Abstract
The most sophisticated oral solid dose forms are mouth dissolving or orodispersible films because of their versatility and ease of usage. The Mouth Dissolving film is a solid oral dosage form that, when placed in the mouth without water or chewing, dissolves and decomposes rapidly. By avoiding first pass metabolism, the drug's bioavailability is increased when taken in this dosage form. Additionally, orodispersible films may result in a lower dosage, a quicker onset of action, and no choking hazards. Solvent casting and semisolid casting methods are used to laminate API chemicals that mask flavour. The solvent casting process is preferred over alternative techniques due to its superior physical qualities, glossy appearance, and excellent thickness uniformity of the films it produces. A number of factors are taken into account while evaluating mouth-dissolving films, including thickness and physical characteristics including disintegration, folding durability, and dissolution time. This review covers the formulation methods and evaluation standards for mouth dissolving films.
Keywords
Fast dissolving films, solvent casting, disintegration.
Introduction
Oral medicine administration is one of the most widely used ways because it is greater patient compliance, easier to administer, and more inexpensive. The oral route is difficult for older and paediatric patients since they have trouble swallowing and are afraid, they will choke. Drug delivery techniques have become safer and more advanced as a result of research on patient convenience and compliance. One product that has lately acquired public choice and appeal is fast dissolving medication delivery systems. This is because the product's quick disintegration or dissolve enables for self-administration even without the need for water or chewing. Fast-dissolving drug delivery devices were first created in the late 1970s to assist patients, particularly young and old, who had difficulty swallowing pills and capsules. The use of buccal medication delivery as a drug administration technique has increased recently. Bioadhesive mucosal dosage forms that have been developed include adhesive tablets, gels, ointments, patches, and, more recently, the use of polymeric films for buccal delivery, also known as mouth dissolving films. An undulating basement membrane effectively divides the stratified squamous epithelium covering the buccal cavity's surface from the underlying tissue, the lamina propria and submucosa. It's interesting to note that intestinal permeability is lower than buccal mucosa permeability, which is roughly 4-4,000 times higher than skin permeability. When administering chemicals with little skin penetration, buccal administration offers the best absorption medium. The main permeability barrier of the otiral mucosa is formed by intercellular material that comes from the so-called "membrane coating granules" at the top 200 ?m layer. These dosage forms have a two- to three-year shelf life, depending on the active therapeutic ingredient; nonetheless, they are very vulnerable to environmental dampness. An ideal quick dissolving delivery system should have high stability, transportability, ease of handling and administration, no specific packing material or processing requirements, no need for water during application, and a pleasant flavour. They are therefore perfect for patients who are bedridden, old, or have Parkinson's disease, mucositis, dysphagia, or vomiting. This cutting-edge medicine delivery technique may help meet the demands of the industry today. When rapidly dissolving films (RDF) were originally introduced to the market, they were marketed as dental strips and soap, along with breath fresheners. However, the pharmaceutical markets in the US and Europe are offering a range of dose forms for therapeutic uses. Pfizer, a major pharmaceutical company, invented the first oral strips (OS) called Listerine® pocket packsTM for mouth freshening reasons. Chloraseptic® Comfort Strips were the first therapeutic oral thin films (OTF) to treat sore throats; they included seven benzocaine Fast-dissolving buccal film is made from ingredients such as strip-forming polymers, plasticizers, active pharmaceutical ingredients, sweeteners, flavouring agents, saliva-stimulating agents, colouring agents, stabilising and thickening agents, permeation enhancers, and superdisintegrants. Regulators believe that all of the excipients used in the fast-dissolving film's composition ought to be approved for use in oral pharmaceutical dosage forms.[1
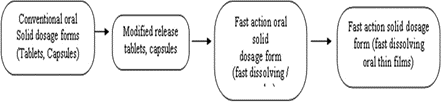
Figure no 1 : Stages in the development oral solid dosage forms
CLASSIFICATION OF FAST DISSOLVING TECHNOLOGY [1]
For ease of explanation, fast dissolve technologies can be divided into three primary kinds.
- Systems lyophilized.
- Systems based on compressed tablets.
- OTF.
SPECIAL FEATURES OF FAST DISSOLVING FILM [2]
- A film must have a sleek, appealing appearance.
- Is available in many shapes and sizes
- Unnoticeable.
- It should be easy to insert into the oral cavity.
- Must decompose rapidly in the absence of water.
- Rapid discharge.
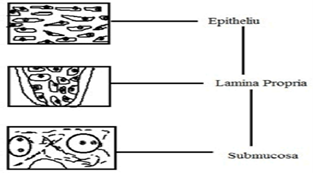
Figure no 2 : Various layers of oral mucosa
The stratified squamous epithelium makes up the oral mucosa's outermost layer. Beneath this is the deepest layer, the submucosa, lamina propria, and basement membrane. The epithelium shares features with the other stratified squamous epithelia in the body, such as a mitotic basal cell layer. Before this layer reaches the superficial layers, where cells are lost from the surface of the epithelium, it passes through a number of developing intermediate levels.
ADVANTAGES [4]
-
- Presented without the need for water, at any time, wherever.
- Its large surface area causes it to melt and dissolve quickly in the mouth.
- Dosage accuracy.
- You are able to avoid the stomach's acidic environment.
- Local as well as site-specific activities.
- Being lightweight and flexible makes it easier to handle and store the customer while in transit.
- For everyone, regardless of age, who experiences difficulties with swallowing, is ill, has a developmental or mental disorder, is resistant to therapy, or has issues consuming fluids.
- Helpful in situations requiring a quick start, such as motion sickness, severe pain, allergic reactions, or coughing.
DISADVANTAGES [5]
-
- Drugs that are not stable at buccal pH cannot be administered.
- Drugs cannot be given in larger than necessary dosages.
- Due to their harsh taste, several medications need to have their taste masks applied.
- Due to its fragile nature and the requirement to be kept dry and away from water, it needs particular packing.
- drugs that should not be taken this way because they irritate the mucosa.
IDEAL CHARACTERISTICS OF SUITABLE DRUG CANDIDATE [6]
-
- Mouth dissolving film should be thin, flexible, but strong to enable easy handling and administration, as well as a dependable manufacture and storage method. The films should not stick to surfaces, be portable, and be able to keep their flat shape without rolling.
- Simple administration for people who refuse or have mental disease or disability.
- Their flavour and texture must be pleasing.
- It is not necessary to have water.
- Everything ought to disintegrate as quickly as possible.
- The environment's temperature and humidity levels ought to have the biggest impacts on them.
- They must be able to offer pharmaceutical formulations that combine the advantages of liquid and solid forms.
CLASSIFICATION OF MOUTH DISSOLVING FILMS [7]
- Mucoadhesive melt-away wafer:
A Mucoadhesive film is applied to the buccal and gingival mucosa, and it sticks to the mucosal surface. Two kinds of films are immunoadhesive.
- Mucoadhesive melt-away strip:
This kind of strip sticks to the mucosa, melts all by itself in a few minutes, and delivers medication continuously over time.
- Sustained release mucoadhesive films:
These films stick to the mucosa and remain there for a few hours.
- Flash release wafers:
These wafers dissolve for up to 60 seconds and then immediately release the drug into the oral cavity. The application site determines how the two parts of the flash release wafers are categorised.
A. Orodispersible film (ODF):
ODF is a minuscule strip that is about the size of a postage stamp.
-
-
- Sublingual films:
These films are produced using the same methodology as ODFs. These films are positioned under the tongue as opposed to in the mouth.
STANDARD COMPOSITION OF MOUTH DISSOLVING FILMS
- Active Pharmaceutical Ingredient-
The drug-loaded film's area should be between 1 and 20 cm2, depending on how much of the water-soluble polymers accelerate the film's dissolution. An array of APIS can be supplied by oral strip technology. This delivery system may consist of multiple substances. These could include erectile dysfunction drugs, antihistamines, anti-asthmatics, gastrointestinal problems, nausea, discomfort, CNS (e.g., anti-parkinson's disease), anxiety pills, cardiovascular drugs, sore throat and cough/cold remedies (antitussives, expectorants). Since it enhances the oral strip's consistency, dissolving ability and film texture, micronized API is typically beneficial. In these oral strip technologies, APIs are more promising but have a harsher taste. This makes the composition unpleasant, particularly in the case of kid-friendly compositions.
- Film Forming Polymers-
For the creation of the oral soluble film, water soluble polymers are used, such as HPMC E-3, E-5, E-15, K-3, methyl cellulose A-3, A-6, and A6, carboxymethylcellulose, pullulan, maltodextrin, hydroxypropyl cellulose Cekol 30, polyvinyl alcohol, etc. They can be used separately or in combination to affect the desired aspects of the film. The peculiarities of the film-forming polymer:
- It must have an extended shelf life.
- It must possess good wetting properties.
- It needs to have a decent spread ability property.
- It must not encourage the growth of secondary infections in the oral mucosa or teeth.
- The mouthfeel must be pleasant.
- The polymer that is utilised must be nontoxic, nonirritating, and devoid of any impurities that may leak out. [8]
- Plasticizers-
It improves the film flexibility by reducing its brittleness and boosts the strip property for oral film processing, making it an essential component of oral film. It also improves the polymer's flow and strengthens it. The plasticizer of choice will be determined by how effectively it combines with the polymer and the solvent used in the strip casting process. Plasticizers should normally be used at concentrations between 0 and 20% w/w of dry polymer weight. [8].
D. Strip Forming Polymers –
The polymers in the formulation can be used singly or in combination to achieve the required strip properties. The resultant film must be strong enough to withstand any damage during handling or transportation. The durability of the strip will depend on the type and amount of polymer used. There are several different types of polymers that can be used to make oral strips. On the other hand, a fast-dissolving strip dosage form should be able to instantaneously dissolve in the mouth and deliver the medication to the oral cavity.
When creating oral strips, pullulan, hydromellose, and gelatin are the polymers that are most frequently used. A dry oral strip should typically have at least 45% weight percent polymers. [8].
-
-
- Saliva Stimulating Agent-
Saliva stimulaters are used to boost saliva production rates because saliva facilitates the fast disintegration of rapidly dissolving films. Saliva stimulating agents are employed singly or in mixtures ranging from 2 to 6% weight/weight of the film. [8].
-
-
- Sweetening Agent-
The use of sweeteners in pharmaceutical and food preparations that are supposed to dissolve or disintegrate in the mouth has increased. Sweeteners, both natural and artificial, are used to improve the mouth-dissolving formulations' palatability. Water soluble natural sweeteners, such as xylose, ribose, glucose, sucrose, maltose, and stevioside, are among the approved sweeteners. Artificial sweeteners that dissolve in water: acesulfame-K, sodium or calcium saccharin salts, etc. Aspartame is a dipeptide-based sweetener. Agent that Stimulates Saliva: Because most salivary stimulants have an acidic character, they promote the production of saliva in the buccal cavity and facilitate the breakdown of ODFs. Saliva-stimulating agents that are frequently used include tartaric acid, lactic acid, ascorbic acid, malic acid, and citric acid.
-
-
- Surfactant-
Surfactants are used as wetting, dispersion, or solubilizing agents to facilitate the fast dissolution of the film and immediate release of the active ingredient. Additionally, surfactants aid in the fast dissolution of buccal films by making poorly soluble medicines more soluble. As an example, consider tweens and spans, sodium lauryl sulphate, Polaxamer 407, benzalkonium chloride, and benzthonium chloride.
-
-
- Flavor-
Flavours are used to mask the unpleasant or harsh taste of integrated pharmaceuticals. Its type and potency affect taste intensity. Any US FDA-approved flavour, such as mint, sour, or sweet, may be used. According to one study, the mix of mint, licorice, and sucralose flavours successfully masks the bitter taste of diclofenac sodium. To distinguish between distinct flavours, electronic tongues are used. masked agents (TMAs) [9].
-
-
- Colouring Agent-
In oral strips, when specific formulation ingredients or drugs are present in an insoluble or suspension form, pigments such as titanium dioxide or FD&C-approved colouring additives are added at amounts no more than 1%w/w. [9]
MECHANISM OF FILM FORMATION
A thin, transparent film is formed on the skin as the solvent in the film-forming system evaporates. The volatile components of the vehicle are lost after formulation application to the skin, which results in a significant alteration in the film forming system's composition and the formation of residual film on the skin's surface. The drug's concentration on the skin's surface increases during this process to the point of saturation and possibly even beyond super saturation. Supersaturation improves drug flow through the skin by increasing the formulation's thermodynamic activity without weakening the skin's barrier, which lessens side effects and irritation. One can utilise the modified version of Fick's law of diffusion to demonstrate supersaturation. Fick's law of diffusion equation:
"J"=DKCv/h
Where
J is the drug permeation rate (flux) per unit skin area per unit time.
D is the drug's diffusion coefficient.
Cv= medication concentration h is the diffusion barrier's thickness
It is clear from this equation that there is a proportionality between the drug's concentration and the rate of skin penetration. To show supersaturation, one can apply the modified version of Fick's law of diffusion. This equation makes it very evident that the rate of skin penetration and the drug's concentration are proportionate. However, if the drug entirely dissolves in the car, this is accurate. The following equation characterises the modified form of Fick's law of diffusion: ? D/?h = J, where ? represents the drug's thermodynamic activity in the membrane and a denotes the drug's thermodynamic activity in the formulation. According to this formula, the saturation-related thermodynamic activity of the system is directly associated with the drug's flow. Conversely, as super saturation increases, thermodynamic instability increases. FFS rapidly creates supersaturated systems via the human epidermis in vitro when applied to the patch (EVRA®). By forming the skin, the film resolves the instability problem. As a result, the enhanced formulations showed a higher penetration than the conventional patch. The ethinyl ethinyl estradiol distribution efficiency of the film-forming solutions is higher than that of the commercial patch. An enhancer was used to study estradiol. The ethinyl estradiol permeation of the formulation provided approximately seven times the amount of ethinyl estradiol as the film-forming solution of the commercial patch, regardless of whether enhancer was used. Considering this, these techniques exhibit potential in comparison to the drug permeation enhancement systems that are in use today. [10].
PREFORMULATION STUDIES:
Formulation scientists first identify the physical, chemical, and mechanical properties of innovative therapeutic substances in order to design stable, secure, and efficient dosage forms. We call this procedure preformulation. The preformulation stage should ideally begin early in the discovery process in order to have the required physical and chemical data available to help with the identification of new chemical entities that join the development process. This evaluation also considered possible interactions with various inert compounds intended to be utilised in the final dosage form. The following facts must be considered.
Drug - Excipient Compatibility Study-
Practically every dosage form used in medicine requires an excipient. A stable and effective solid dosage form formulation requires the careful selection of excipients, which are additives made to a solid dosage form to promote the drug's uniform release and bioavailability, aid in administration, and shield it from degradation. The mixture was put through a 40# sieve after the API and excipients were carefully combined in the ratios shown in the table below. The liquid was filled into transparent glass vials, which were then charged to the previously stated stress condition and sealed with grey rubber stoppers and aluminium seals. API should be maintained in the same manner as samples, which should always be kept on file. Samples were taken out for analysis within two days of the sampling date in accordance with the compatibility research design. For a maximum of one month, physical observation should be done once every week. FTIR and DSC investigations were performed to determine whether the excipients and the drug are compatible.
FTIR Study-
Evaluations of Drug-Excipient Interactions by DSC Ropinirole hydrochloride DSC thermograms and its physical mixing with polymers were done to investigate possible interactions between the medication and the polymer (Pullulan, HPMC, PVA). The selected heating rate is from 50°C to 300°C at a rate of 20°C per minute using a Differential Scanning Calorimeter (Shimadzu Corporation, Japan).
ANALYTICAL METHOD DEVELOPMENT
Calibration curve of API [11].
FORMULATION METHODS [12]
Solvent casting, which entails dissolving medication, polymers, and water-soluble excipients in de-ionized water, is the most widely used method for creating ODFs. To create a homogenous mixture, high shear forces generated by a shear processor are then employed. The prepared solution is then put onto a petri plate and the solvent is exposed to a high temperature to allow it to dry, producing high-quality films. The solvent casting method was successfully used to produce an orodispersible film of tianeptine sodium using different grades of Lycoat and HPMC. When employing the solvent casting technique, film-forming polymer is usually steeped in the appropriate solvent for a whole night. The type of API required for ODF dictates the optimal solvent based on the API's significant physico-chemical properties, such as melting point, shear sensitivity, and polymorphic form. The drug's compatibility with the solvent and other excipients is taken into account before a formulation is finalised. Air bubble entrapment during formulation may result in fewer uniform films being created. As a result, the mixture is assisted in deaerating by use of a vacuum pump. Another effective method for producing the mosapride orodispersible film formulation was solvent casting.The viscosity of the solution to be poured is an important part of the casting process. Concentrations of pullulan ranging from 2% to 8% result in low viscosity solutions that make film casting easy.
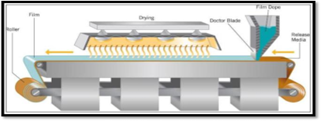
Figure no 3 : Solvent casting method
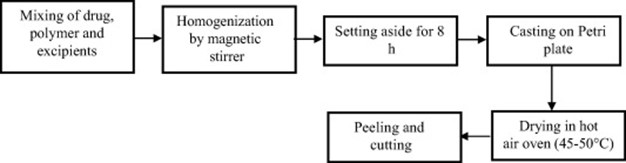
Figure no 4 : Solvent Casting Method-Flow Chart
- Semi-solid casting method-
Flow map of semi-solid casting method is given below.
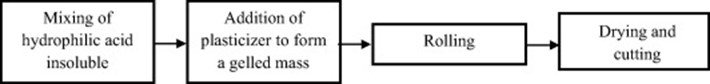
Figure no 4: Flow Chart of Semisolid Casting
- Hot melt extrusion
A mixture of medication, polymer, and excipients is extruded at a high temperature using the hot melt extrusion technique to generate a homogenous mass that is then casted to create smooth films. The processing of thermolabile materials is one of this method's primary drawbacks, despite the fact that it doesn't require the use of solvents because extrusion requires a high temperature.
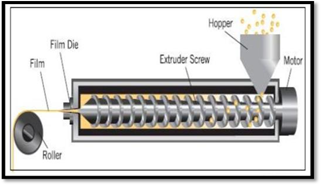
Figure no 5 : Hot Melt Extrusion
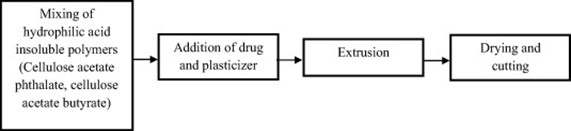
Figure no 6 : Hot Melt Extrusion Method-Flow Chart
- Solid dispersion extrusion-
It was possible to effectively turn domperidone into a solid dispersion by combining beta-cyclodextrin, PEG 400, and HPMC E15. The films were then made using the solid dispersion extrusion technique.
Figure no 7 : Solid dispersion method

Figure no 8 : Solid Dispersion Extrusion- Flow Chart
- Rolling method-
Fig shows the plot of the rolling method. The prepared solution needs to have specific rheological properties in order for it to roll onto the drum. [12]
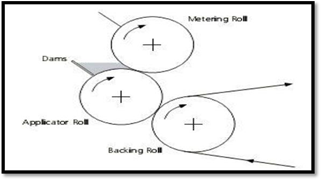
Figure no 9 : Rolling method
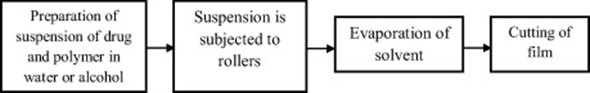
Figure no 10 : Rolling Method- Flow Chart
- Spray technique
The drug substance, polymers, and all other excipients are dissolved in the proper solvent to produce a transparent solution. Then, this clear solution is sprayed onto the proper surfaces, which include glass, Teflon sheets, non-siliconized Kraft paper, and polyethylene film.

Figure no 11 : Spray Technique- Flow Chart
TECHNOLOGIES TO PRODUCE FILMS [8]
- Soluleaves™Technology-
A range of oral delivery films containing active ingredients, colours, and flavours can be produced utilising SoluleavesTM technology. SoluleavesTM films break down rapidly upon contact with saliva, releasing the flavours and active ingredients immediately. In terms of pharmaceutical uses, this method of administration is beneficial for individuals who may have difficulty swallowing standard pills or capsules, such as young or elderly patients. The distribution approach can be applied to therapeutic areas for pain, gastrointestinal issues, and colds and coughs, in addition to nourishing foods. Furthermore, it is possible to make the active component of SoluleavesTM films adhere to mucosal membranes and release gradually over a 15-minute period.
- Wafertab™-
Drug delivery actives are incorporated into an edible filmstrip by a process known as WafertabTM. The mechanism allows for a quick dissolution and release of the active chemicals when the strip comes into contact with saliva in the mouth. To achieve even better taste masking, the WafertabTM filmstrip can be flavoured. The active ingredient, which is precisely dosed and integrated into the body of a prefabricated XgelTM film, shields the product from unnecessary exposure to heat and moisture, potentially enhancing its stability. The WafertabTM technology makes it possible to link together many films with different actives, opening up a plethora of imaginative possibilities for product creation. When administering drugs that need to be released quickly or for people who have trouble swallowing, WafertabTM is a flexible medication administration device that works effectively.
- Foamburst™-
FoamburstTM is a special kind of SoluleavesTM technology in which an inert gas is infused into the film during the manufacturing process. As a result, a film with a honeycomb structure is produced that dissolves fast and has an unparalleled flavour. Food and confectionery manufacturers are interested in utilising FoamburstTM to express and release flavours.
- Xgel™-
The XgelTM film, which is utilised in all of Meldex International's film systems and ingestible dosage delivery technologies, is the core of the company's intellectual property. Special benefits come with XgelTM film for pharmaceutical and healthcare products. It is free of genetically modified organisms (GMOs), can be used by vegetarians, and is not derived from animals. Its continuous production processing also makes manufacturing it competitive and economical. Apart from its capacity to disperse active pharmaceutical ingredients, Xgel film possesses enteric properties and can be efficiently stacked, taste-masked, and coloured. XgelTM film systems can be used to encapsulate any oral dosage form and can be dissolved in either hot or cold water. Numerous unique water-soluble polymers that are specially designed for the specified application, make up XgelTM film.
CLINICAL AND REGULATORY ASPECTS [8]:
If the product is bioequivalent to the medicine's current oral formulation, the US Food and medicine Administration uses an abbreviated new drug application (ANDA) approach. Clinical trials are not a part of the Food, Drug and Cosmetics Act's section 505(j) generic clearance process. A comparison of the bioequivalence between an ODT formulation and an OTF product would serve as an example of the scenario that was previously described. However, the intended oral film product's target pharmacokinetic profile may also be different from the one of the product that is currently on the market. Since the OTF falls under the category of a "new dosage form," section 505(b)(2) approval processes need to be followed. In this case, a new clinical examination would be necessary.
One advantage of a new clinical research is that it would grant the product three years of exclusive marketing rights.
CHARACTERIZATION AND EVALUATION:
- Thickness test
A film's thickness is measured with a calibrated digital micrometre, and the mean average is subsequently calculated. For every batch, three readings are typically obtained, and an average is calculated. Three separate computations of the weight variation of a film are made by cutting the film and weighing each individual film. As thickness uniformity is directly related to the film's dosing accuracy, it is important to determine.
- Dryness test/tack test
The purpose of this test is to see if a film will adhere to a piece of paper that has been pressed in between two strips.[13]. The tenacity with which the film adheres to the paper or any other object inserted in between the films is known as tact. The drying of film can be separated into about eight stages: dry-to-touch, dry hard, dry-through, tack-free, dust-free, and dry-to-recoat. It is also possible to analyse oral fast-disintegrating films with these assays. They are typically used in the paint industry to verify that films are dry. Tests for dryness or tack can also be conducted with recently designed equipment. [2].
- Tensile strength
Tensile strength is the maximum stress at which a film breaks. In essence, this test measures the mechanical strength of a film. The following formula, which divides the applied load at rupture by the strip cross-sectional area, can be used to calculate it:
Tensile strength = (load at failure/ strip thickness × strip width) × 100
- Percent elongation
When stress is applied to a film, a specimen stretches, resulting in strain. The difference between the beginning and ending lengths of the film divided by the length change of the film is the definition of strain. There is a quantitative relationship between the amount of plasticizer used in the film formulation and the percentage of elongation. When the plasticizer content in the film is larger, the strip elongates more easily. It is determined by applying the following formula:
Percentage elongation=(change in length/initial length)×100
- Tear resistance-
Tear resistance is the intricate mechanism that gives a film its final resistance to break. The maximum force required to rip the film is indicated by the tear resistance value. This test is typically attributed to the plastics industry. The force required to cause the film specimen to tear is calculated using a loading rate of two inches per minute. The strongest power needed for tearing is usually found at the tear resistance value, which is ranked near the tearing initiation. [2].
- Young’s modulus
This is the measured stiffness of the film. It can be computed as the ratio of applied stress to strain in the elastic deformation area. It is determined by the following formula.
Young’s modulus=(slope/strip thickness×cross head speed)×100
Another way to write it is:
Young’s modulus=force at corresponding strain/cross-sectional area × corresponding strain
Hardness and brittleness are two characteristics of the films that are linked to Young's modulus and tensile strength. A stiff, brittle film has higher Young's modulus and tensile strength values with minimal elongation [2].
- Folding Endurance
An additional technique to ascertain a film's mechanical properties is folding endurance. To find the measurement, a film is repeatedly folded at the same location until it breaks. Folding endurance value is the number of times a film can be folded without breaking. A film that has a higher rating for folding endurance is mechanically stronger. There exists a clear correlation between the mechanical strength and folding durability of films. The concentration of plasticizer clearly affects mechanical strength, and it also indirectly affects folding endurance value.
- Swelling property
Simulated saliva is used to validate film swell experiments. The film is weighed before being placed within a previously weighed stainless steel wire mesh. Next, a saliva-like solution is dipped into the mesh that contains the film. When there is no further weight gain, the weight of the film is recorded rising at regular intervals.
Degree of swelling is determined by these parameters:
Degree of swelling=final weight(wt)-initial weight(w0)/initial weight(w0)
wt = weight of film at time interval t;
w0 = weight of film at time 0. [4]
- Transparency
The transparency of a strip is determined using a UV spectrophotometer. The purpose of this test is to assess the formulation's visual appeal. Film specimens are cut into rectangular shapes and placed inside the photometer cell. A wavelength of 600 nm is used to compute the transmittance of the film. The transparency is calculated using the following formula.
Transparency=(logT600)/b= -€c
Where, T600 = transmittance at 600 nm,
b = film thickness (mm) and
c = concentration. [1][20]
- Content Uniformity
For every particular medication, many pharmacopoeias describe a standard test protocol that is used to determine the contents of the film. This test is performed on twenty samples using analytical techniques. The test's acceptance value, as per Japanese pharmacopoeia, is less than 15%. According to USP27, the contents should range from 85% to 115% and have a standard deviation of less than or equal to 6%. [12][13].
Content homogeneity is determined in order to estimate the drug content of each individual video. [12][2].
- Contact angle
When a film is at room temperature, the contact angle is frequently measured using a goniometer. The surface of the dried film is touched with a drop of double-distilled water. Water droplet photos are taken using a digital camera ten seconds after the drop is deposited. These digital images are analysed using Image 1.28 V software to determine the contact angle. The contact angle is measured on both droplet sides, and the mean is calculated. In order to fully comprehend the nature of films, contact angle must be measured at least five times at different locations.
- Disintegration time
Using the disintegration equipment specified in reputable pharmacopoeias, one can determine a film's disintegration period. The disintegration time, which normally ranges from 5 to 30 seconds, frequently changes with formulation and is influenced by the makeup of the film. Usually, the USP disintegration apparatus is used for this test. There are no reliable standards to use when estimating the disintegration time of oral fast-disintegrating films. [12][2].
There are two methods for calculating the dissolution time of a film.
- Slide frame method
The film is placed on a petri dish and fastened into slide frames. Onto the film, a drop of distilled water is dropped. The length of time it takes for the film to dissolve is noted. [21]
- Petri dish method
A film is placed over two millilitres of distilled water in a petri dish. The disintegration time is the length of time it takes for a film to completely dissolve [12][14] [22].
- In-vitro dissolution test
The approved tool for film dissolving tests is the basket or paddle device. Maintaining sink conditions during the dissolution process is crucial. Correct testing can be difficult if the film hovers above the medium while being processed. The basket equipment is generally advised because the paddle approach is more likely to result in this problem. The media used are 300 millilitres of 6.8 pH phosphate buffer and 900 millilitres of N HCl. The rotation speed is normally regulated at 50 rpm, and the temperature is maintained at 37 ± 0.5 °C. At predetermined intervals, drug-dissolved sample collections are made, and UV spectrophotometer analysis is carried out. Despite being widely used, the dissolving test still has a considerable mistake rate and test letdown rate. [12][15].
- Surface pH
The process of measuring the pH of a manufactured film involves placing it on a petri dish, wetting it with distilled water, and using an electrode from a pH metre to touch the film's surface. Since an acidic or basic pH may irritate the oral mucosa, it is necessary to measure the surface pH. [12][16].
- Moisture uptake and moisture loss
The percentage of moisture loss in a film is one indicator of its hygroscopicity. Usually, to find this metric, the film is first weighed and then it is placed in a desiccator for three days. There is calcium carbonate in the desiccator. After three days, the strips are taken out and the weight is measured again. Use the following formula to determine moisture loss. [12][17].
Initial weight - final weight / initial weight×100 equals the percentage moisture loss.
To determine a film's moisture absorption, it must first be sliced to a 2 × 2 cm2 size. After that, these strips are left for seven days at room temperature in an environment with a 75% relative humidity. The moisture uptake is computed using the strips' % weight gain. [12][18].
Percentage moisture uptake = (Final weight - Initial weight)/Initial weight × 100
PACKAGING OF MOUTH DISSOLVING FILMS-
The specifics of the packaging affect the dosage form's stability, preservation, and storage. Oral thin-film packaging consists of single pouches, barrier films, blister packaging with multiple units, aluminium or foil paper pouches, and blister packaging. Barrier films are most commonly used in the treatment of drugs that are very vulnerable to moisture. [12[14]. Rapid film technology from Labtec GmbH describes the main package, which is a sealing pouch with plenty of space for logos, codes, directions, or other information. The films are made by laminating, and the cost of the packaging is comparable to that of tablets. [12][19].
APPLICATIONS OF MOUTH DISOLVING FILM [1][4]
-
- Oral Mucosal drug delivery via sublingual, buccal and mucosal routes
- Topical applications
- Gastro retentive drug delivery system
- Diagnostic devices
CONCLUSION:
The present review indicates that the use of oral fast-disintegrating films is one of the state-of-the-art techniques in the pharmaceutical sciences. They are safer and more effective than traditional dosage forms, with improved patient compliance and acceptance. There is also no risk of choking. The main reason that ODFs were developed was to solve the difficulty that paediatric, elderly or mental dysphagia patients had while trying to swallow conventional oral dosage forms. ODFs are already widely available to treat a wide range of ailments, such as acidity, hypertension, allergies and discomfort. One of the primary advantages of these dosage forms is that they can be delivered without the need for water, meeting the needs of the intended audience who prefers ease of use when taking their medications.
REFERENCES-
- Bala R, Pawar P, Khanna S, Arora S. Orally dissolving strips: A new approach to oral drug delivery system. International journal of pharmaceutical investigation. 2013 Apr;3(2):67.
- Bhyan B, Jangra S, Kaur M, Singh H. Orally fast dissolving films: innovations in formulation and technology. Int J Pharm Sci Rev Res. 2011 Jul;9(2):9-15.
- Thakur N, Bansal M, Sharma N, Yadav G, Khare P. Overview “a novel approach of fast dissolving films and their patients”. Advances in biological research. 2013 Aug;7(2):50-8.
- Chaudhari AP, Patil BA, Tadavi SA, Pawar SP. Mouth Dissolving Film: An Innovative and Effective Drug Delivery System.
- Keshari A, Sharma PK, Parvez N. Fast dissolving oral film: a novel and innovative drug delivery system. International Journal of Pharma Sciences and Research. 2014;5(3):92-5.
- Jain P, Gupta A, Darwhekar G. A Detailed Overview on Mouth Dissolving Film. Journal of Drug Delivery and Therapeutics. 2023 Jul 15;13(7):172-6.
- Pattewar SV, Kasture SB, Pande VV, Sharma SK. A new self microemulsifying mouth dissolving film. Indian J. Pharm. Educ. Res. 2016 Jul 1; 50:191-9.
- Kumar RS, Yagnesh TN. Oral dissolving films: an effective tool for fast therapeutic action. Journal of Drug Delivery and Therapeutics. 2019 Feb 15;9(1-s):492-500.
- Pawar R, Sharma R, Sharma P, Darwhekar GN. A review on mouth dissolving film. Journal of Drug delivery and Therapeutics. 2019 Nov 15;9(6):206-10.
- Bilal Q, Unhale S, Shelke S, Kale P, Sarode P, Biyani D. A review on mouth dissolving films. Eur. J. Pharm. Med. Res. 2020; 7:232-8.
- Panchal MS, Patel H, Bagada A, Vadalia KR. Formulation and evaluation of mouth dissolving film of ropinirole hydrochloride by using pullulan polymers. International Journal of Pharmaceutical Research & Allied Sciences. 2012;1(3):60-72.
- Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, Khan A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi pharmaceutical journal. 2016 Sep 1;24(5):537-46.
- Chaudhary H, Gauri S, Rathee P, Kumar V. Development and optimization of fast dissolving oro-dispersible films of granisetron HCl using Box–Behnken statistical design. Bulletin of Faculty of Pharmacy, Cairo University. 2013 Dec 1;51(2):193-201.
- Patil PC, Shrivastava SK, Vaidehi S, Ashwini P. Oral fast dissolving drug delivery system: a modern approach for patient compliance. International Journal of Drug Regulatory Affairs. 2014 Jun 1;2(2):49-60.
- Bai GE, Armenante PM, Plank RV, Gentzler M, Ford K, Harmon P. Hydrodynamic investigation of USP dissolution test apparatus II. Journal of Pharmaceutical sciences. 2007 Sep 1;96(9):2327-49.
- Patel RS, Poddar SS. Development and characterization of mucoadhesive buccal patches of salbutamol sulphate. Current drug delivery. 2009 Jan 1;6(1):140-4.
- Yellanki SK, Jagtap S, Masareddy R. Dissofilm: a novel approach for delivery of phenobarbital; design and characterization. Journal of Young Pharmacists. 2011 Jul 1;3(3):181-8.
- Gorle AP, Gattani SG. Design and evaluation of polymeric ocular drug delivery system. Chemical and Pharmaceutical Bulletin. 2009 Sep 1;57(9):914-9.
- Bhasin RK, Bhasin N, Ghosh PK. Advances in formulation of orally disintegrating dosage forms: a review article. Indo Global Journal of Pharmaceutical Sciences. 2011;1(4):328-53.
- Corniello C. Quick dissolving strips: from concept to commercialization. Drug Delivery Technology. 2006;6(2):68-71.
- Laohakunjit N, Noomhorm A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch?Stärke. 2004 Aug;56(8):348-56.
- Wu Y, Weller CL, Hamouz F, Cuppett S, Schnepf M. Moisture loss and lipid oxidation for precooked ground?beef patties packaged in edible starch?alginate?based composite films. Journal of food science. 2001 Apr;66(3):486-93.


 Vrushali S. Gangurde * 1
Vrushali S. Gangurde * 1
 Pradnya H. Kapse 2
Pradnya H. Kapse 2
 Khanderao R. Jadhav 3
Khanderao R. Jadhav 3
 Rishikesh S. Bacchav 4
Rishikesh S. Bacchav 4












 10.5281/zenodo.11080675
10.5281/zenodo.11080675