Abstract
Topical treatments are used for skin emollients, antifungal agents, antiseptics, and protective. The medications' ability to reach the target layers of the skin at effective quantities determines how successful that therapy will be. But the skin's outermost layer, the stratum corneum, acts as a strong barrier to prevent medications from penetrating the skin's deeper layers. Some Factors Affecting Topical Absorption of Drugs may- includes Physiochemical Factors of Drug Substances, Physiological Factors, Vehicle, Site of application. Fungal illnesses are a major hazard to the public health of the world, with their prevalence rising recently. Osteopportunistic infections that manifest superficially are mostly caused by Candida spp. The potential of nanotechnology for microemulsion-based medication delivery has been demonstrated as a means of overcoming therapeutic efficacy. Evaluation of Microemulsion may include Electrical conductivity, Zeta potential, Scanning Electron Microscopy, etc. In conclusion, microemulsions constitute a unique and promising drug delivery approach for cutaneous applications. Gels are a relatively new sort of dosage form made of colloidal solid particles encasing enormous volumes of aqueous or hydroalcoholic liquid. Psedoternary phase diagram was performed by taking the ratios of surfactant to co-surfactant were varied, ranging from 2:1 to 5:1. Evaluation of Emulgel may include Particle size determination, Zeta potential, SEM, Measurement of Bio adhesive strength, etc. Stability Study tests are performed at 5°C, 25°C/60% RH, 30°C/65% RH, and 40°C/75% RH. The unique properties of emulgel, such as good stability and efficient drug solubilization, make them a promising choice for targeted drug administration.
Keywords
Topical drug delivery, Evaluation, Fungal infection, Emulgel, Micro emulsion.
Introduction
Topical drug delivery systems are dosage forms that are administered topically to treat skin conditions or when other methods of drug are not effective. The benefit of first-pass metabolism negotiation is present in topical medication delivery devices. Topical formulations also have another benefit of not requiring parenteral therapy, which entails avoiding its dangers and drawbacks as well as different absorption circumstances including pH fluctuations, the presence of enzymes, and stomach emptying time. Topical drug delivery methods have some drawbacks, such as low drug permeability through the skin, allergic responses, skin irritation in contact dermatitis, and difficulty absorbing big particle size medications via the skin [1].
The epidermis, dermis, and hypodermis are the three primary layers of the well-organized membrane that makes up human skin. The stratum corneum, the outermost layer of the epidermis, is made up of keratinized and dead skin cells. It serves as a great barrier to prevent medications from penetrating the skin. Additionally, topical preparations are non-invasive and self-administrable, patient compliance is higher [2].
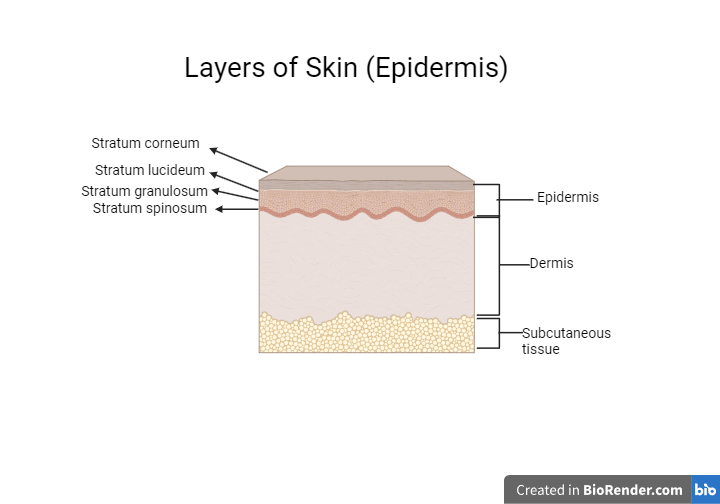
Figure-1: Layers of Skin (Epidermis)
CLASSIFICATION OF TOPICAL DRUG DELIVERY SYSTEM

2.1. Factors Affecting Topical Absorption of Drugs:
Table-1: Factors Affecting Topical Absorption [3]
-
|
Physiochemical Factors of Drug Substances
|
- Molecular Weight (400-500 Daltons)
- Diffusion coefficient
- Protein binding capacity.
- Water/lipid partition coefficient
- Permeability coefficient ionization - unionized drugs are well absorbed.
|
-
|
Physiological Factors of drugs
|
- Thickness of skin
- Content of lipid
- Density of Hair follicle
- Density of Sweat gland
- pH of skin
- Blood flow
- Skin hydration and skin inflammation
|
-
|
Vehicle
|
- Polarity and Solubility
- Volatility
- Concentration of vehicle
- Spreading in stratum corneum
- Excipients
- Penetration enhancer
|
-
|
Application site
|
- Area of skin dose (thickness of film and concentration)
- Duration of exposure
|
There are several variables that impact the topical absorption of medicines, such as:
- Skin conditions: Including psoriasis and eczema, as well as damaged and healthy skin, all have an impact on metabolism.
- Drug concentration: Absorption may be enhanced by higher concentrations.
- Vehicle: Absorption may be impacted by the base or solvent employed in the formulation (e.g., cream, gel, ointment).
- pH: The drug's pKa and the skin's inherent pH might affect absorption.
- Enzyme activity: Skin enzymes have the ability to digest medications, which can impact absorption.
- Solubility: How well the medication dissolves in the lipids of the skin and in the vehicle.
- Ionization: Absorption of the medicine may be impacted by its ionization state.
- Drug molecular weight: In general, smaller molecules are more readily absorbed.
- Skin age and location: The age and location of the skin (face, arms, etc) might affect absorption.
- Dosing frequency: Increasing the number of applications may result in higher absorption.
- Humidity: Absorption can be enhanced by high humidity.
- Skin temperature: A higher body temperature might improve absorption.
- Occlusion: Applying material over the application location might improve absorption.
- Particle size: Absorption may be enhanced by smaller particles.
- Permeability: The drug's capacity to permeate the strata of the skin.
3.COMMON SKIN FUNGAL INFECTIONS
With an increasing occurrence seen in recent years, fungal diseases pose a serious threat to global public health. Among the several therapeutic options available, topical antifungal medication has a number of benefits, such as improved patient compliance, targeted distribution, and less systemic adverse effects [4]. Candida spp. is the primary cause of opportunistic infections that appear superficially. Antimycotics are not widely available, despite the fact that Candida is the primary cause of superficial opportunistic infections. When treating superficial and systemic infections, azoles are the antifungal drugs that are most frequently utilized [5].

Figure-2: List of different Antifungal drugs.
SIGNIFICANCE OF MICROEMULSIONS
In order to overcome therapeutic effectiveness, the potential nanotechnology of microemulsion-based drug delivery has been presented. Microemulsions are immiscible mixtures of two separate liquids with diameters ranging from 10 to 100 nm that are stabilized by surfactants and co-surfactants [6]. Microemulsions are adaptable Nano colloidal carriers that can maintain drug solubilization and release at the buccal mucosa level. This has been observed for a number of natural and synthetic antifungal agents, including spray-able liquid formulations of various oils- clotrimazole, and itraconazole, which are intended to act as mucoadhesive structures [7]. The microemulsion technology was selected because of its superior solubility and skin penetration capabilities [8].

Figure-3: Showing micro-emulgel pathway on skin.
Making the right choices for the oil phase, emulsifier, and gelling agent is the main prerequisite for creating an emulgel. Thus, while making micro-emulgels, oils, surfactants, and co-surfactants need to be well screened and optimized. The solubility profile of the API is taken into consideration while selecting these. When there is oil present, the API gets deeper into the skin. The formation of small droplets inside the microemulsion creates a high interfacial area, increasing the surface area accessible for drug absorption [9].
Based on their structure, microemulsions can be categorized as (Figure 4)
- Bicontinuous,
- Oil-in-Water (O/W)
- Water-in-Oil (W/O)
Both the water and oil phases of bicontinuous microemulsions are continuous and distributed throughout the system, giving them a complex, networked structure. If an O/W microemulsion comprises oil droplets scattered inside a continuous aqueous phase, then water droplets are distributed within an oil phase in a W/O microemulsion. It has been demonstrated that hydrophilic and hydrophobic medications may be delivered with the use of bicontinuous microemulsions. While hydrophilic medications are usually delivered by W/O microemulsions, whereas hydrophobic pharmaceuticals are better delivered by O/W microemulsions [10]

Figure-4: Different configurations of MEs: water-in-oil, bicontinuous, and oil-in-water.
4.1. Evaluation of Microemulsion: [11]
- pH: The digital pH meter (Model EQ610) was used to measure the pH of the microemulsion. Phosphate buffers 4 and 7 were used to calibrate the pH meter prior to measuring the pH of the optimized microemulsion. The pH of the microemulsion was then measured after a minute of immersion of the pH meter electrode into a glass beaker containing the microemulsion.
- Viscosity: The Brookfield viscometer (Model LV) was used with spindle number 62 to measure the viscosity of the microemulsion. At 10, 20, 30, 50, and 100 revolutions per minute, the apparent viscosity was found. The stationary cup and spinning spindle make up the Brookfield viscometer. Various spindles with varying sizes are utilized and submerged in liquid. High viscosity liquids require small spindles with a small diameter and surface area, whereas low viscosity liquids require big spindles with a large diameter and surface area. Spindle in microemulsion should be rotated until the viscometer display shows a consistent dial reading. For a repeatable outcome, this process is performed three times.
- Electrical conductivity: The kind of formulation—oil continuous or water continuous—can be ascertained with the use of the conductivity measurement. Using an electrical conductivity measurement, the solubilization of a particular oily combination was quantified. The Systonics Conductivity Meter Model 304 was utilized to test the conductivity of the prepared samples.
- Drug content: By precisely dissolving one millilitre of the microemulsion in ten millilitres of methanol, the drug concentration of the microemulsion was ascertained. Utilizing a UV-visible spectrophotometer (UV – 1800 Shimadzu, Japan) at 270 nm, absorbance was measured following an appropriate dilution.
- Zeta potential: The Zeta potential of the microemulsion is measured using the HORIBA Scientific SZ-100 to ascertain the charge on the particle surface. After the substance is added to the disposable cell, the particle electrophoretic moiety is measured to determine the Zeta potential.
- Particle size determination: To test the particle size and Zeta potential in triplicate and determine average results, samples were diluted with distilled water. Averaging values ±SD were recorded after microemulsion particle sizes were measured with HORIBA sz-100 (z type) [12].
- Scanning Electron Microscopy: With the use of high-resolution imaging provided by scanning electron microscopy, a variety of materials may be examined for surface cracks, flaws, impurities, and corrosion. Using Nova NanoSEM NPEP, many singles are generated when a concentrated stream of secondary electrons contacts with atoms in the sample, providing details about the surface topography. Every image is pre-processed at 10,000 x and has a 303 x 5 m dimension scale.
4.2. Stability Evaluation of Microemulsion: [13]
- Thermodynamic Stability: In order to solve the issue of metastable formulations, thermodynamic stability of the obtained microemulsions at various concentrations of oil mixture and Smix was formed. The first step in the microemulsion screening process was based on thermodynamic stability.
- Heating Cooling Cycle: The microemulsions were maintained at two distinct temperatures, namely 4°C for refrigeration and 45°C for higher temperatures, in order to accomplish this. With storage at each temperature for at least 48 hours, six cycles were examined. Further investigation was done on those formulations that showed stability at these temperatures.
- Centrifugation: Centrifugation testing was subsequently applied to the formulations that cleared the heating-cooling cycle. The microemulsions underwent a 30-minute centrifugation at 3500 rpm. For the freeze-thaw stress test, the formulations that showed no phase separation were selected.
- Freeze Thaw Cycle: Microemulsions were prepared by undergoing three freeze-thaw cycles at temperatures ranging from -21°C to +25°C, with a minimum 48-hour storage period at each temperature. This test is conducted to observe if the microemulsions remained stable at extremely low temperatures and whether they reverted to their stable state upon freezing
- Marketed Emulgels:
Table-2: Marketed Emulgels [14]
|
Sr. No.
|
Brand Name
|
Active Ingredient
|
Use
|
|
1.
|
Avindo Gel
|
Azithromycin
|
Cosme Pharmaceutical
|
|
2.
|
Adwiflam Emulgel
|
Diclofenac diethylamine, Methyl salicylate And Menthol
|
Anti-inflammatory And Pain removal
|
|
3.
|
Benzolait Emulgel
|
Benzoyl peroxidase And Biguanide
|
Antiacne
|
|
4.
|
Cataflam Emulgel
|
Diclofenac potassium
|
Anti-inflammatory
|
|
5.
|
Denacine Emulgel
|
Clindamycin phosphate
|
Antiacne
|
|
6.
|
Dermafeet Emulgel
|
Urea 40%
|
Moisturizing and Exfoliation activity
|
|
7.
|
Diclomax Emulgel
|
Diclofenac sodium
|
Anti-inflammatory
|
|
8.
|
Diclon emulgel
|
Diclofenac diethylamine
|
Anti-inflammatory
|
|
9.
|
Dosanac emulsion gel
|
Diclofenac diethylammonium
|
Anti-inflammatory
|
|
10.
|
Isofen emulgel
|
Ibuprofen
|
Anti-inflammatory
|
|
11.
|
Miconaz-H-emulgel
|
Miconazole nitrate, Hydrocortisone
|
Topical corticosteroid And Antifungal
|
|
12.
|
Voltarol 1.16% emulgel
|
Diclofenac Diethylammonium salt
|
Anti-inflammatory
|
4.4. Gels based delivery system:
Gels are a relatively new sort of dosage form made of colloidal solid particles encasing enormous volumes of aqueous or hydroalcoholic liquid. When compared to conventional ointments and creams, gel formulations typically offer faster medication release [15]. Emulgels are the term for dosage forms that are created when gels and emulsions are mixed.
Gel is a colloidal preparation that is composed of 99 percent liquid and a macromolecular network of fibers that are immobilized by surface tension between the liquids and a gelling agent. In actuality, a conventional emulsion becomes an emulgel when a gelling agent is present in the water phase [16].
4.5. Methods of Formulation of Emulgel:
Emulgel is formulated by following steps,
- Selection of components
- Preparation of emulsion
- Preparation of emulgel
- Selection of components: The drug's solubility was assessed in a variety of oils by adding an excess of the substance and vigorously stirring the mixture for 72 hours to reach equilibrium. Following that, the samples were centrifuged, the supernatant was collected, and the solubility was ascertained using the appropriate analytical techniques.
Next, the excipients in each category that have the highest drug solubility are chosen for additional research [17].
Psedoternary phase diagram: The ratios of surfactant to co-surfactant were varied, ranging from 2:1 to 5:1. Every ratio that is favoured while reviewing phase diagrams is used to increase the quantity of surfactant relative to cosurfactant. The most common type of dilution medium is aqueous phase, or diluted water. For each combination, oil, surfactant, and co-surfactant were combined in several vials at ratios ranging from 9:1 to 1:9. This is primarily important since it covers the study that determines the limits of the phases generated in the diagrams. The oil, surfactant, and co-surfactant are titrated slowly, and the emulsion's transparency is checked visually [18].
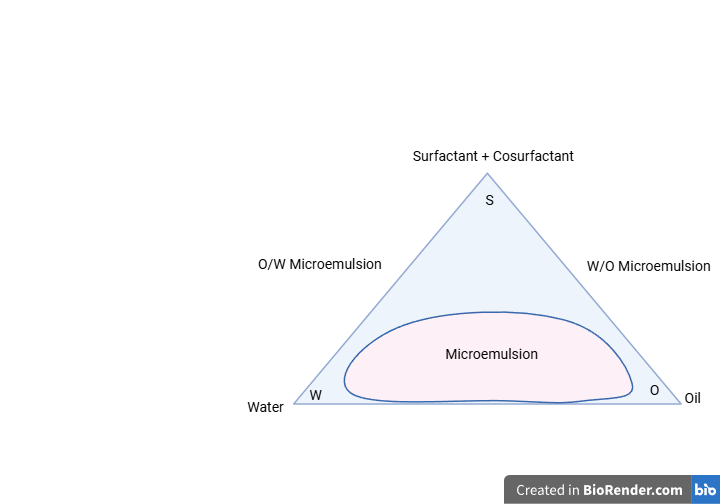
Figure-5: Psedoternary phase diagram of Emulgel
- Preparation of emulsion: After solubilizing the medication in oil, the mixture of surfactant and co-surfactant is combined with the oil and diluted with water in order to produce an emulsion of the known drug.
- Preparation of emulgel: The produced emulsion is gradually added while stirring continuously to the gel base, which is made by combining 1g of Carbopol with the necessary amount of water and letting it soak for the entire night. To keep the formulation's pH stable, triethanolamine is used. Distilled water is used to adjust the necessary residual volume in the end.
4.6. Evaluation of Emulgel:
- Physical appearance: The prepared formulations were examined visually for their colour, homogeneity and consistency.
- Particle size determination: The (NanoPlus) equipment at 25°C calculated the ideal batch's particle size analysis via Brownian motion. The samples were diluted in particle-free filtered water with a scattering intensity of approximately 150–300 keeps [19].
- Polydispersity index: Using cumulative analysis and NanoPlus software, the polydispersity index and mean z-average diameter have been determined [20].
- Zeta potential: One important measure of the formulation's stability is the zeta potential. The degree of the electronic repulsion between the adjusters and the scattered charge particle is indicated by the size of the zeta potential. Using a zetasizer, the zeta potential of the optimized batch was determined using folded capillary cells.
One millilitre of the formed nanosuspension was extracted, and it was mixed with ten millilitres of double-distilled water. The samples were ultrasonically treated for five minutes to find the main particle size, and then their size was evaluated. Subsequently, the material was extracted from a receptacle and introduced into the apparatus to assess its dimensions and zeta potential [21].
- SEM: Examine the shapes and surface characteristics of any NLC using scanning electron microscopy (SEM). It took approximately five minutes for the sputtering procedure to produce a uniform coating on the sample and enable high-quality SEM images. The SEM was operated at an insignificant accelerating voltage of approximately 25KV with a load current of about 80MA [22].
- Measurement of Bio adhesive strength: Pieces of hairless rat skin are placed between slides with an appropriate 1gram application of emulgel.
Applying pressure to a single glass slide causes the sandwich of two slides to separate. The rate at which more weight is added is 200 mg/min until the skin's surface separates. The bioadhesive strength will be determined by the weight required to separate the emulgel from the skin. It is calculated by using following formula:
Bio adhesive Strength = W
A
Where, W= Weight is required (in gms) and A=Area (cm2) [23].
- Skin irritation test: Apply 0.25 gm of the prepared emulgel to two to three separate sites/rabbit. Wipe and clean the rabbit skin sites after 24 hours. Note any unfavourable changes in morphology or skin colour.
- Stability Studies: For a duration of three months, the manufactured emulgels were placed in aluminum collapsible tubes (5 g) and exposed to stability tests at 5°C, 25°C/60% RH, 30°C/65% RH, and 40°C/75% RH. Samples were taken out at intervals of 15 days, and their physical characteristics, Particle size determination, Polydispersity index, Zeta potential, SEM, Measurement of Bio adhesive strength, and Skin irritation test patterns were assessed.
CONCLUSIONS
To increase patient compliance, topical medication administration will be utilized extensively in the upcoming years. Due to its advantages over oral treatment, including the ability to focus the medicine directly at the infection site, prevent systemic side effects, and increase patient compliance, topical treatment for cutaneous infections has been favoured. Topical antifungal medicine is one of the many treatment choices available. It offers various advantages, including better patient compliance, adapted distribution, and less systemic side effects. Generally, antifungal drugs are highly lipo-philic compounds, which can affect the penetration of drugs across stratum corneum. Additionally, the advantages of using microemulsions as drug delivery methods were discussed, such as their high stability, effective drug solubilization, and possibility for tailored drug administration. Emulgel enhances extrudability, viscosity, adhesiveness, and spreadability. The benefits of emulgel provide significant potential for the topical administration of hydrophobic drugs in the future, with increased effectiveness and reduced production costs. The synergistic action of emulgel is provided by oils having therapeutic value. In conclusion, a novel and promising drug delivery method for cutaneous applications is represented by microemulsions. For targeted drug delivery, they are a desirable alternative because to their special qualities, which include excellent stability and effective drug solubilization.
REFERENCES
- Salve K, Shinde S, Shirsath P, Shirsath P, Sonawane S. FORMULATION AND EVALUATION OF CLOTRIMAZOLE TOPICAL EMULGEL.
- Güng S. New formulation strategies in topical antifungal therapy.
- Singh RP, Parpani S, Narke R, Chavan R. Emulgel: A recent approach for topical drug delivery system. Asian Journal of Pharmaceutical Research and Development. 2014 Mar 1:112-23.
- Netra S. ENHANCING ANTIFUNGAL THERAPY: DEVELOPMENT AND EVALUATION OF NANO-GEL LOADED WITH FLUCONAZOLE FOR TOPICAL APPLICATION. The American Journal of Medical Sciences and Pharmaceutical Research. 2024 Jun 1;6(06):1-7.
- Jagadish G, Shukla R, Shukla P. Formulation and evaluation of microemulsion based gel of posaconazole for topical delivery. EPRA International Journal of Research and Development. 2021; 6 (1): 164. 2021;174.
- Naksuriya O, Nitthikan N, Supadej K, Kheawfu K, Khonkarn R, Ampasavate C, Intasai N, Monton C, Kiattisin K. Approach for Development of Topical Ketoconazole-Loaded Microemulsions and Its Antifungal Activity. Trends in Sciences. 2023 Sep 10;20(12):7046-.
- Talianu MT, Dinu-Pîrvu CE, Ghica MV, Anu?a V, Prisada RM, Popa L. Development and Characterization of New Miconazole-Based Microemulsions for Buccal Delivery by Implementing a Full Factorial Design Modeling. Pharmaceutics. 2024 Feb 14;16(2):271.
- Dandagi PM, Pandey P, Gadad AP, Mastiholimath VS. Formulation and evaluation of microemulsion based luliconazole gel for topical delivery. Indian J. Pharm. Educ. Res. 2020 Apr 1;54(2):293-301.
- Singh S, Chauhan SB, Gupta C, Singh I, Gupta A, Sharma S, Kawish SM, Rahman S, Iqbal M. Design and Characterization of Citronella Oil-Loaded Micro-Emulgel for the Treatment of Candida Albicans Infection. Gels. 2023 Oct 5;9(10):799.
- Ait-Touchente Z, Zine N, Jaffrezic-Renault N, Errachid A, Lebaz N, Fessi H, Elaissari A. Exploring the versatility of microemulsions in cutaneous drug delivery: opportunities and challenges. Nanomaterials. 2023 May 21;13(10):1688.
- Sukre M, Barge V, Kasabe A, Shinde T, Kandge M. Formulation and evaluation of econazole nitrate microemulsion. International Journal of Health Sciences. 2022(III):9181-90.
- Theansungnoen T, Daduang J, Priprem A, Boonsiri P, Daduang S, Klaynongsruang S. Formulation and evaluation of niosomes encapsulated with KT2 and RT2: Antimicrobial and anticancer peptides derived from crocodile leukocyte extract.
- Ware P, Yadav G, Jain A, Tambvekar O. Formulation And Evaluation of Microemulsion Based Itraconazole Gel: Formulation And Evaluation of Microemulsion Based Itraconazole Gel. Journal of Drug Delivery and Biotherapeutics. 2024 Jan 13;2(01):42-71.
- Sah SK, Badola A, Nayak BK. Emulgel: Magnifying the application of topical drug delivery. Indian Journal of Pharmaceutical and Biological Research. 2017 Jan 31;5(01):25-33.
- Pant S, Badola A, Baluni S, Pant W. A review on emulgel novel approach for topical drug delivery system. World journal of pharmacy and pharmaceutical sciences. 2015 Aug;4(10):1728-43.
- Khullar R, Saini S, Seth N, Rana AC. Emulgels: a surrogate approach for topically used hydrophobic drugs. Int J Pharm Bio Sci. 2011 Jul;1(3):117-28.
- Redkar MR, Patil SV, Rukari TG. Emulgel: A modern tool for topical drug delivery. World J. Pharm. Res. 2019 Jan 29;8(4):586-97.
- Modi JD, Patel JK. Nanoemulsion-based gel formulation of aceclofenac for topical delivery. International Journal of Pharmacy and Pharmaceutical Science Research. 2011;1(1):6-12.
- Gondkar SB, Kandalkar SG, Bachhav RS, Hiray N. DEVELOPMENT AND EVALUATION OF NANOSTRUCTURED LIPID CARRIER LOADED EMULGEL OF ECONAZOLE NITRATE.
- Jadhav KR, Choursiya KA, Jadhav RG, Nikam SM, Bachhav RS. African Journal of Biological Sciences.
- Ghurghure SM, Jadhav T, Kale S, Phatak AA. Formulation and evaluation of posaconazole loaded nanostructured lipid carriers for topical drug delivery system. Methods. 2022; 33:35.
- Sanap GS, Mohanta GP. Design and evaluation of miconazole nitrate loaded nanostructured lipid carriers (NLC) for improving the antifungal therapy. Journal of Applied Pharmaceutical Science. 2013 Jan 28;3(1):046-54.
- Redkar MR, Patil SV, Rukari TG. Emulgel: A modern tool for topical drug delivery. World J. Pharm. Res. 2019 Jan 29;8(4):586-97.


 Sakshi jain*
Sakshi jain*
 Prakhar Nema
Prakhar Nema






 10.5281/zenodo.14178549
10.5281/zenodo.14178549