulting high burden of childhood illnesswith clinical pneumonia.
REFERENCES
- Advance Market Commitment for Pneumococcal Vaccines. (2008). Retrieved from www.vaccineamc.org: ] http://www.vaccineamc.org/timeline_media/Expert Group Report.
- (??UNICEF), W. H. (2009, Jun 17). Global Action Plan for Prevention and Control of Pneumonia (?GAPP)?. Retrieved from www.who.int: https://www.who.int/publications/i/item/WHO-FCH-CAH-NCH-09.04
- (2009). Retrieved from www.whqlibdoc.who.int: http://whqlibdoc.who.int/publications/2009/
- Andrade AL, S. S. (2004). Population-based surveillance of pediatric pneumonia: use of spatial analysis in an urban area of Central Brazil. Cad Saude Publica, 411–21.
- Angela Gentile, V. B. (2011). Causes of mortality in children <5>
- Bourcier, J. E. (2014). Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. The American journal of emergency medicine, 115-118.
- Ça?layan Serin, D. P. (2014). Bacterial and viral etiology in hospitalized community acquired pneumonia with molecular methods and clinical evaluation. Journal of infection in developing countries, 510–518.
- Chen K, J. R. (2015). The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public, 15:113.
- Cherian T, M. E. (2005). Bull World Health Organ, 353-9.
- Claessens, Y. E. (2015). Early Chest Computed Tomography Scan to Assist Diagnosis and Guide Treatment Decision for Suspected Community-acquired Pneumonia. American journal of respiratory and critical care medicine, 974–982.
- Constenla D, G. E. (2011). Cost-effectiveness of pneumococcal conjugate vaccination in Latin America and the Caribbean: a regional analysis. Original research, 313.
- Cortellaro, F. C. (2012). Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emergency medicine journal, 19-23.
- Dagan, R. (2010). Informe Regional de SIREVA II, 2009: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Organización Panamericana de la Salud., 306.
- Dela Cruz, C. S. (2018). Future Research Directions in Pneumonia. NHLBI Working Group Report. American journal of respiratory and critical care medicine, 256-263.
- Dickson, R. P.-D. (2014). Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. The Lancet. Respiratory medicine,, 238-246.
- Garcia S, L. O. (2006). Working Group members. Pneumococcal disease and vaccination in the Americas: an agenda for accelerated vaccine introduction. Rev Panam Salud Publica, 340–8.
- García-Elorriaga G1, *. D.-P. (2015). Synopsis of main r?cŽmm?n???Žn?. iMedPub Journals, 1-2.
- Gentile ABJ, F. M. (2008). Jornada de la Sociedad Latinoamericana de Infectología, 16 al 19.
- Gentile, A., & Bazán, V. (2011). Framework for pneumonia control outlined by WHO/UNICEF based on protection from exposure to agents that cause pneumococcal disease, prevention of illness in. ELSEVIER, C18.
- Gentile, A., & Bazán, V. (2011). WHO/UNICEF estimates of childhood pneumonia deaths that could be prevented in the 68 “Countdown” countries by case management, H. influenzae b vaccination,. ELSEVIER, C19.
- Hoffman, J. (2009, June 17). Global Action Plan for Prevention and Control of Pneumonia (?GAPP). Retrieved from www.who.int/publications/i/item/WHO-FCH-CAH-NCH-09.04: https://www.who.int/publications/i/item/WHO-FCH-CAH-NCH-09.04
- Hoffman, J. (2018, Feb 19). Millennium Development Goal 4: reduce child mortality. Retrieved from www.who.int: https://www.who.int/news-room/fact-sheets/detail/millennium-development-goals-(mdgs)#:~:text=Millennium Development Goal 4: reduce child mortality&text=Between 1990 and 2013, under,4.0% during 2005–2013.
- Hoffman, J. (2018, February 19). Millennium Development Goals (MDGs). Retrieved from www.who.int: https://www.who.int/news-room/fact-sheets/detail/millennium-development-goals-(mdgs)#:~:text=The United Nations Millennium Declaration,are derived from this Declaration.
- Holter, J. C. (2015). Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC infectious diseases, 64.
- Hortal M, E. M. (2007). A population-based assessment of the disease burden of consolidated pneumonia in hospitalized children under five years of age. Int J Infect Dis, 273–7.
- Jain, S. S. (2015). Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. The New England journal of medicine, 415-427.
- Jokinen C, H. L. (1993). Incidence of community-acquired pneumonia in the population of four municipalities in Eastern Finland. American Journal of Epidemiology, 977-88.
- Jones B, W. G. (2020). Advances in community-acquired pneumonia. Therapeutic Advances in Infectious Disease, 7.
- Jones G, S. R. (2003). Bellagio Child Survival Study Group. How many child deaths can we prevent this year? . Lancet, 65–71.
- Juven T, M. J. (2000). Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J , 293-298.
- K, M. (2002). Community-acquired pneumonia in children. N. Engl J Med, 429-37.
- Lagos R, D. F. (2003). Rev Panam Salud Publica, 294-304.
- Levine OS, O. K.-K. (2012). The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis, S93-101.
- Levyt, J. L. (12 JUNE 2009 – 31 MARCH 2010). ADVANCE MARKET COMMITMENT FOR PNEUMOCOCCAL VACCINES. Advance Market Commitment, 38.
- M., E. (2009). Global health: some neglected diseases are more neglected than others. Science, ;323(5915):700.
- M., E. (2009). Global health: some neglected diseases are more neglected than others. Science, 323(5915):700.
- Manejo racional de la neumonía aguda de la comunidad. (1999). An EspPediatr, 609-616.
- Metlay, J. P. (2019). Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American journal of respiratory and critical care medicine, e45–e67.
- Millennium Development Goals Indicators. (n.d.). Retrieved from www.unstats.un.org: http://unstats.un.org/unsd/mdg/Host.aspx?Content=Indicators/OfficialList.
- Montejo M, G. C. (2005). Estudio clínico yepidemiológico de la neumonía adquirida en la comunidad enniños menores de 5 años de edad. An Pediatr, 131-136.
- Murray CJL, L. A. (2001). The global burden of disease 2000 project: aims, methods and data sources. Geneva: World Health Organization, 36.
- Nayar , A. (2011). Pneumococcal vaccine rolls out in developing world. Nature, 1476-4687.
- Nervi, L. (1993). IMPACT OF THE RESEARCH GRANTS PROGRAM OF THE PAN AMERICAN HEALTH ORGANIZATION. Pan American Health Organization , 42.
- Niessen LW, t. H. (2009). Comparative impact assessment of child pneumonia interventions. . Bull World Health organ., 472–80.
- Ray GT, W. C. (2006). Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J, 494-501.
- Salud, O. M. (2015). Neumonía. Nota descriptiva, 331.
- Sazawal S, B. R. (2003). Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis, 547–56.
- Self, W. H. (2013). High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. The American journal of emergency medicine, 401–405.
- Smith-Bindman, R. K. (2019). Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000-2016. JAMA, 843-856.
- United Nations Development Program. (n.d.). Retrieved from www.beta.undp.org: http://www.beta.undp.org/undp/en/home/ourwork/povertyreduction/focus areas/focus mdg strategies.html
- Upchurch, C. P. (2018). Community-Acquired Pneumonia Visualized on CT Scans but Not Chest Radiographs: Pathogens, Severity, and Clinical Outcomes. Chest, 601-610.
- Weigl JA, B. H. (2003). Population-based burden of pneumonia before school entry in Schleswig-Holstein, Germany. European journal of Pediatrics, 309-16.
- WHO. Millennium Development Goals (MDGs). (n.d.). Retrieved from www.who.int: http://www.who.int/topics/millennium development goals/about/en/index.
- Williams BG, G. E.-P. (2002). Estimates of world wide distribution of child deaths from acute respiratory infections. Lancet Infectious Diseases, 25-32.
- World Health Organization. Global immunization data. (2008, Jan). Retrieved from www.who.int: http://www.who.int/immunization/newsroom/Global


 Pooja Sharma *
Pooja Sharma *




 10.5281/zenodo.14700357
10.5281/zenodo.14700357