Skin whitening has become a global beauty trend driven by cultural, social and personal preferences for beautiful skin. Over the years, extensive research has been done to develop effective and safe skin care products. Doctors and dermatologists often look for long-term cosmetic products, including formulations and topical products, to control hyperpigmentation. A particular concern expressed by many women is the desire to show beautiful skin, reduce yellow or pale tones, and reduce the symptoms of hyperpigmented spots such as age spots or sunspots. While kojic acid, hydroquinone, and corticosteroids being conventional depigmenting agents have been shown to be effective, their long-term use may pose serious safety concerns, including aging, atrophy, carcinogenicity, and other local side effects or disease. However, exploring the benefits of natural and herbal products offers promising opportunities to create new products designed to address pigmentation issues while reducing safety risks.
Skin lightening, Hyperpigmentation, Natural Bioactive components, Tyrosinase inhibitors
orus australis and its chemical components:
The extract of Morus australis has shown to have potential to be a rich source of tyrosinase inhibitors. These inhibitors are suitable for many applications, including anti-inflammatory agents and skin lightening agents in cosmetics. Sanggenon type prenyl flavanones, and its derivatives (sanggenon O, sanggenon C, sanggenon M), chalcomoracin, sorocein H and kuwanon J obtained from Morus australis were investigated as tyrosinase inhibitors. More importantly, sanggenon D exhibited stronger inhibitory activity compared to kojic acid and arbutin. Also, in the tyrosinase inhibition test, compounds such as oxidized resveratrol, sanggenon T and sanggenon O proved to be more potent against tyrosinase than the still known tyrosinase inhibitor. This data supports the utility of Morus australis root extract as a potential source for the development of tyrosinase inhibitors. 28,29
Scutellaria baicalensis and its chemical components:
Baicalein, an important flavonoid found in Scutellaria baicalensis, is considered a potent tyrosinase inhibitor, an important enzyme involved in melanin production. The mechanism by which baicalein inhibits tyrosinase was investigated through a combination of enzyme kinetics, spectroscopic methods, and computational simulations. 30,31,32 The results showed that baicalein had a significant effect on the diphenolase activity of tyrosinase with an IC50 value of 0.11 µM. Inhibition kinetics showed that baicalein was a complex inhibitor of binding with a Ki value of 0.17µM and 0.56. Also, spectroscopic analysis revealed the formation of a complex between baicalein and tyrosinase, as evidenced by ultraviolet absorption spectroscopy. Baicalein's ability to control melanin production and potent tyrosinase inhibition make best candidate for skin lightening and hyperpigmentation control.32,33
Rhus succedanea and its chemical components:
The alkyl hydroquinones 10'(Z)-heptadecenylhydroquinones , extracted from the sap of the lacquer tree Rhus succedanea, has emerged as a captivating compound with remarkable potential in inhibiting activity of tyrosinase and production of melanin in animal cells. 34In a clear study by Chen et al., the inhibitory effect of alkylhydroquinone 10'(Z)-heptadecenylhydroquinone on tyrosinase and its superior potency compared to the well-established tyrosinase inhibitor, hydroquinone were unraveled35. The elucidation of alkyl hydroquinone 10'(Z)-heptadecenylhydroquinone's potent impact on tyrosinase activity helps in utilization in preserving the quality of this botanical component susceptible for skin whitening.
Agaricus hondensis mushrooms and its chemical components:
Hydroquinone (HQ) is the primary toxic compound found in Agaricus hondensis mushrooms. This well-studied substance has gained significant recognition as a whitening agent and has been widely employed in cosmetic treatments for hyperpigmentation. In clinical settings, hydroquinone cream is commonly utilized as the standard depigmentation agent. Its therapeutic applications extend to addressing various dyschromia conditions, including melasma, freckles, and post-inflammatory hyperpigmentation. The depigmentation action of hydroquinone stems inhibits melanin synthesis by impeding the conversion of L-3,4-dihydroxyphenylalanine (L-DOPA) into melanin by directly affecting the enzymatic activity of tyrosinase . While hydroquinone remains a commonly prescribed ingredient for skin-lightening purposes, it is essential to acknowledge its potential adverse effects. Skin irritation and burning sensations are among the reported drawbacks associated with its usage. Additionally, hydroquinone has been identified as mutagenic to mammalian cells and exhibits cytotoxicity toward melanocytes 36,37.
Mulberry tree (Morus alba Linn.) and its chemical components:
The Mulberry tree (Morus alba Linn.) a part of the Moraceae family, has been found to possess remarkable tyrosinase inhibition activity, as demonstrated by a recent study. Research focused on investigating the active components present in the extricate obtained from Morus alba Linn twigs. In the evaluation of tyrosinase inhibitory properties were examined, the compound steppogenin exhibited considerable activity with an IC50 value of 0.98 ± 0.01 µM. Notably, the inhibitory effects of steppogenin surpassed those of the positive control kojic acid, a tyrosinase inhibitor. Later, the active components responsible for this inhibition were investigated.38 Among the tested compounds, sanggenon T, oxyresveratrol, kuwanon O and moracenin D exhibited strong activity of tyrosinase inhibition compared to kojic acid, a tyrosinase inhibitor. Notably, a flavone called morusone showed tyrosinase inhibition activity with an IC50 value of 290.00 ± 7.90. These suggest that the extract obtained from the twigs of Morus alba Linn. holds great potential as a natural source of tyrosinase inhibitors and the application of Morus alba twig extract as Anti browning agents can be expected.39
Mung bean (Vigna radiata ) and its chemical components:
Mung bean (Vigna radiata) holds significant cultural value worldwide, particularly in Asian countries, where it is widely consumed as a dietary staple and is used in various culinary applications, including soups, stews, salads, desserts, and more. They are highly nutritious and provide an important source of protein, fiber, and essential nutrients. Beyond its dietary importance, mung bean has a rich history of traditional medicinal usage. In a recent study, a 70% ethanol extract of mung bean was further fractionated using solvents Dichloromethane (CH2Cl2), Ethyl Acetate (EtOAc), and n-Butanol (n-BuOH) to produce four different fractions which included CH2Cl2-soluble in ethyl acetate being soluble, soluble in n-butanol and residual extract fractions. More importantly, the ethyl acetate-soluble fraction exhibits the highest activity against tyrosinase enzyme involved in synthesis of melanin.40 Two pure flavonoids, vitexin and iso-vitexin, were isolated from the EtOAc soluble fraction by enzyme assay-guided fractionation method. These compounds have potent tyrosinase inhibitory activity with IC50 values between 6.3 and 5.6 µg mL-1. This investigation represents the first exploration of the active components present in mung bean targeting mushroom tyrosinase. This highlights the potential of vitexin and iso-vitexin as effective inhibitors of tyrosinase activity.41,42,43
Cudrania cochinchinensis and its chemical components:
The Phytochemical components of each part of Cudrania cochinchinensis, was examined using High-Performance Liquid Chromatography Analysis (HPLC). Remarkably, the stem extract of C. cochinchinensis exhibited the presence of unknown products as tyrosinase inhibitors. Motivated by these findings, further investigations were conducted to identify the chemical constituents in the stem extract, obtained using 95% ethanol.44 The results unveiled the activity of tyrosinase inhibition of specific compounds present in the C. cochinchinensis stem extract. Notably, (±)2,3-cis-dihydromorin, oxyresveratrol and 2,3-trans-dihydromorin, exhibited superior potency as tyrosinase inhibitors, with IC50 values of 31.1 ?M, 21.1 ?M, and 2.33 ?M, respectively. This observation underscores the remarkable potential of Cudrania cochinchinensis stem as a source for effective and potent natural tyrosinase inhibitors. The identified compounds, hold promise for further exploration and application in the development of skincare products and treatments targeting hyperpigmentation disorders.45,46
Licorice root (Glycyrrhiza glabra) and its chemical components:
Licorice root (Glycyrrhiza glabra) possesses medicinal properties attributed to the presence of glabridin, an iso-flavonoid compound. Glabridin exhibits anti-inflammatory and antiplatelet effects by inhibiting cyclooxygenase activity.45 Notably, glabridin has been found to reversibly inhibit tyrosinase, an enzyme involved in melanin synthesis, In the non-competitive situation with the IC50 value is 0.43 ?M L-1. The main feature of this interaction is static quenching, in which glabridin effectively quenches the intrinsic fluorescence of tyrosinase. Interestingly, molecular docking studies show that glabridin does not directly act on the active site of tyrosinase. In addition, licorice extract's inhibitory effect on tyrosinase activity was greater than that of glabridin alone. This has led to the search for other substances that contribute to the extract's inhibitory activity. Studies have shown that Isoliquiritigenin found in extract of licorice can inhibit the activity of monophenolase and bisphenolase tyrosinase. In addition, inhibition of tyrosinase activity by Iso liquiritigenin is dose-dependent and correlates with their ability to inhibit production of melanin in melanocytes.47
Apios americana (A. americana) and its chemical components:
Apios americana (A. americana) is a plant from the Fabaceae family, and is indigenous to eastern North America. In a study, A. americana dried tubers were extracted using methanol at room temperature. The final methanolic extract is concentrated and fractionated using stepwise solvent extraction to obtain the n-hexane, butanol, ethyl acetate and water fractions. From these fractions, the ethyl acetate fraction was further purified using silica gel, Sephadex LH-20 and C-18 column chromatography, and compounds were isolated.48 The structures of these compounds were determined by comparing previous information as well as their spectroscopic data, including circular dichroism (CD), Nuclear Magnetic Resonance Spectra (NMR spectra) and Electrospray Ionization Mass Spectrometry48. Compounds found in the extract such as aromadendrin 5-methyl ether and lupinalbin A, among others were investigated thoroughly. Competitive inhibitors such as 2-hydroxygenistein-7-O-gentibioside and lupinalbin A exhibited a potential Ki value of 10.3 ± 0.8 µM using the Dixon plot. This correlates with its ability of tyrosinase inhibition and potent skin whitening agent. 52
Humulus japonicus and its chemical components:
In the search for natural ingredients with potential cosmetic use, Humulus japonicus was explored. The ethyl acetate soluble fraction of Humulus japonicus exhibited inhibition of tyrosinase activity. Japonicus by High-Performance Liquid Chromatography Mass spectroscopy (HPLC-MS/MS) combined with the tyrosinase assay revealed the presence of phytoconstituents. Results from the HPLC-MS/MS tyrosinase assay were consistent with the tyrosinase-inhibitory activity of the isolated compounds analyzed. N-coumaroyl tyramine and cis-N-coumaroyl tyramine were the main compounds and showed activity of inhibition and as an active ingredient in cosmetics49. In addition, the cosmetic activity of trans-N-coumaroyl tyramine derivatives was investigated using the coating method. Derivatives were first evaluated for Anti tyrosinase activity and then cytotoxicity was evaluated by assays based on mitochondrial activity. The most potent derivatives were then tested in-vitro to inhibit melanogenic activity in two-dimensional monolayers of human melanocytes containing melanocytes and keratinocytes to evaluate their depigmentation activities. Molecular docking shows the interaction between derivatives and tyrosinase and shows their anti-melanogenic activities by inhibiting tyrosinase.50
Allium cepa, and its chemical components:
Quercetin, a flavonoid compound, was discovered in a variety of foods, particularly in fruits, vegetables, and some beverages. has been found to enhance production of melanin per cell in cultured murine B16-F10 melanoma cells. However, this effect can be due to Melano cytotoxicity . At a concentration of 20 ?M, quercetin resulted in a 50% loss of viable cells, and nearly complete cell death was observed at 80 ?M.53 In the pursuit of discovering new whitening agents, the focus turned to Allium cepa, commonly known as red onion. A compound called quercetin 4'-O-?-D-glucopyranoside was extracted from dried onion peel by bio guided fractionation using mushroom tyrosinase. When using L-tyrosine or L-DOPA as substrate, Quercetin 4'-O-?-D-glucopyranoside exhibits inhibitory activity of tyrosinase enzyme with IC50 values of 4.3 and 52.7 µM, respectively.53 These data indicates that the dried skin of red onion contains ingredients with the potential to be used in skin-whitening cosmetics due to their anti-tyrosinase activity.
Koji bean seeds and its chemical components:
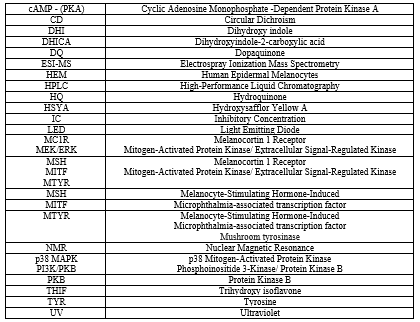
Two metabolites, 7,8,4'-trihydroxyisoflavone and 5,7,8,4'-tetrahydroxyisoflavone, were extracted from koji bean seeds. These compounds have been shown to have a significant effect on the monophenolase and bisphenolase tyrosinase activity. Among these metabolites, 7,3?,4?-trihydroxyisoflavone (7,3?,4?-THIF) drew particular attention.56 Recent studies have shown that 7,3',4'-THIF causes hypopigmentation effects in B16F10 cells. It was discovered that 7,3?,4?-THIF, in contrast to daidzein, directly and effectively inhibited ?-melanocyte-stimulating hormone (MSH)-induced melanin production, both intracellularly and extracellularly, in B16F10 cells. This intriguing effect was attributed to the compound's interaction with the melanocortin 1 receptor (MC1R). Furthermore, the compound modulated the activity of various signaling molecules, including protien kinase B (PKB), p38 Mitogen-Activated Protein Kinase (p38MAPK), and cAMP-dependent protein kinase A(PKA), further influencing the melanin production pathway. 53 Mechanistic investigations, including cAMP and pull-down assays, unveiled that 7,3?,4?-THIF significantly curtailed intracellular cAMP production. Additionally, the compound exhibited a direct binding affinity for MC1R, competing with ?-MSH for receptor occupancy. Strikingly, 7,3?,4?-THIF exerted its inhibitory activity not only in B16F10 cells but also in human epidermal melanocytes (HEMs), highlighting its potential agent for regulating melanin production. Collectively, these findings show modes of action of 7,3?,4?-THIF, which specifically targets MC1R and curtails melanin production 54,55.
Phellinus linteus and its chemical components:
Phellinus linteus, a medicinal mushroom, has emerged as a source of natural tyrosinase inhibitors. Among its compounds, protocatechualdehyde, a benzaldehyde type compound, has displayed remarkable tyrosinase inhibitory activity, surpassing that of kojic acid by 7.8-fold. This potent inhibitor holds promise for applications in the field of cosmeceuticals and skincare 57. Furthermore, the complex culture broth of Phellinus linteus has demonstrated significant effects on melanin synthesis and tyrosinase activity. When administered as a drug, culture medium 3-isobutyl-1-methylxanthine (IBMX) reduced proteins related to melanogenesis, including microphthalmia and tyrosinase in cells. Interestingly, this medium does not affect tyrosinase-associated protein 1 and tyrosinase-related protein 2, indicating a selective inhibitory effect on specific components of the melanogenesis pathway. In the exploration of Phellinus linteus the chemical components were isolated from the fruit body. Importantly, compounds such as protocatechualdehyde, 5-hydroxymethyl-2-furaldehyde (HMF) exhibited inhibitory effects on L-tyrosine oxidation catalyzed by mushroom tyrosinase, with IC50 values of 0.40 and 90.8 µg mL-1 weight. 57,58 These findings point to the potential of Phellinus and its derivatives to be important natural tyrosinase inhibitors.
Aloe vera and its chemical components:
Aloe species are known to possess anti-tyrosinase properties, making them potential candidates for managing hyperpigmentation and serving as skin lightening agents. Aloe vera leaf extricate, as well as its bioactive component aloin, were investigated on tyrosinase inhibition activity and was found to be a mixed competitive tyrosinase inhibitor59,60. The researchers conducted a screening of exudates of South African Aloe species to identify those anti-tyrosinase activity. Qualitative screening revealed that 29 Aloe species exhibited inhibitory activity against tyrosinase, Further analysis was conducted through molecular docking, comparing the activity of plicataloside and aloesin. The results indicated that plicataloside exhibited considerably lower docking scores than aloesin (P < 0>
Carthamus tinctorius L and its chemical components:
Hydroxysafflor yellow A (HSYA) is an active compound derived from Carthami Flos, the flower of safflower (Carthamus tinctorius L.) L in the name refers to the botanist Carl Linnaeus. It is a water-soluble pigment with significant pharmacological properties, making it a subject of extensive research since its isolation in 1993.61 Studies have demonstrated the potential of HSYA in topical applications for mitigating Ultra-violent induced (UV) skin damage. Its antioxidative activity promotes skin recovery, reduces epidermal hyperproliferation, and keeps up the basic integrity of the skin. Additionally, HSYA exhibits strong inhibitory effects on tyrosinase, an enzyme involved in melanin synthesis. It achieves this by binding it to tyrosinase and inducing conformational changes in its tertiary structure. Furthermore, HSYA has shown favorable therapeutic effects in animal models, indicating as protective agent against UV-induced skin damage. Additionally, its potent inhibition of tyrosinase activity positions HSYA as a potential ingredient in the development of skin-whitening products.62,63
Thymus quinquecostatus and its chemical components:
Two varieties of Thymus quinquecostatus are found in Korea: bak-ri-hyang (T. quinquecostatus Celakovsky), which is distributed throughout the Korean Peninsula, and island thyme. The main component identified in the essential oils of both varieties was thymol, with bak-ri-hyang containing 39.8% and island thyme containing 54.7% thymol. Studies have highlighted the potential whitening activity of thymol and its inhibitory effect on tyrosinase, a key enzyme involved in melanin production.66 Further on exploration complex containing thymol ester (3,4,5-methoxycinnamate thymol ester), on melanogenesis in melanin-? and ?-melanocyte stimulating hormone inhibitory effect in stimulated B16 cells was reported. It is important to note that while thymol exhibits promising activities, it also demonstrates moderate cytotoxicity towards B16-F10 melanoma cells, with an IC50 value of 400 ?M (60.09 µg mL-1). However, this toxic effect can be mitigated by the addition of vitamin D and vitamin C, resulting in increased cell viability by 20% and 40%, respectively. Moreover, the toxic effect of thymol on melanoma cells can be reversed by the treatment of l-cysteine. Thymol, an aromatic monoterpene, exhibits the potential for whitening activity and is considered an important functional compound.67
Morus lhou Koidz., and its chemical components:
The stem barks of Morus lhou Koidz., a cultivated plant with edible properties, have revealed discovery of tyrosinase inhibition. Within this botanical treasure, five distinctive flavones have emerged, showcasing remarkable potential in suppressing the enzymatic activity of tyrosinase.68,69 These compounds have been identified as mormin , kuwanon C, cyclomorusin, morusin and norartocarpetin, adding to the rich phytochemical diversity. To unravel the inhibitory potential of these flavonoids against the monophenolase activity of mushroom tyrosinase, rigorous experimentation was undertaken.64,65 The ensuing revelations divulged the IC50 values for compounds 1-5: a mere 0.088 mM, 0.135 mM, 0.092 mM, 0.250 mM, and 1.2µM, were recorded. Such potency underscores their impact on the catalytic activity of tyrosinase. Norartocarpetin unveils an inhibitory behavior as a time-dependent inhibition against the oxidation of L-tyrosine. Operating under the enzyme isomerization model, this compound exhibits its effect by an apparent inhibition constant of 1.354µM this botanical revelation from Morus lhou imparts a vivid glimpse into the intricate world of flavones as potent inhibitors of tyrosinase. 66,67
Lippia origanoides,(Aerial parts) Essential Oil
The inhibitory potential of the Essential oils obtained from Lippia origanoides against tyrosinase activity was assessed using L-tyrosine and L-DOPA as substrates was explored. Essential oils, namely Lippia origanoides-1(LiOr-1), LiOr-2, and LiOr-3, were investigated for their ability to inhibit tyrosinase activity, a key enzyme involved in melanin production. These essential oils displayed effective inhibition, particularly in the initial oxidation step, suggesting their potential for skin whitening and managing hyperpigmentation. Additionally, they interacted with copper ions and engaged in hydrogen bonding interactions, controlling inhibitory mechanisms. The essential oils were rich in specific compounds, such as 1,8-cineole, thymol, and (E)-nerolidol, which contributed to their tyrosinase-inhibiting properties. It highlights the promising cosmetic applications of these essential oils in skincare products aimed at addressing skin pigmentation concerns and promoting a more even skin tone.74
Kojic acid
Kojic acid and ?-arbutin are widely recognized for their ability to inhibit tyrosinase activity and deoxy arbutin effectively suppressed mushroom tyrosinase (MTYR) activity and reduced melanin content.73 Conversely, ?-arbutin and ?-arbutin dose-dependently inhibited B16F10 cells melanin content, tyrosinase (BTYR) activity, resulting in decreased melanin content.75,76 Based on these results, kojic acid is the most suitable positive control among the investigated It effectively inhibits both monophenolase and diphenolase activities of mushroom tyrosinase (MTYR), leading to reduced intracellular melanin content without compromising cell viability. 77,78
Andrographis paniculata
Andrographis paniculata, a medicinal plant, has numerous therapeutic properties, including antifungal, antimicrobial, antiprotozoal, antidiabetic, hepatoprotective, insecticidal, and toxicological attributes.79,80,81,82 However, there is a lack of information regarding its potential anti-melanogenic activity.83 A recent study was conducted to explore the anti-melanogenic properties of A. paniculata leaf extract, focusing on its ability to inhibit melanin synthesis, a process relevant to conditions like hyperpigmentation. The study used advanced technology, such as the In-vitro tyrosinase assay and the Mushroom TYR inhibition assay, to quantitatively assess the extract's ability to modulate tyrosinase activity.84,85,86 This assay provides insights into its potential as an anti-melanogenic agent and serves as a reliable model for studying melanin synthesis inhibition.87,88,89,90 The results provide valuable insights for further exploring the potential application of Andrographis paniculata in dermatology and developing novel therapeutic interventions for hyperpigmentation and related skin conditions.91 Below Table II represents the plants and Plant extracts which have shown significant inhibitory effects on melanin formation
Table II : Provided table presents a summary of several plant species and their potential as sources of natural compounds with tyrosinase inhibitory activity. Tyrosinase is an enzyme responsible for melanin synthesis, and its inhibition can play a role in skin lightening and depigmentation.97,98,99


Fig .2 : Provided figure presents a summary of several plant species potential as sources of natural compounds with tyrosinase inhibitory activity.36,53,60,63,49,75,66,41
CONCLUSION


 Anushree P Munchinamane*
Anushree P Munchinamane*




 10.5281/zenodo.12105303
10.5281/zenodo.12105303