Abstract
The rising prevalence of antibiotic-resistant infections poses a significant challenge to global health, necessitating alternative approaches to traditional antibiotic therapies. Herbal antibiotics, derived from medicinal plants, have emerged as promising candidates due to their antimicrobial properties and diverse mechanisms of action. This review explores the potential of herbal antibiotics as natural agents against pathogenic bacteria, focusing on their efficacy, modes of action, and potential to combat antibiotic resistance. Key phytochemicals such as essential oils, polyphenols, and alkaloids are examined for their ability to inhibit bacterial growth, disrupt biofilms, and enhance host immunity. Additionally, the safety, accessibility, and sustainability of herbal antibiotics highlight their suitability for integration into modern medical practices. While evidence suggests their effectiveness against a range of pathogens, further clinical studies are needed to establish standardized protocols for their therapeutic use. Herbal antibiotics offer a compelling alternative in the fight against antimicrobial resistance, blending traditional knowledge with scientific exploration to address one of the most pressing healthcare challenges.
Keywords
antibiotics, Herbal antibiotics, antimicrobial, infections.
Introduction
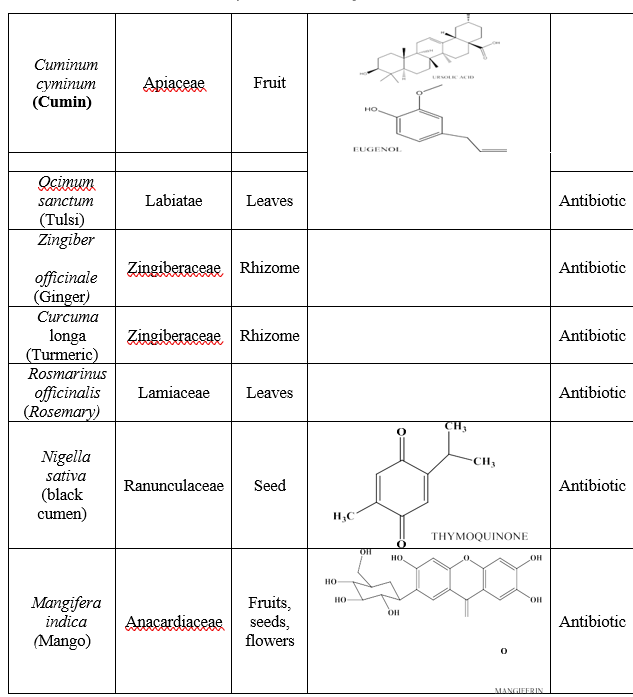
Medicinal plants are used throughout the world as the source of effective and powerful drugs. Because of the overuse of antibiotics in prescription it leads to the development of antibiotic-resistant strains of bacteria and then they become useless next time so experts are seeing with a hope towards natural and safe antibiotic as alternate. Medicines prepared from the natural herbs are relatively inexpensive and stored for long time at normal temperature. Herbal antibiotics having complex nature which are used to kill bacteria, cleanse blood, enhances immune system and functions of particular organ systems. They work by simply killing bacteria and recover imbalances of body [2]. Garlic It is very powerful herb for the treatment of antibiotic-resistant disease. Allicin is the most important active constituent found in garlic which is more powerful than standard Penicillin. And has excellent antimicrobial functions. There are different cultures in world recognized garlic as healer and preventive effects because of its antibacterial, antifungal and antiviral properties. Biofilms which act as defence mechanisms of bacteria’s and fungus, the allicin inhibits the formation of biofilms because biofilms can makes the treatment of infections very difficult and other active ingredient exist in garlic known as Ajoene that was using in treatment of fungal infections by suppressing bacteria by inhibit the production of enzymes which are responsible for various role of the bacteria such as cell structure formation and energy production and survival of bacteria without energy is impossible. Extract of garlic can also be used for the cure of herpes viruses and influenza and many forms of bacteria like Salmonella and Escherichia coli (E. coli) as a very effective treatment [1]. Garlic is used to reduce blood pressure, enhance immune system, lung and digestive system infections. Garlic was used as antimicrobial agent to prevent gangrene during World Wars [1]. 2.5% of fresh garlic used as a mouth wash and give excellent antimicrobial activity but having Bad breath and unpleasant taste in a recent clinical trial [5,6]. Honey It not having only nutritional value but also having health benefits as a traditional medicine that are used in the curing of tuberculosis, eye diseases, throat infections, bronchial asthma, worm infestation, eczema, constipation, wounds, healing of ulcers [1]. From ancient time honey applied on wounds to heal and prevents infection as antibiotics and nowadays it useful in treating chronic wounds, burns, ulcers, skin sores. The antibacterial effects are due to its hydrogen peroxide content. Wounds infected with methicillin-resistant Staphylococcus aureus treated with honey very effectively by providing a protective coating. The character of honey like as glucose oxidase, hydrogen peroxide, low water acidity and honey prevent the growth of bacteria and yeast [1]. The minimum concentration of honey sufficient for complete inhibitory growth of bacteria. The antibacterial activity of honey is effective against many bacterial pathogens and fungi [7]. Ginger It is used as a natural antibiotic. Ginger having the ability to fight against bacteria and helpful in seasickness and nausea and to lower blood sugar levels. Chemical constituents like zingerone, gingerol, terpenoids, zerumbone, gingerdiol, shogaol and flavonoids which gives excellent antimicrobial properties by inhibiting the formation of biofilms. The ginger can normalize the production of acid in stomach so the activity of bacteria H. pylori bacteria grow in stomach in the presence of acid can be reduced acidity by ginger. Clove Water extract of clove effective against various kinds of bacteria including E. coli, Staphylococcus aureus, Pseudomonas aeruginosa. Active constituents of clove, eugenol which having excellent antibacterial properties because of having the capacity to destroy the outer layer of bacterial cells by inhibit the production of protein synthesis and it results the inhibition of bacterial growth and in last death of bacteria. Eugenol has antibacterial activity against S. typhi [1]. Tulsi Tulsi plant mostly leaf (dried or fresh) are used [8] and mainly three types are commonly found. O. tenuiflorum (or O. sanctum L.) contain two phytochemically and botanically different varieties like Rama tulsi (green leaves) and Shyamatulsi (purplish leaves) and Ocimum gratissimum is another variety of Tulsi called as Vanatulsi (dark green leaves) [9]. Tulsi having antibiotics activity against many bacteria like Candida albicans, Staphylococcus aureus, Escherichia coli because of presence of active constituents in it. Ocimum sanctum L. fixed oil having antibacterial activity in which found higher content of linoleic acid in against Pseudomonas aeruginosa, S. aureus and Bacillus pumius, the aqueous extract of Ocimum sanctum L. showed zones of inhibition against Klebsiella. Ursolic acid, Eugenol and carvacrol present in Tulsi possess antimicrobial activity against Streptococcus mutans. At the 4% concentration level these having maximum antimicrobial activity and enhances immunity and metabolic functions with lowering stress and possessing antioxidant property [10]. Turmeric It is a very well-known Indian spice which having antimicrobial properties and flavour. The chemical constituent Curcumin derived from the rhizome of C. longa is an active constituent of turmeric which having very important role in treatment of UTI (Urinary Tract Infections), stomach inflammations as antibacterial as well as antibiofilm activity [1]. Curcumin works as amphipathic and lipophilic so its easily penetrate cell membrane bacteria which results in its leakage and disruption [11]. Due to modulation of gene expression and inhibition of quorum sensing antibiofilm effect shown. It inhibits biofilm and also down regulate many quorum sensing-dependent virulence factors as the production of motility, alginate and swarming [16]. Rosemary It acts as effective natural antibiotic without side-effects in salmonella infections and staph infections and much effective in fighting quorum sensing bacteria. The Rosemary oil shown antibacterial activity of against Bacillus cereus, E. coli, Staphylococcus aureus, Salmonella choleraesuis, Aeromonas hydrophila, Bacillus cereus, Staphylococcus aureus and Clostridium perfringens [12]. Active constituents like isorosmanoletc, rosmarinic acid, carnosol, carnosic acid, rosmanol, epirosmanol and rosmaridiphenol work by interaction with the microbial cell membrane that caused change in hereditary material with changing the transport of electrons which results produced the loss of structure and its membrane functionality [1]. Black cumen Nigella sativa is herbal plant which is also called black cumen. The seed or it oil also used as a carminative, diuretic, lactagogue and vermifuge from past. It also used in the cure of rheumatic diseases, fever, warts, bites of snake and asthma. Thymoquinone and thymohydroquinone obtained from the Extraction and isolation of the volatile oil of N. sativa having suppressive activity against gram-negative and gram-positive bacteria. Diethyl ether extract of N. sativa having combining effect with gentamicin and streptomycin exhibit synergetic effect with tobramycin, spectinomycin, erythromycin, nalidixic acid, doxycycline, chloramphenicol, co-trimoxazole, lincomycin, and ampicillin [13]. N. sativa seeds contain oil, protein, saponin, arachidonic acid and carbohydrate. The fixed oil is composed of eicosadenoic acid, linoleic acid, almitoleic acid, palmitic acid, myristic acid, stearic acid, sterols, fiber and oleic acid whereas the essential oil of N. sativa entails nigellone, carvacrol, thymol, ?- and ?-pinene, p-cymeme, d-limonene, d-citronellol thymoquinone and thymohydroquinone [14]. Mango Magnifera indica commonly known as mango belonging to family Anacardiaeceae is the most popular fruit bearing trees in the world. It is a good source of vitamin A. The main active constituents are the polyphenolics, flavonoids, triterpenoids. Mangiferin a xanthone glycoside major bio-active constituent, Seed kernel extract of mango showing inhibitory effect against coliform and E. coli. Trituration of mango kernel or its extract is being used in food products or cosmetics because of its bacteriostatic and antibacterial properties. Acetone leaf extract of mango show antibacterial activity against S. typhi. [1]. The acetone and methanolic extracts reduced the growth of gram-positive bacteria. Bioactive components present in this plant are thermostable. The application of these plant materials requires boiling for long periods but does not affect its efficacy. Temperature stability by plant extracts had earlier been reported in studies [1]. Onion Allium cepa is also called as bulb onion or garden onion, and is the widely grown species of the genus Allium. It having powerful flavonoids that have antibiotic effects and contain therapeutic sulfur compounds called cysteine sulphoxides and also having proteins, carbohydrates and phosphorus. If we eat white onion as raw regularly it showed its antioxidant and anti-inflammatory properties. Raw onion is also helpful in reducing swelling from bee stings and onion extract are used in the treatment of topical scars; onion used to treat intestinal infections from ancient time and antibacterial activity was evaluated against V. cholerae. By using disc diffusion method its revealed that Allium sativum was viricidal and had MIC of its aqueous extract is obtained to be 5–15 mg/dl and with acetone extract it was obtained to be 2.5–5 mg/dl [15]. Allium extract considered as a natural preservative or food additive. In addition to its nutritional values it also having the antibacterial activities against lots of both gram-negative and grampositive bacteria including Bacillus subtilis, Salmonella, and E. coli and this inhibiting action also noted on Staphylococcus aureus and results a complete inhibition of all strains tested at a concentration of 6.5 mg/ml. Effectiveness in antibacterial activity was depending on the type of onions and extracts concentration. Mostly extracts of onion in concentrations of 50% and it shown excellent antibacterial activity above 50% [1]. Persian cumin Carum carvi is also called as Persian cumin belongs to family Apiaceae, mostly contain volatile oil carvone and limonene. The fruits can be used as whole with pungent or anise-like flavor and aroma because of essential oils present in it. C. Carvi is used as antispasmodic, carminative and appetite enhancing agents. C. Carvi essential oils controlled the Gram-positive and Gram-negative bacteria [1]. Caraway essential oil showed the maximum effect on Acinetobacter spp, E. coli, staphylococcus aureus, Proteus spp. and minimum effect on Pseudomonas aeroginosa [1].
Risks Of Natural Antibiotics
It is not necessary that anything that is natural is counted as safe. Its only depends on amounts and concentrations of active ingredients of drug taken. Taking garlic in high concentration may enhance the risk of bleeding so not suggested for people having surgery or taking blood thinners. There are lots of phyto-chemicals having antibacterial efficacy but they are not used as commercial antibiotics still. So, plants must be explored for getting proper recognition of their therapeutic values, safety & efficacy which results so herbal can replace & used as an alternate of synthetic drugs [16].
When To Use Prescribed Antibiotics
Antibiotics prescribed for better recovery from illness and to prevent the spreading diseases. But currently drug-resistant diseases come in knowledge so doctors do not prescribe antibiotics if that are not much necessary. Antibiotics are given to inhibit the spread of very infectious diseases from having more harmful and give high recovery rate from illness. Antibiotic should be taken only when it prescribed and prescription should not be shared and neither should left-over before time even if feels better then completion of prescription prescribed. Medical expert tries their best to developed herbal antibiotics for antibacterial resistance because bacterial infection gives lots of threat to life [1].
Why Is Herbal Antibiotics Needed?
Antibiotics used during any bacterial infection causing lots of side effect by disturbing natural functions of the body and destruction of good bacteria. These unwanted effects of antibiotics can completely kill or inhibited by the replacing with natural antibiotics. Herbal antibiotics gives effect by not just as bactericidal, but also boosting the body's natural power or immunity for future to save from bacterial infections. Mostly drug resistance does not develop with herbals antibiotics which many times we seen in pharmaceutically produced antibiotics and natural antibiotics does not give the bactericidal effect against beneficial bacteria which lived in our bodies and help us in many ways.
Mode Of Action of Crude Drug
There are lots of crude drugs which having the antibacterial properties. The active constituents of the crude drugs like garlic, ginger, turmeric having approximately same mode of action on bacteria by reducing or inhibits the bio-films growth and rendering it so sensitive as antibacterial herbal drugs. The active constituents of clove and tulip also having the same pharmacological activity by blocking the production of genetic materials DNA and protein and inhibits the bacterial growth.
Antibacterial Spectrum of Crude Drugs
There are two types of bacteria according to gram staining ie. gram positive and gram negative. These drugs having the broad-spectrum antibiotics, mostly drugs are given positive results in both cases like gram positive and gram negative. Tulsi, Turmeric, Carum carvi, Allium cepa, Nigella sativum give their positive antibacterial properties on both type of bacteria like Bacillus subtilis, Salmonella, E. Coli and Staphylococcus aureus. [17]

Antibacterial Activity of Medicinal Plants
Antimicrobials of plant origin have enormous therapeutic potential. They are effective in the treatment of infectious diseases while simultaneously mitigating many of the side effects that are often associated with synthetic antimicrobials. The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. In plants, these compounds are mostly secondary metabolites such as alkaloids, steroids, tannins and phenol compounds, flavonoids, steroids, resins fatty acids gums which are capable of producing definite physiological action on body. Compounds extracted from different parts of the plants can be used to cure diarrhea, dysentery, cough, cold, cholera, fever, bronchitis, etc. Dagmar Janovyska et al. tested the antimicrobial activity of crude ethanolic extracts of ten medicinal plants used in traditional medicine against five species of microorganisms: Bacillus cereus, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Of the 10 plants tested, 5 showed antimicrobial activity against one or more species of microorganisms. The most active antimicrobial plants were Chelidonium majus, Sanguisorba officinalis and Tussilago farfara[19]. Nair et al. screened nine plants for potential antibacterial activity. The plants screened were Sapindus emarginatus, Hibiscus rosa sinensis, Mirabilis jalapa, Rhoeo discolor, Nyctanthes arbor-tristis, Colocasia esculenta, Gracilaria corticata, Dictyota sp. and Pulicaria wightiana. Antibacterial activity was tested against 6 bacterial strains, Pseudomonas testosteroni, Staphylococcus epidermidis, Klebsiella pneumoniae, Bacillus subtilis, Proteus morganii and Micrococcus flavus. Two methods, Agar disc diffusionand Agar disc diffusion, were used to study the antibacterial activity of all these plants. Pseudomonas testosteroni and Klebsiella pneumonia were the most resistant bacterial strains. Sapindus emarginatus showed strong activity against the tested bacterial strains[20]. Ramasamy and Charles Manoharan found the antibacterial activity of valuable compounds from various solvent extracts of Anosomeles indica, Blumea lacera and Melia azadirachta against Escherichia coli, Pseudomonas aeruginosa, Serratia maraceseuns and Staphylococcus aureus by tube diffusion method. Acetone and methanol extracts of all plants showed strong antibacterial effect, whereas petroleum ether and aqueous did not exhibit any effect. Pseudomonas aeruginosa and Serratia marcesenes were relatively moresensitive.[18]
Voravuthi kunchai et al. investigated the aqueous and ethanolic extract of ten traditional Thai medicinal plants for their ability to inhibit 35 hospital isolates of MRSA. Nine medicinal plants displayed activity against all isolates tested. Ethanolic extracts of Garcinia mangostana, Pucinia granatum and Quercus infectoria were more effective, with MICs for MRSA isolates of 0.05 -0.4, 0.2- 0.4 and 0.2 -0.4 mg/ml and for Staphylococcus aureus of 0.1, 0.2 and 0.1mg/ml. MBCs for MRSA isolateswere 0.1-0.4, 1.6-3.2 and 0.4-1.6 mg/ml for Staphylococcus aureus were 0.4, 3.2 and 1.6 mg/ml.[21] Astal et al. tested the aqueous extracts of sage and thyme had action against microorganisms. Phenolic extract of sage and thyme showed antibacterial activity against Staphylococcus aureus and Enterococcus sp. Escherichia coli was more affected by the ethanolic extract of parsley. While, that extract does not elicit pronounce effect on the tested Gram positive organisms. The results of commercial oils of sage, thyme and parsley displayed no antimicrobial activity against Escherichiacoli, Proteus mirabilis and Salmonella typhi. The dataobtained revealed that, among the 10 tested microorganisms, Staphylococcus aureus was the most susceptible microbe to most extract of the three plants.[18] Kabir et al. stated that both water and ethanol extracts ofTerminalia avicennioides, Phyllanthus discoideus, Ocimum gratissimum and Acalypha wilkesiana were effective on MRSA. The MIC and MBCof the ethanol extract of these plants range from 18.2 to 24.0mcg/ml were recorded for ethanol and water extracts of Bridella ferriginea and Ageratum conyzoides. Higher MBC values were obtained for the two plants. All the four active plants contained at least trace amounts of Anthraquinones.[22] Poonko thai et al. pointed out that petroleum ether, benzene ethyl acetate and acetone extract of Galinisoga ciliate leaves displays higher activity against Gram positive bacteria (Staphylococcus aureus and Bacillus subtilis) rather than Gram the negative bacteria(Pseudomonas aeruginosa and Escherichia coli). The toxicity against microorganisms may be done to the high amount of phenolic compounds present.[18] Deshpande et al. isolated that petroleum ether, acetone and methanol extracts of Abrus precatorius, Boswellia serrata, Careya arborea, Emblica officinalis, Syzygium cumini, Woodfordia fruticosa and Sphaeranthus indicus shows appreciable antibacterial activity against Gram positive bacteria (Staphylococcus aureus and Bacillus cereus) and Gram negative bacteria(Escherichia coli, Proteus vulgaris and Pseudomonas aeruginosa). Extracts of some other plants were activeonly against Gram positive bacteria.[18] According to Tambekatr and Kharate et al. Ocimum sanctum showed inhibitory effect on Escherichia coli, Staphylococcus aureus, Proteus mirabilis, Salmonella typhi, Enterococcus faecalis, Pseudomonas aeruginosa and Yersinia enterocolitica. The leaves extract of various plants such as Tulsi, Pudina and Beetle showed antimicrobial activity of Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Salmonella typhi, Vibrio cholerae, Proteus mirablis, Pseudomonas aeruginosa, Yersinia enterocolitica while piper betel showed resistance to Streptococcus pneumoniae.[18] Panthi and Chaudhary et al. tested eighteen plant species used in folklore medicine for their antibacterial activity by the disk diffusion method. The bacteria employed were Gram positive (Staphylococcus aureus) and Gram negative (Escherichia coli, Pseudomonas aeruginosa and Shigella boydii). Extracts of eight plantsshowed encouraging result against three strains of bacteria, while other showed activity against one or two strains[18] Balakrishnan et al. performed antibacterial activity of Mimosa pudica, Aegle marmelos and Sida cordifolia against Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli and Salmonella typhi. The maximum inhibitory zone of inhibition Sida cordifolia was against Bacillus subtilis (35 mm) and Salmonella typhi (26 mm). Minosa pudica and Aegle marmelos were found to be active against all themicroorganisms tested and the maximum activity was noted against Pseudomonas aeruginosa and Salmonella typhi.[23] Attar Singh Chauhan et al. screened Sea buckthorn (Hippophae rhamnoides) seeds aqueous extract for antioxidant and antibacterial activities. The antioxidant activities (Reducing power, DPPH and liposome model system) showed a good antioxidant activity. The extract was also found to possess antibacterial activity with a MIC values with respect to Listeria monocytogenes and Yersinia enterocolitica found to be 750 ppm and 1000 ppm respectively. The antioxidant and antimicrobial effects of the extract implicate its potential for natural preservation.[18] Bupesh et al. evaluated the antibacterial activity in the leaf extracts of Mentha piperita against pathogenic bacteria like Bacillus subtilis, Pseudomonas aureus, Pseudomonas aeruginosa, Serratia marcescens and Streptococcus aureus. The aqueous as well as organic extracts of the leaves were found to possess strong antibacterial activity against a range of pathogenic bacteria as revealed by in vitro agar well diffusion method. The ethyl acetate leaf extract of Mentha piperita showed pronounced inhibition than chloroform, petroleum ether and water, leaf extracts being more on Bacillus subtilis, Pseudomonas aeruginosa than Streptococcus aureus, Pseudomonas aureus and Serratia marcescens.[24] Mohammad Ahanjan et al. tested ethanol, methanol, chloroform, petroleum ether and aqueous extracts of leaves of Parrotia persica for antibacterial activity. The zone of inhibition varied from 13 mm to 22 mm. The highest inhibition was obtained with methanol and ethanol. Chloroform and petroleum ether extracts did not show any activity. The MIC value of the methanol extract for the test bacteria ranged between 3.12 mg/ml and 6.25 mg/ml and that of ethanol extract ranged between 6.25 mg/ml and 12.5 mg/ml. The results scientifically validate the use of this plant in the traditional medicine.[18] Priscila Ikeda Ushimaru et al. evaluated the invitro antimicrobial activity of methanolic extracts of some medicinal plants against Escherichia coli, Salmonella typhimurium, Staphylococcus aureus and Enterococcus sp. The methanolic extract of Caryophyllus aromaticus presented the highest anti-Staphylococcus aureus activity and was effective against all bacterial strains tested.[25] Sumathi and Pushpa et al. evaluated tested ten bacterial isolates for their sensitivity against standard antibiotics, aqueous and alcoholic extracts of five plant samples and the mixture. Only the growth of Escherichia coli was inhibited by the aqueous extracts of Acalypha indica. Mollungolatoides was found to be effective in inhibiting the growth of Escherichia coli at a concentration of 12.5 mg/ml and 6.25 mg/ml. The MIC of alcoholic extracts of Nelumbo nucifera was found to be 0.390 mg/ml for Klebsiella pneumoniae. All the plants extracts showed promising antibacterial properties.[26] Rupanjali Shan et al. tested the antibacterial activity of different solvent extracts of the air dried bark of Parkia javanica, against five antibiotic resistant bacteria viz, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Micrococcus luteus and Escherichia coli by cup-plate diffusion method. MIC values of each active extract were determined. The results showed dose dependent positive activity against all the bacteria except Escherichia coli.[18] Vimala et al. carried out the antimicrobial activity of Ipomea ken trochulous leaf extracts against several pathogenic microorganisms and microbial isolates by disc diffusion method. The crude, cold methanol, distillate and residual extracts of Ipomea ken trochulos were tried on various microorganisms. The crude extract showed zones of inhibition ranging from 0.0 to 21mm, with maximum activity against isolated Rhizopus sp. and least activity against Serratia marcense, Yersinia sp. and Salmonella typhimurium. The zone of inhibition to cold methanol, residual extract and distillate ranged between 6-18 mm and 9-19 mm suggesting that the distillate was more effective than the crude, cold and residual extracts of Ipomea ken trochulous leaf extract against various pathogens and microbial isolates.[18] Kumar et al. evaluated the antimicrobial activities of some Indian medicinal plants against these etiologic agents of Acne vulgaris. Ethanolic extracts of Hemidesmus indicus (Roots), Eclipta alba (Fruits), Coscinium fenestratum (Stems), Curcubito pepo (Seeds), Tephrosia purpurea (Roots), Mentha piperita (Leaves), Pongamia pinnata (Seeds), Symplocos racemosa (Barks), Euphorbia hirta (Roots), Tinospora cordyfolia (Roots), Thespesia populnea (Roots) and Jasminum officinale (Flowers) for antimicrobial activities by disc diffusion and broth dilution methods. The results from the disc diffusion method showed that 07 medicinal plants could inhibit the growth of Propionibacterium acnes. Among those Hemidesmus indicus, Coscinium fenestratum, Tephrosia purpurea, Euphorbia hirta, Symplocos racemosa, Curcubita pepo and Eclipta alba had strong inhibitory effects. Based on a broth dilution method, the Coscinium fenestratum extract had the greatest antimicrobial effect. The MIC values were the same (0.049 mg/ml) for both bacterial species and the MBC values were 0.049 and 0.165 mg/ml against Propionibacterium acnes and Staphylococcus epidermidis.[27] Bin Shah et al. investigated the in vitro antibacterial activities of a total of 46 extracts from dietary spices and medicinal herbs agar-well diffusion method against five food borne bacteria (Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Escherichia coli and Salmonella anatum). Many herb and spiceextracts contained high levels of phenolics and exhibited antibacterial activity against food borne pathogens. Gram-positive bacteria were generally more sensitive to the tested extracts than Gram negative ones. Staphylococcus aureus was the most sensitive, while Escherichia coli were the most resistant.[18] Khalid Mahmood et al. evaluated the antibacterial activity of Ocimum sanctum essential oil against five human pathogenic bacterial species Escherichia coli, Klebsiella sp., Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus by disc-diffusion method. Six mm discs were impregnated with 5 and 10 µl of undiluted essential oil and seeded over the plates aseptically having test microorganisms. The zones of inhibition were measured after 24 hours at 378°C. The essential oil exhibited significant antibacterial activity against all the test pathogens, with maximum zone of inhibition against Staphylococcus aureus (20.0 mm and 41.5 mm) and minimum against Escherichia coli (10.2 mm and 17.8 mm) for 5 and 10 µl of essential oil, respectively. Similarly, the inhibition zones recorded in Proteusmirabilis were 15.1 mm and 26.0 mm, in Pseudomonas aeruginosa10.2 mm and 20.0 mm, in Klebsiellasp. 11.1and 19.4 mm for two given concentrations of essential oil.[18] Cock et al.reported the antimicrobial activity of Ocimum sanctum leaves against bacteria and yeast.The diameter of inhibition zone recorded in Escherichia coli was 18 mm for 22 µl of oil. Thesedifferences may be attributed due to presence of antibacterial component in high concentration in local variety enhancing the medicinal importance of indigenous essential oil.[28] Hadi Mehrgan et al. collected the aerial parts of the plant from Alv and mountain side. The air-dried plant materials were ground to fine powder and then extracted by Soxhelet apparatus using methanol. The extract was tested at a concentration of 100 mg/ml against a panel of Gram-positive and Gram-negative bacteria using the disk diffusion technique. This methanolic extract demonstrated antibacterial activity against Gram positive bacteria including Staphylococcus aureus, Methicillin resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Enterococcus faecalis, Vancomycin - resistant Enterococcus faecalis and Micrococcus luteus and produced inhibition zones with 8-16 mm diameters. It showed no activity against Gram negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa and Salmonella spp. Minimum concentrations (MC) of theextract forming a clear zone were determined against susceptible bacteria.[18] Roopa shree et al. studied the antibacterial activity with respect to their traditional use as anti-psoriatic agents. The herbs were subjected to successive extraction using different solvents and the extracts were subjected to antibacterial evaluation against both Gram positive and Gram negative organisms by Cup plate technique. Among the various extracts, aqueous extracts were found to be more effective against all the bacteria. Staphylococcus aureus was more susceptible to the aqueous extracts among the tested organisms.[18] Koshy Philip et al. screened 32 extracts from eight selected medicinal plants, namely Pereskia bleo, Pereskia grandifolia, Curcuma aeruginosa, Curcuma zedoria, Curcuma mangga, Curcuma inodora, Zingiber officinale and Zingiber officinale for their antimicrobial activity against both Gram-positive bacteria and Gram- negative bacteria using agar disc diffusion assay. The efficacy of the extracts was compared to the commercially prepared antibiotic diffusion discs. No inhibition was observed with the water fractions. None of the plants tested showed inhibition against Escherichia coli. Curcuma mangga showed some remarked inhibition against the bacteria.[29] Bishnu Joshi et al. assessed the antibacterial properties of selected medicinal plants viz. Ocimum sanctum (Tulsi), Origanum majorana (Ram Tulsi),Cinnamomum zeylanicum (Dalchini) and Zanthoxylum armatum (Timur), for potential antibacterial activityagainst 10 medically important bacterial strains, Bacillus subtilis, Bacillus cereus, Bacillus thuringiensis, Staphylococcus aureus, Pseudomonas sp, Proteus sp, Salmonella typhi, Escherichia coli, Shigella dysentriae, Klebsiella pneumoniae. The antibacterial activity of ethanol extracts was determined by agar well diffusion method. The plant extracts were more active against Gram positive bacteria than against Gram negative bacteria. The most susceptible bacteria were Bacillus subtilis, followed by Staphylococcus aureus, while the most resistant bacteria were Escherichia coli, followed by Shigella dysenteriae, Klebsiella pneumonia and Salmonella typhi. Origanum majorana showed the best antibacterial activity. The largest zone of inhibition was obtained with Xanthoxylum armatum against Bacillus subtilis (23 mm) .[30] of Acalypha indica. In case of Staphylococcus aureus, maximum inhibition of 8 mm was obtained in aqueous extracts of Acalypha indica and 6 mm from methanol extract of Lawsonia inermis.[33] Swati Chauhan et al. assessed the antibacterial activity of standard routine antibiotics along with 23 plant extracts by disc diffusion procedure (Bauer-Kirby method) against Klebsiella pneumoniae isolated from nasal samples of pneumonic Barbari goats. The isolate was characterized using biochemical methods and was identified as Klebsiella pneumoniae. The organism was found to be resistant against Amoxicillin, Erythromycin, Cephadroxil, Cefaclor, Roxithromycin and Cephalexin. Terminalia catappa (Leaves), Punica granatum (Bark), Syzygium cumini (bark) and Azadirachta indica (leaves) showed potential activity with MICs at 62.5 mg/ml, 31.2 [34] mg/ml, 62.5 mg/ml and 125 mg/ml respectively .
Warda et al. tested four plants (Marrubium vulgare, Thymus pallidus, Eryngiumilicifolium and Lavandulastoechas) against Streptococcus pneumonia responsible for pharyngitis, rhinitis, otitis and sinusitis infections. Aqueous and methanol extracts have been prepared and tested on Streptococcus pneumoniae collected in four regions. A significant activity has been observed with methanol extracts of three plants; Marrubiumvulgare, Thymus pallidusandLavandula stoechas.[31] Doss et al. isolated compounds of pharmacological interest (Tannins) from the plant species, Solanumtrilobatum and assayed against the bacteria, Staphylococcus aureus, Streptococcus pyrogens, Salmonella typhi, Pseudomonas aeruginosa, Proteus vulgaris and Escherichia coli using agar diffusionmethod. Tannins exhibited antibacterial activities against all the tested microorganisms. Staphylococcus aureus was the most resistant to tannins isolated from the plant material followed by Streptococcus pyrogens, Salmonella typhi, Escherichia coli, Proteus vulgaris and Pseudomonas aeruginosa. Minimum inhibitoryconcentration of the tannins ranged between 1.0 and 2.0 mg/ml while the minimum bactericidal concentration ranged between 1.5 and 2.0 Sheeba et al. detected the antibacterial activity against Staphylococcus aureus, Streptococcus sp., Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysenteriae and Vibrio cholerae. The highest antibacterial activity wasobserved in 500 µg concentration of leaf extracts of all bacteria screened except Shigella dysenteriae. The minimum zone of inhibition observed in 25 µg concentration of leaf extract except Pseudomonas aeruginosa and Shigella dysenteriae. These results indicate that the extracts were bacteriostatic at higher concentrations.[18] Akinjogunla et al. assessed the antibacterial activity of extracts of the root and leaf of Phyllanthusamarus against extend spectrum lactamase (ESBL) producing Escherichia coli isolated from the stool samples of HIV sero-positive patients with or without diarrhoea using Bauer disc diffusion method. The phenotypic confirmation of ESBL - Escherichia coli were done by Double Disc Synergistic Methods (DDST). The phytochemical analysis of both root and leaf revealed the presence of alkaloids, flavonoids, saponins, tannins, cardiac glycoside, terpenes and anthraquinones. The strains isolated from both HIV sero- positive patients were susceptible to various concentrations of the extracts (5 mg ml-1, 10 mg ml-1, 20 mg ml-1, 40 mg ml-1 mg/ml.[32]and 80 mg ml-1).[35] Sukanya et al. examined the ethno botanical efficacy of Indian medicinal plants; Achyranthes aspera, Artemisia parviflora, Azadirachta indica, Calotropis gigantean, Lawsonia inermis, Mimosa pudica, Ixora coccinea, Parthenium hysterophorus and Chromolaena odorata using agar disc diffusion method against clinicalbacteria (Escherichia coli and Staphylococcus aureus) and phyto-pathogenic bacteria (Xanthomonas vesicatoria and Ralstonia solanacearum). Leaves were extracted using different solvents such as methanol, ethanol, ethyl acetate and chloroform. Among treatments, maximum invitro inhibition was scored in methanol extracts of Chromolaena odorata which offered inhibition zone of 10, 9, 12 and 12 mm against Escherichia coli, Staphylococcus aureus, Xanthomonas vesicatoria and Ralstonia solanaccearum, followed by chloroform extract of the same plant leaf with inhibition zone of 8, 4, 4 and 4A significant inhibition of Escherichia coli was found in aqueous and in all tested solvent extracts. Adegoke et al. investigated the phytochemical screening and antimicrobial potentiality of Phyllanthus amarus against multidrug resistant pathogens usingstandard microbiological techniques. The extracts were tested by agar well diffusion method for activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella sp. isolated from clinical samples. The susceptibility patterns of the test isolates against the crude extract was determined at extract concentrations of 10 mg/ml, 50 mg/ml, 100 mg/ml and 150 mg/ml respectively. The results revealed that the extracts did not inhibit the growth of Escherichia coli,Pseudomonas sp. and Klebsiellasp. at 10mg/ml but thelargest zones of growth inhibition for the ethanolic extract was recorded with Staphylococcus aureus, Escherichia coli and Klebsiellasp. with a mean zone diameter of 20 mm concentrations. The minimum inhibitory concentration (MIC) of the ethanolic plant extracts on Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiellasp. were at 10 mg/ml, 50 mg/ml, 150 mg/ml and100 mg/ml while the MBC were at 50 mg/ml, 100 mg/ml, 150 mg/ml and 150 mg/ml respectively.[18] Ajayi and Akintola et al. screened the leave extracts from medicinal plants in vitro in the laboratory for their antibacterial activity against two prominent enteric bacteria, Escherichia coli and Salmonella typhimurium using the agar disc diffusion method. The tyndalized leave extract of C. zambesicus showing antibacterial inhibition zone of 4 and 2 mm against Salmonella typhimurium and Escherichia coli exhibited highestactivity than the autoclaved samples and other plant sources tested independently or combined, showing that the combinations of the extract samples do not exhibit synergistic effects.[36] Saranraj et al. evaluated the antibacterial potentiality of ethanol and ethyl acetate solvent extracts of mature leaves of Acalypha indica against nine pathogenic bacterial isolates viz., Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Escherichia coli, Salmonella typhi, Shigella flexneri, Klebsiella pneumoniae, Vibrio cholera and Pseudomonas aeruginosa. The turbidity of the bacterial inoculums was compared with 0.5 McFarland standards and the antibacterial potential of Acalypha indica ethanol extract was tested by using Agar well diffusion method. The ethanol extract of Acalypha indica (100 mg/ml) showed maximum zone of inhibition (30 mm) against Pseudomonas aeruginosa, Escherichia coli and Bacillus subtilis. Staphylococcus aureus showed less zone of inhibition (12 mm). The ethyl acetate extract of Acalypha indica(100 mg/ml) showed maximum zone of inhibition(23 mm) against Escherichia coli.[37] Murugan and Saranraj et al. tested the herbal plant Acalypha indica for its antibacterial activity against Nosocomial infection causing bacteria. The Acalypha indica was shade dried and the antimicrobial principleswere extracted with Methanol, Acetone, Chloroform, Petroleum Ether and Hexane. The antibacterial activity of Acalypha indica was determined by Agar Well Diffusion Method. It was found that 50mg/ml of methanolic extract of the plant able to inhibit the growth of nosocomial infection causing bacteria when compared to other solvent extracts. From this it was concluded that the solvent methanol able to leach out antimicrobial principle very effectively from the plant than the other solvents. The phytochemicals present in the Acalypha indica was tested and it conferred that the possible antibacterial principle resided in tannins and alkaloids.[38] Siva Sakthi et al. evaluated the antibacterial potentiality of ethanol and ethyl acetate solvent extracts of mature leaves of Datura metel against nine pathogenic bacterial isolates viz., Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Escherichia coli, Salmonella typhi, Shigella flexneri, Klebsiella pneumoniae, Vibrio cholera and Pseudomonas aeruginosa. The turbidity of the bacterial inoculums was compared with 0.5 McFarland standards and the antibacterial potential of Datura metel ethanol extract was tested by using Agar well diffusion method. The ethanol extract of Datura metel (100 mg/ml) showed maximum zone of inhibition (26 mm) against Pseudomonas aeruginosa, Escherichia coli and Bacillus subtilis. Staphylococcus aureus showed less zone of inhibition (8 mm). The ethyl acetate extract of Datura metel (100 mg/ml) showed maximum zone of inhibition (19 mm against Escherichia coli. There was no zone of inhibition against Pseudomonas aeruginosa.[39] Saranraj and Sivasakthivelan et al. tested the antibacterial activity of Phyllanthus amarus was tested against Urinary tract infection causing bacterial isolates viz., Staphylococcus aureus, Serratia marcescens, Escherichia coli, Enterobacter sp., Streptococcus faecalis, Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa. The Phyllanthus amarus wasshade dried and the antimicrobial principles were extracted with methanol, acetone, chloroform, petroleum ether and hexane. The antibacterial activity of Phyllanthus amarus was determined by Agar Well Diffusion Method. It was found that methanol extract of Phyllanthus amarus showed more inhibitory activity against UTI causing bacterial pathogens when compared to other solvent extracts.[40] Saranraj et al. evaluated the bioactivity of Mangifera indica ethanol extract against human pathogenic bacteria and fungi. The plant material was collected, shade dried and powdered. The powdered material was extracted using the organic solvent ethanol. Antimicrobial activity of Mangifera indica ethanol extract was determined by Disc diffusion method. The zone of inhibition of Mangifera indica ethanol extract against bacteria was maximum against Vibrio cholerae followed by Klebsiella pneumoniae, Staphylococcus aureus, Proteus mirabilis, Pseudomonas aeruginosa and Escherichia coli. The least zone of inhibition was recorded against Salmonella typhi. The Minimum Inhibitory Concentration (MIC) was ranged from 2 mg/ml to 4 mg/ml. The Minimum Bactericidal Concentration (MBC) value ranged between 2mg/ml and 4mg/ml. For fungi, the zone of inhibition was maximum against Candida albicans, followed byAspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Candida tropical is and Candida cruzei. The least zone of inhibition was recorded against Penicillium sp. The MIC was 0.5 mg/ml and the MFC value was 1 mg/ml.[41]
CONCLUSION
Herbal antibiotics hold great potential as a natural and sustainable approach to addressing antimicrobial resistance. With diverse mechanisms that target bacterial cells, disrupt biofilms, and bolster host immune responses, these plant-derived compounds represent a viable supplement or alternative to synthetic antibiotics. The review underscores the importance of continued research into the clinical applications, efficacy, and safety profiles of these herbal agents. While promising results have been observed in laboratory and preclinical studies, rigorous clinical trials are essential to validate their therapeutic efficacy and establish dosage guidelines. Integrating herbal antibiotics into modern healthcare systems may offer an effective strategy to mitigate antibiotic resistance and reduce reliance on conventional antibiotics. As we advance in this field, collaboration between traditional medicine practitioners and modern healthcare researchers will be crucial in unlocking the full potential of herbal antibiotics as nature’s solution to antibiotic-resistant infections.
REFERENCES
- Reena Gupta, Sharad Sharma. Herbal antibiotics: A Review. Bull. Env. Pharmacol. Life Sci., Vol 9[11] October 2020 : 136-142
- Basappa, K., Venu Gopal, J. (2013). Natural Alternatives to antibiotic agents. Asian J. Biomed. Pharm. Sci.,3(24):1-4.
- Joy, P. P., Thomas, J., Mathew, S. Skaria, B. P. (1998). Zingiberaceous Medicinal and aromatic plants. Aromatic and medicinal plants research station, Odakkali, Asamannor, Kerala, India. 1-31.
- Groppo, F., Ramacciato, J., Motta, R. et al. (2007). Antimicrobial activity of garlic against oral streptococci. Int. J. Dent. Hyg.; 5(2):109–115.
- Basappa, K. et al. (2013). Natural alternatives to antibiotic agents. Asian J. Biomed. Pharm. Sci.,3(25):1-4.
- Wilkinson, J. M., Cavanagh, H. M. (2005) Antibacterial activity of honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food,8:100–103.
- Jamshidi, N. Cohen, M. M. (2017). The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evid Based Complement. Alternat. Med.,2017:1-13.
- Bhamra, S., Heinrich, M., Howard, C., Johnson, M., Slater, A. (2015). DNA authentication of tulsi (Ocimum tenuiflorum) using the nuclear ribosomal internal transcribed spacer (ITS) and the chloroplast intergenic spacer trnH-psbA. Planta Medica,81:20.
- Srinivas, N., Sali, K., Bajoria, A. A. (2016). Therapeutic aspects of Tulsiunraveled: A review. J. Indian Acad. Oral Med. Radiol.,28 [1]: 17-23.
- Tyagi, P., Singh, M., Kumari, H., Kumari, A., Mukhopadhyay, K. (2015). Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One,10: 1-15.
- Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods-A review. Int. J. Food Microbiol.,94:223–253.
- Halawani, E. (2009). Antibacterial Activity of Thymoquinone and Thymohydroquinone of Nigella sativa L. and Their Interaction with Some Antibiotics. Adv. Biolog. Res.,3(5-6):148-152.
- Heimesaat, M. M. (2017). Finding novel antibiotic substances from medicinal plants – antimicrobial properties of nigella sativa directed against multidrug-resistant bacteria. Eur. J. Micro. Immun.,7[1]:92–98.
- Doughari, J. H. and Manzara, S. (2008). In vitro antibacterial activity of crude leaf extracts of Mangiferaindica Linn. Afr. J. Microbiol. Res.,(2):067-072.
- Sharma, A., Kumar, P. (2009). In-vitro screening of the antibacterial activity and identification of bioactive compounds from plants against selected Vibrio spp. Pathogens. Turk. J. Biol.,33:137–44.
- Priti, M. [2018]. Need of herbal antibiotics. Clin. Pathol. Res. J.,2:1.
- Uttpa, A., Jacobo-Herrera, N., Altemimi, A., Lakhssassi, N. (2019). A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites,9(11):258.
- Mahato TK, Sharma K, Study of medicinal hurbs and its antibacterial activity: a review, Journal of Drug Delivery and Therapeutics. 2018; 8(5-s):47-5
- Dagmar Janovska., Katerina Kubikova., Adislav Kokoska. Screening for antimicrobial activity of some medicinal plants species of traditional Chinese medicine. Czech Journal of Food Science, 2003; 107-110.
- Nair RT., Kalariya., Chanda S. Antibacterial activity of some selected Indian medicinal flora. Turkey Journal of Biology, 2005; 29:41-47.
- Saranraj P., D Stella. Antibiogram of nosocomial infection and its antimicrobial drug resistance. International Journal of Pharmacological and Biological Archives, 2011; 2(6):1598- 1610.
- Kabir O Akinyemi., Olukayode Oladapo., Chidi Okwara., Christopher C., Ibe Kehinde A. Fasure. Screening of crude extracts of six medicinal plants used in South-west Nigerian unorthodox medicine for anti- methicillin resistant Staphylococcus aureus activity. BMC Complementary and Alternative Medicine. 2005; 1-7
- Balakrishnan N., VH Bhaskar., B Jayakar., B Sangeswaran. Antibacterial activity of Mimosa pudica, Aegle marmels and Sida cordiffolia. Pharmacogysonya Magazine, 2006; 2:198- 199.
- Bupesh GC., Amutha S., Nandagopal A., Ganesh Kumar P.,Suresh Kumar., K Saravana Murali. Antibacterial activity of Mentha piperita L. (peppermint) from leaf extracts-a medicinal plant. Acta Agriculturae Slovenica, 2007; 89(1):73-79.
- Priscila Ikeda Ushimaru., Mariama Tomaz Nogueira da Silva., Luiz Claudio Di Stasi., Luciano Barbosa., Ary Fernandes Junior. Antibacterial activity of medicinal plant extracts. Brazilian Journal of Microbiology, 2007; 38:717- 719.
- Bishnu Joshi., Sunil Lekhak., Anuja Sharma. Antibacterial property of different medicinal plants: Ocimum sanctum, Cinnamomum zeylanicum, Xantho xylumarmatum and Origanum majorana. Kathmandu University Journal of Science, Engineering and Technology, 2009; 5(1):143- 150.
- Kumar GS., KN Jayaveera., CK Ashok Kumar., Umachigi P. Sanjay BM. Vrushabendra Swamy., DV Kishore Kumar. Antimicrobial effects of Indian medicinal plants against acne- inducing bacteria. Tropical Journal of Pharmaceutical Research, 2007; 6(2):717-723.
- Cock IE. Antimicrobial Activity of Aloe barbadensis miller leaf gel components, Journal of Microbiology, 2008; 4:2.
- Koshy Philip., Sri Nurestri Abd Malek., Wirakarnain Sani., Sim Kae Shin., Saravana Kumar., Hong Sok Lai., Lee Guan Serm., Syarifah NSA Rahman. Antimicrobial activity of some medicinal plants from Malaysia. American Journal of Applied Sciences, 2009; 6(8):1613-1617.
- Bishnu Joshi., Sunil Lekhak., Anuja Sharma. Antibacterial property of different medicinal plants: Ocimum sanctum, Cinnamomum zeylanicum, Xantho xylumarmatum and Origanum majorana. Kathmandu University Journal of Science, Engineering and Technology, 2009; 5(1):143-150.
- Warda K., M. Markouk., K Bekkouche., M Larhsini., A Abbad., A Romane., M Bouskraoui. Antibacterial evaluation of selected Moroccan medicinal plants against Streptococcus pneumonia. African Journal of Pharmacy and Pharmacology, 2009; 3(3):101-104.
- Doss A., H. Mohammed Mubarack., Dhanabalan. Antibacterial activity of tannins from the leaves of Solanum trilobatum. Indian Journal of Science and Technology, 2009; 2(2): 41-43.
- Sukanya SL., J Sudisha., P Hariprasad., SR Niranjana., H S Prakash., SK Fathima. Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. African Journal of Biotechnology, 2009; 8(23): 6677-6682.
- Chauhan S, Dwivedi D, Ashok kumar., Gururaj., VS Vihan. In vitro Resisto typing and antibacterial screening of medicinal plant extracts in Pneumonic Goats. Asian Journal of Experimental Biological Science, 2010; 1(1):15-22
- Saranraj P., D. Stella., K. Sathiyaseelan., Sajani Samuel. Antibacterial potentiality of Ethanol and Ethyl acetate extract of Acalypha indica against human pathogenic bacteria. Journal of Ecobiotechnology, 2010; 2(7):23-27.
- Ajayi OA, Akintola TA. Evaluation of antibacterial activity of some medicinal plants on common enteric food-borne pathogens. African Journal of Microbiology Research, 2010; 4(4):314-316.
- Saranraj PD., Stella K., Sathiyaseelan., Samuel S. Antibacterial potentiality of Ethanol and Ethyl acetate extract of Acalypha indica against human pathogenic bacteria. Journal of Ecobiotechnology, 2010; 2(7):23-27.
- Murugan T., P Saranraj. Antibacterial activity of various solvent extracts of the Indian herbal plant Acalypha indica against human pathogens causing nosocomial infection. International Journal of Pharmacological and Biological Archives, 2011; 2(5):1498-1503.
- Siva S., Saranraj SP, Geetha M, Antibacterial evaluation and phytochemical screening of Datura metel leaf extracts against bacterial pathogens. International J. of Pharmacology and Biological Archives, 2011; 2(4):1130-1136.
- Saranraj P, Sivasakthivelan P. Screening of antibacterial activity of medicinal plant Phyllanthus amarus against Urinary tract infection (UTI) causing bacterial pathogens. Applied Journal of Hygiene, 2012; 1(3):19-24.
- Saranraj PD., Stella D Reetha. Bioactivity of Mangifera indica ethanol extract against human pathogenic microorganisms. Novus International Journal of Pharmaceutical Technology, 2012; 1(1):11-18


 Sayali Gade *
Sayali Gade *


 10.5281/zenodo.14614775
10.5281/zenodo.14614775