Abstract
Carbon nanotubes (CNTs) are cylindrical nanostructures made of rolled-up sheets of single-layer carbon atoms (graphene) with remarkable mechanical, electrical, and thermal properties. Since their discovery, carbon nanotubes (CNTs) have transformed the study of nanomaterials and shown unmatched promise in the biological sciences. This study outlines current developments in CNT-based pharmaceutical applications, with an emphasis on biosensing, tissue engineering, drug delivery systems, and diagnostic imaging. These nanoscale structures have the potential to revolutionize therapeutic efficacy, precision targeting, and diagnostic accuracy due to their remarkable surface area, adaptable surface chemistry, and exceptional biocompatibility. A thorough analysis of the literature shows that CNTs greatly improve drug delivery through increased solubility, stability, and targeted distribution, which improves bioavailability and therapeutic results. Functionalized carbon nanotubes (CNTs) improve resolution and contrast in magnetic resonance imaging (MRI), computed tomography (CT), and fluorescence imaging. This helps in early illness identification and tracking. CNT-based scaffolds are used in tissue engineering to imitate the extracellular matrix, which is essential for tissue regeneration as it promotes cell adhesion, proliferation, and differentiation. Furthermore, CNT-based biosensors show great promise in clinical diagnostics due to their remarkable sensitivity and specificity in identifying a wide range of chemicals and pathogens. However, issues including possible toxicity, biocompatibility over the long term, and the requirement for standardized procedures for CNT characterisation and functionalization continue to be problems. For CNT-based technologies to be successfully translated into clinical practice, these problems must be resolved. All things considered, CNTs have enormous potential to transform pharmaceutical applications, and more multidisciplinary research is needed to fully realize their potential for advancements in medicine.
Keywords
Carbon nanotubes, pharmaceutical applications, drug delivery, imaging, tissue engineering.
Introduction
Carbon nanotubes (CNTs) first described by Iijima. and since their discovery, they have contributed to the development of studies in the field of physics, chemistry and material sciences. Many works were completed on the structure, properties [1] carbon nanotube (CNT) have become one of the leading topics of academic research and are widely used in various industrial applications. CNTs are carbon allotropes made of graphite. they are cylindrical shape, with a diameter of nanometer and a length of several millimeters. carbon nanotubes have been first used as additives to various structural materials for electronics, optics, plastics, and other materials of nanotechnology fields.it has been first applied to bind antineoplastic and antibiotic drug to carbon nanotubes for cancer and infection treatment, respectively.[2] carbon nanotubes are composed of carbon, which is a tube-like material, and the nanometer scale is used to measure the diameters of carbon nanotubes. The nanometer scale is about one billionth of a meter, or one tenth of a hair length. [3] CNTs possess various novel properties that make them useful in the field of nanotechnology and pharmaceutical, highly purified CNTs are extensively used as building blocks of advanced materials with remarkable properties.[4] The wrapping method of the graphene sheets directly affects a number of the nanotubes' characteristics. Because of their nanometric size and cylindrical shape, carbon nanotubes (CNTs) are promising materials for future uses such as hydrogen storage.[5] CNTs come in two primary types: single-walled nanotubes (SWCNTs) and multi-walled nanotubes (MWCNTs). SWCNTs consist of a single layer of carbon atoms wrapped into a seamless cylinder, while MWCNTs comprise multiple concentric cylinders nested within one another. One of the most significant characteristics of CNTs is their exceptional strength-to-weight ratio. They are incredibly strong, yet lightweight, which makes them ideal candidates for reinforcing materials in composites. Additionally, they possess excellent electrical conductivity, thermal conductivity, and have unique optical properties.[6]
Structure:
Carbon nanotubes can be categorized into two main types based on their structure:
- Single-Walled Carbon Nanotubes (SWCNTs): These consist of a single layer of carbon atoms rolled into a seamless cylindrical tube. SWCNTs can have diameters as small as 0.4 nanometers, and their properties can vary depending on the direction of rolling, known as chirality. (the way the graphene sheet is rolled). SWCNTs exhibit extraordinary mechanical, electrical, and thermal properties, making them attractive for a wide range of applications, including electronics, sensors, and composites.
- Multi-Walled Carbon Nanotubes (MWCNTs): These structures consist of multiple layers of graphene sheets nested concentrically to form a tube-within-a-tube configuration. MWCNTs can have larger diameters ranging from a few to several tens of nanometers.[7]
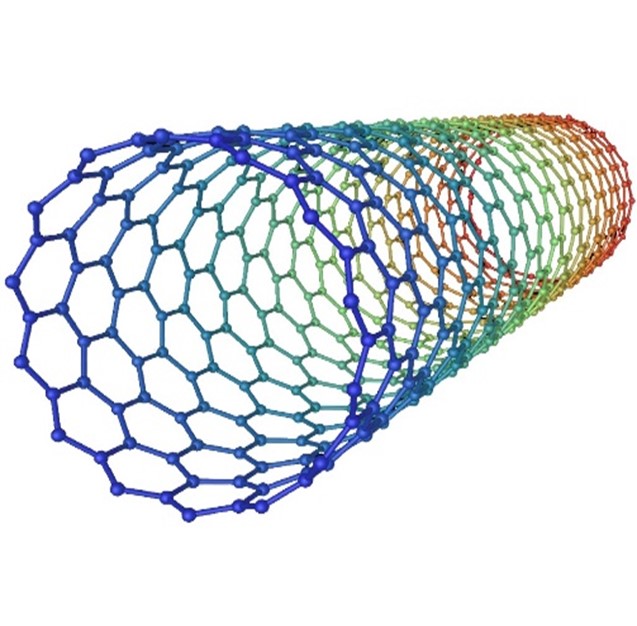
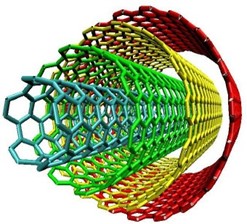
Fig -1 Single Walled And Multiple Walled Carbon Nano Tubes
Carbon nanotubes (CNTs) exhibit a wide range of remarkable properties due to their unique structure and bonding arrangements. Some of the key properties of carbon nanotubes include: -
- Exceptional Mechanical Strength:
CNTs are one of the strongest known materials, with tensile strengths exceeding those of most other materials, including steel. This exceptional strength arises from the strong sp?2; carbon-carbon bonds and the seamless cylindrical structure of CNTs.[8]
- High Electrical Conductivity:
Single-walled carbon nanotubes (SWCNTs) can exhibit either metallic or semiconducting behavior depending on their chirality (the way the graphene sheet is rolled). Metallic SWCNTs are highly conductive, with electrical conductivities comparable to or even surpassing that of copper. Semiconducting SWCNTs can be used in electronic devices such as field-effect transistors.[9]
- High Thermal Conductivity:
CNTs possess excellent thermal conductivity along their axial direction, which can exceed that of diamond. This property makes CNTs promising candidates for applications in thermal management, such as heat sinks, thermal interface materials, and nanocomposites.
- Low Density:
Despite their exceptional strength, carbon nanotubes are lightweight materials due to their low density. This combination of high strength and low weight makes them attractive for applications where weight reduction is critical, such as aerospace and automotive industries.
- Large Aspect Ratio:
Carbon nanotubes typically have very high aspect ratios (length-to-diameter ratios), which contributes to their exceptional mechanical properties. The high aspect ratio also makes them effective reinforcements in composite materials, enhancing stiffness and strength.
- Chemical Stability:
Carbon nanotubes exhibit high chemical stability, resisting corrosion and degradation in a wide range of environments. This property makes them suitable for applications where chemical resistance is important, such as in sensors and protective coatings.
- Optical Properties:
Carbon nanotubes exhibit unique optical properties, including strong absorption and emission in the infrared and near-infrared regions of the electromagnetic spectrum. These properties have implications for applications in optoelectronics, photovoltaics, and biological imaging.
- Flexibility and Elasticity:
Carbon nanotubes can exhibit significant flexibility and elasticity, allowing them to bend and deform without breaking. This property makes them suitable for applications where flexibility is desired, such as in wearable electronics and flexible displays.[10]
Method of carbon nano- tubes synthesis
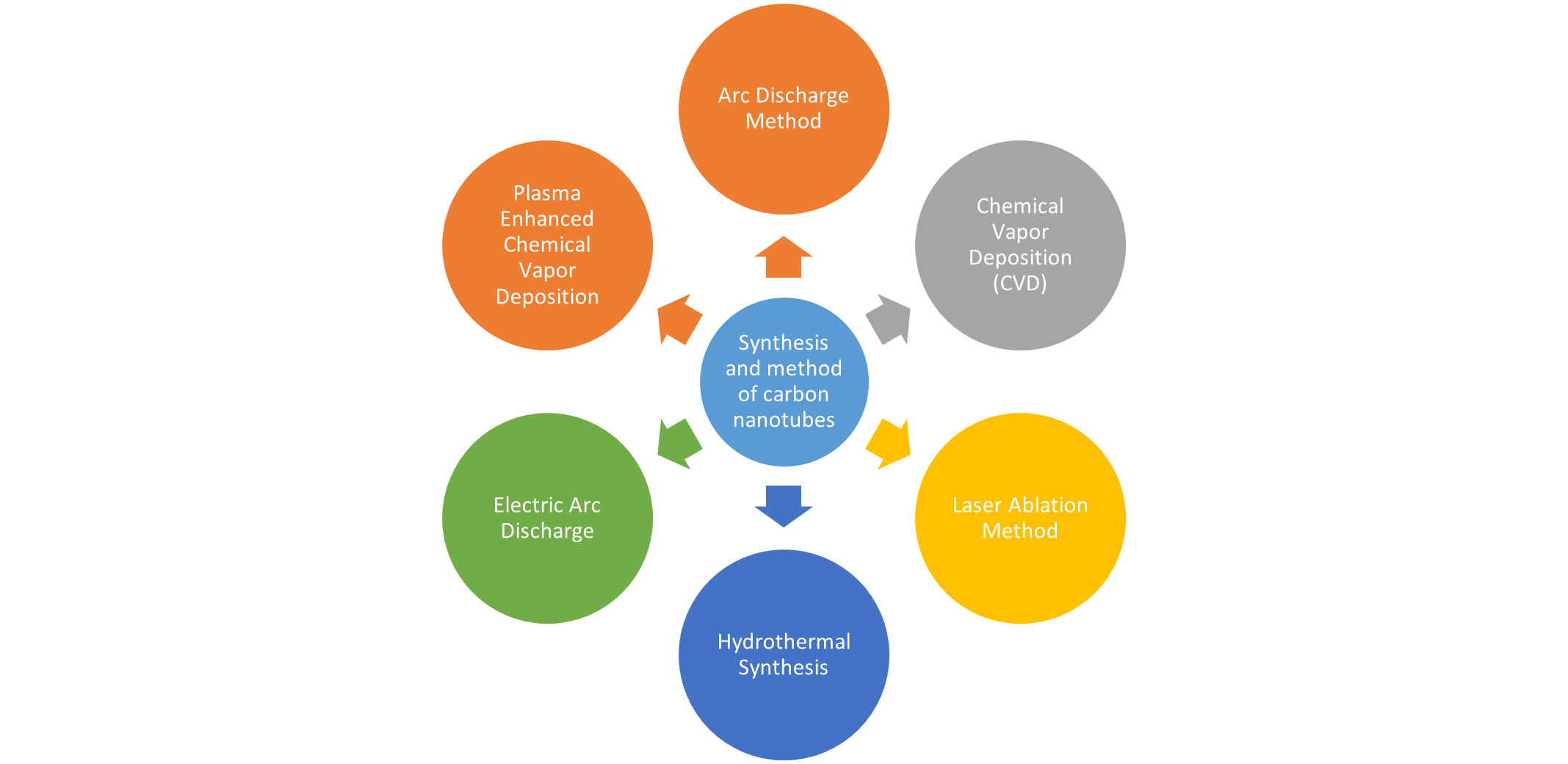
High temperature preparation techniques such as arc discharge or laser ablation were first used to produce CNTs however nowadays these methods have been replaced by low temperature chemical vapour deposition (CVD) techniques (<800>
- Arc Discharge:
This method involves creating an arc discharge between two graphite electrodes in an inert atmosphere (usually helium) at high temperatures (around 3000°C). The high temperature and pressure cause carbon atoms to vaporize and condense into nanotubes.[12]
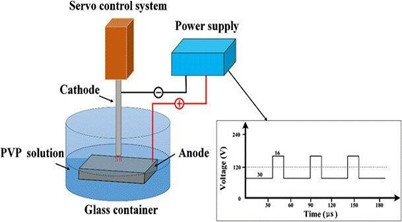
Fig 2– Arc discharge method
- Chemical Vapor Deposition (CVD):
This is one of the most widely used methods. It involves the decomposition of hydrocarbons such as methane or ethylene at high temperatures (typically 600-1000°C) in the presence of a catalyst, often transition metals like iron, nickel, or cobalt. CNTs grow on the surface of the catalyst.[13]

Fig-3 Chemical Vapor Deposition (CVD)
- Laser Ablation:
In this method, a high-power laser is focused on a target containing carbon (typically graphite) in a chamber filled with an inert gas. The intense heat generated vaporizes the carbon, which then condenses and forms CNTs as the vapor cools. The laser ablation method uses a pulsed and continuous laser to vaporize a graphite target in an oven, which is filled with helium or argon gas to keep pressure. The laser ablation is similar to the arc discharge, both taking advantage of the very high temperature generated, with the similar optimum background gas and catalyst mix observed. The very similar reaction conditions needed to indicate that the reactions probably occur with the same mechanism for both the laser ablation and electric arc methods. SWNTs were prepared by continuous wave carbon dioxide laser ablation without applying additional heat to the target. They found that the average diameter of SWNTs produced by carbon dioxide laser increased with increasing laser power.[14]

Fig- 4 Laser Ablation Method
- Hydrothermal Synthesis:
CNTs can also be synthesized under high-temperature and high-pressure conditions in an aqueous solution containing carbon precursors and catalysts.
- Plasma Enhanced Chemical Vapor Deposition (PECVD):
This method involves the use of plasma to enhance the decomposition of hydrocarbons and the growth of CNTs on a substrate. [15]
Current trends in carbon nanotubes
Functionalization and Surface Modification: Researchers are exploring various methods to functionalize and modify the surface of carbon nanotubes to tailor their properties for specific applications. Functionalization can enhance compatibility with polymers, improve dispersibility in solvents, or introduce specific functionalities for targeted applications such as sensing, drug delivery, or energy storage.[16]
Composite Materials:
CNTs are increasingly being incorporated into composite materials to enhance mechanical, electrical, and thermal properties. These composites find applications in aerospace, automotive, electronics, and structural materials. The development of scalable and cost-effective manufacturing processes for CNT-based composites is an ongoing trend.[17]
Energy Storage and Conversion:
Carbon nanotubes are being explored for use in various energy storage and conversion devices, including batteries, supercapacitors, and fuel cells. Their high surface area, conductivity, and mechanical strength make them promising materials for improving the performance and efficiency of these devices.[18]
Sensors and Electronics:
CNT-based sensors have attracted significant attention for their high sensitivity, low detection limits, and fast response times. They find applications in gas sensing, biosensing, environmental monitoring, and medical diagnostics. Additionally, CNTs continue to be investigated for use in flexible and transparent electronics, wearable devices, and next-generation displays.[19]
Biomedical Applications:
Carbon nanotubes hold promise for various biomedical applications, including drug delivery, imaging, tissue engineering, and biosensing. Researchers are exploring the biocompatibility, stability, and safety of CNTs for use in therapeutic and diagnostic applications.[20]
Scale-up and Commercialization:
Efforts continue to scale up the production of carbon nanotubes to meet industrial demand and reduce production costs. Advances in synthesis techniques, purification methods, and post-processing technologies are driving progress towards large-scale production and commercialization of CNT-based products.[21]
Regulatory and Safety Considerations:
As CNT-based products enter the market, there is increasing attention on regulatory frameworks, safety standards, and environmental impacts. Efforts are underway to assess the health and environmental risks associated with CNT exposure and to develop guidelines for safe handling, disposal, and waste management.[22]
Pharmaceutical application of carbon nanotues-
Structure means that the tubes have an inner and an outer core which can both be modified by different functional groups. Thus, the CNTs can be designed for very specific purposes. In the area of biomedicine, the applications of CNTs are investigated and make them useful in a range of different applications. Their fields: drug delivery, biomedical imaging, biosensors and scaffolds in tissue engineering.[23]
- Drug Delivery:
CNTs can serve as carriers for various therapeutic agents, including small molecules, proteins, nucleic acids, and imaging agents. Their high surface area allows for efficient loading of drugs, while their unique structure facilitates targeted delivery and controlled release.[24]
- Imaging and Diagnosis:
CNTs can be functionalized with imaging probes such as fluorescent dyes, quantum dots, or magnetic nanoparticles to enable multimodal imaging for diagnostic purposes. Their high aspect ratio and surface area also make them suitable for biosensing applications.[25]
- Therapeutic Applications:
CNTs possess inherent properties that can be exploited for therapeutic purposes, such as photothermal therapy (PTT) and photodynamic therapy (PDT). In PTT, CNTs absorb near-infrared light and convert it into heat, selectively destroying cancer cells. In PDT, CNTs generate reactive oxygen species upon light irradiation to induce cell death.[26]
- Tissue Engineering:
CNT-based scaffolds can mimic the structural and mechanical properties of natural tissues and provide a suitable environment for cell adhesion, proliferation, and differentiation. Functionalized CNTs can also promote tissue regeneration and modulate cellular responses.[27]
- Biosensing and Detection:
Functionalized CNTs have been extensively used for the detection of various biomolecules, pathogens, and analytes. Their high sensitivity, selectivity, and rapid response make them valuable tools for applications such as disease diagnosis, environmental monitoring, and food safety.[28]
- Diseases
Blood cancer
- Leukemia is a cancer that begins in the bone marrow (the soft inner part of some bones), but in most cases, moves into the blood. It can then spread to other parts of the body, such as organs and tissues. Acute lymphoblastic leukemia (ALL), one of the four main types of leukemia, is a slow-growing blood cancer that starts in bone marrow cells called lymphocytes or white blood cells. Once these white blood cells are affected by leukemia, they do not go through their normal process of maturing. An intensified targeted delivery of daunorubicin (Dau) to acute lymphoblastic leukemia was achieved by Taghdisi they developed a tertiary complex of Sgc8c aptamer (this aptamer targets leukemia biomarker protein tyrosine kinasedaunorubicin, and SWCNT named as Dau-aptamer SWCNTs. Flow cytometric analysis viewed that the tertiary complex was internalized effectively into human T cell leukemia cell (MOLT-4 cells) but not to U266 myeloma cells. Release of Dau-loaded nanotubes was pH-dependent. In a slightly acidic solution of pH 5.5, Dau was released from complex in 72 h at 37 ?C, whilst Dau-aptamer-SWNTs tertiary complex was pretty stable after the same incubation at pH 7.4. [29]
- Breast Cancer
Over expression of human epidermal growth factor receptor 2 (HER2), also known as c-erbB-2 or HER2/neu, is approximately 20-25% responsible for invasive breast cancer. studied SWNT delivery of paclitaxel (PTX) into xenograft tumors in mice with higher tumor suppression efficacy than the clinical drug formulation Taxol. The PTX conjugated to PEGylated SWNTs showed high water solubility and maintains alike toxicity to cancer cells as Taxol in vitro. SWNT-PTX affords much longer blood circulation time of PTX than that of Taxol and PEG ylated PTX, leading to high tumor uptake of the drug through EPR effect. The strong therapeutic efficacy of SWNT-PTX is shown by its ability to slow down tumor growth even at a lower drug dose. Investigated the efficiency of MWCNTs to deliver the gene to the tumor cell for cancer therapy.[30]
- Liver Cancer
Polyamidoamine dendrimer modified CNTs (dMWCNTs) were fabricated for the efficient delivery of antisense c-myc oligonucleotide (asODN) into liver cancer cell line HepG2 cells. As ODN-dMWCNTs composites were incubated with HepG2 cells and confirmed to enter into tumor cells within 15 min by laser confocal microscopy. These composites inhibited the cell growth in time and dose dependent means and down regulated the expression of the c-myc gene and C-Myc protein. These composites exhibit maximal transfection efficiencies and inhibition effects on tumor cells when compared to CNT-NH -asODN and dendrimer (asODN) alone.[31]
- Brain Cancer
Synthesized phospholipids-bearing polyethylene glycol (PL-PEG) functionalized SWCNTs conjugated with protein A, which was further coupled with the fluorescein-labeled integrin monoclonal antibody to form SWCNT-integrin monoclonal antibody (SWCNT-PEGmAb). Confocal microscopy revealed that SWNT-PEG-mAb showed a much higher fluorescence signal on integrin positive U87MG cells and presented a high targeting efficiency with low cellular toxicity, whilst, for integrin -negative MCF-7 cells, no obvious fluorescence was observed, which clearly reveals low targeting efficiency of the functionalized SWCNTs, demonstrating that the specific targeting of integrin positive U87MG cells was caused by the specific recognition of integrin on the cellular membrane by the monoclonal antibody. [32]
- Cervical Cancer
Developed a novel approach of utilizing natural biocompatible polymer chitosan for imaging the tumor cells. In this assay, SWCNTS were modified by chitosan (chit) fluorescein is othyocyanate (FITC). This was further conjugated with folic acid, as mostly cancer cells over express folic acid receptors, to construct the functional fitc-chit-swcnt-fa conjugate. These novels functionalized swcnts were found to be soluble and stable in phosphate buffer saline and can be readily transported inside the human cervical carcinoma hela cells. Combining the intrinsic properties of CNTS, versatility of chitosan, and folic acid, fitc-chit-swcnt-fa can be used as potential devices for targeting the drug into the tumor cells and also for imaging.[33]
- Gene Therapy
CNTs can deliver a large amount of therapeutic agents, including DNA and RNA, to the target disease sites, Gene therapy and RNA have presented a great potential for antitumor treatment. The wire shaped structure (with a diameter matching that of DNA/siRNA) and their remarkable flexibility, CNTs can influence the conformational structure and the transient conformational changes of DNA RNA, which can further enhance the therapeutic effects of DNA is RNA. The treatment of a human lung carcinoma model in vivo using siRNA sequences, which led to cytotoxicity and cell death using amino-functionalized multiwalled carbon nanotube (MWNT-NH3+). This is believed to activate biologically in vivo by triggering an apoptotic cascade that leads to extensive necrosis of the human tumor mass followed by a concomitant prolongation of survival of human lung tumor-bearing animals.[34]
- Immune Therapy
Chemotherapy faces the issues of accumulative toxicity and drug resistance, anti-tumor immunotherapy usually has few adverse effects, good patient tolerance, and the potential to improve the prognosis significantly. CNTs have also shown the potential to boost the antigenicity of the carried proteins or peptides. Studied that MWNTs conjugated to tumor lysate protein will enhance the efficacy of an anti-tumor immunotherapy that employs tumor cell vaccine (TCV) in a mouse model bearing the H22 liver cancer. The study showed that MWNTs conjugated to tumor lysate protein enhanced the specific anti-tumor immune response and the cancer cure rate of a TCV immunotherapy in mice.
- Biosensors
Biosensors are used for mentioning biological processes or for recognition of biomolecules and differ from other sensors by having a sensing element consisting of a biological material such as proteins, oligo- or polynucleotides or microorganisms. The most popular type of biosensors is the electrochemical biosensor and carbon materials have been used in these devices for a long time. Electrochemical biosensors are popular for detecting biomolecules in solutions because of their simplicity and the relative ease of calibration. These sensors are normally based on enzymatic catalysis of a reaction that either produces or consumes electrons and CNT-based biosensors incorporating enzymes have been produced for detection of glucose and other biomolecules.[35]
CONCLUSIONS
Carbon nanotubes as drug delivery systems are designed to improve the pharmacological and therapeutic properties of conventional drugs. The incorporation of drug molecules into nanocarrier can protect a drug against degradation as well as offers possibilities of targeting and controlled release. In comparison with the traditional form of drugs, nanocarrier-drug conjugates are more effective and selective; they can reduce the toxicity and other adverse side effects in normal tissues by accumulating drugs in target sites. In consequence, the required doses of drugs are lower.
REFERENCES-
- Belin T Characterization methods of carbon nanotubes: a review Materials Science and Engineering B 119 (2005) 105–118.
- He Hua, Lien Ai Pham-Huy, Pierre Dramou, Deli Xiao, Pengli Zuo, and Chuong Pham- Huy Carbon Nanotubes: Applications in Pharmacy and Medicine. Hindawi Publishing Corporation BioMed Research International Volume 2013, Article ID 578290.
- Yang W, Thordarson P, Gooding JJ, Ringer SP, Braet F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007; 18: 412001
- Pai P, Nair K, Jamade S, Shah R, Ekshinge V, Jadhav N. Pharmaceutical applications of carbon tubes and nanohorns. Current Pharma search Journal 2006; 1:11-15
- Murata, K. Kaneko, F.Kokai, K.Takahashi, M.Yudasaka, S. Iijima,Chem. Phys. Lett. 331 (2000) 14.
- Charlier, J. C., Blase, X., & Roche, S. (2007). "Electronic and transport properties of nanotubes." Reviews of Modern Physics, 79(2), 677.
- Popov, V. N. (2013). "Carbon nanotubes: properties and application." Materials Science and Engineering: R: Reports, 73(3), 61-102.
- Bandaru, P. R. (2007). "Electrical properties and applications of carbon nanotube structures." Journal of Nanoscience and Nanotechnology, 7(4-5), 1239-1267.
- M.S. Dresselhaus et al., "Carbon Nanotubes: Synthesis, Structure, Properties, and Applications," Springer, 2001.
- P. Avouris et al., "Carbon Nanotube Electronics," Proc. IEEE, 2007P. Avouris et al., "Carbon Nanotube Electronics," Proc. IEEE, 2007.
- Jan P, Jana D, Jana C, Hubalek J, Jasek O, Adam V et al. Methods for carbon nanotubes synthesis. J Mater Chem. 2011; 21:15872-15879
- Jie MA, Wang JA, Tsai CJ, Nussino R, Buyong MA. Diameters of single walled carbon nanotubes (SWCNTs) and related nanochemistry and nanobiology. Front Mater Sci China. 2010; 4(1):17-28
- Mubarak NM, Abdullah EC, Jayakumar NS, Sahu JN. An overview on methods for the production of carbon nanotubes, Journal of Industrial and Engineering Chemistry. 2014; 20:1186-1197.
- Sarangdevot K, Sonigara BS. The wondrous world of carbon nanotubes: Structure, synthesis, properties and applications, Journal of Chemical and Pharmaceutical Research. 2015; 7(6):916-933.
- Varshney K. Carbon nanotubes: A review on synthesis, properties and applications, Int J Eng Res Gen Sci. 2014; 2(4):660-677
- Li, Y., Drelich, J., & Qi, Y. (2021). Advances in carbon nanotube functionalization: Progress and challenges. Journal of Materials Chemistry A, 9(35), 20832-20860. [DOI: 10.1039/d1ta05506.
- Kumar, S., Balaji, S., & Rajendran, V. (2022). Recent advances in carbon nanotube-based composites: Fabrication techniques, properties, and applications. Materials Science and Engineering: R: Reports, 149, 100795. [DOI: 10.1016/j.mser.2022.100795
- Sun, X., Zhao, Y., & Li, X. (2022). Recent advances in carbon nanotube-based materials for energy storage and conversion applications. Carbon Trends, 1, 100009. [DOI: 10.1016/j.cartre.2022.100009.
- Kauffman, D. R., Star, A., & Naumov, A. V. (2021). Recent advances in carbon nanotube-based sensors and electronics. Nano Convergence, 8(1), 1-23. [DOI: 10.1186/s40580-021-00276.
- Lai, J., Du, T., Yuan, H., Zhu, X., & Qian, J. (2022). Carbon nanotube-based materials for biomedical applications: A review. Bioactive Materials, 12, 71-82. [DOI: 10.1016/j.bioactmat.2021.12.006.
- Zhang, X., Li, Y., & Zhan, Z. (2022). Recent advances in large-scale production of carbon nanotubes: Synthesis methods, challenges, and prospects. Carbon, 193, 22-38. [DOI: 10.1016/j.carbon.2021.12.073.
- Ma, J., & Guo, Y. (2022). Health and safety implications of carbon nanotubes: A review of recent advances. NanoImpact, 25, 100358. [DOI: 10.1016/j.impact.2022.100358.
- Beg S, Rizwan M, Sheikh AM, Hasnain MS, Anwer K, Kohli K. Advancement in carbon nanotubes: basics, biomedical applications and toxicity, J Pharm Pharmacol. 2011; 63:141-163
- Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F., & Farokhzad, O. C. (2012). Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chemical Society Reviews, 41(7), 2971-3010. [DOI: 10.1039/c2cs15294.
- Bhirde, A. A., Patel, S., Sousa, A. A., Patel, V., Molinolo, A. A., Ji, Y., ... & Leapman, R. D. (2009). Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine: Nanotechnology, Biology and Medicine, 5(2), 153-163. [DOI: 10.1016/j.nano.2008.11.001.
- Alidori, S., Akhavein, N., Thorek, D. L., Behling, K., Romin, Y., Queen, D., ... & Grimm, J. (2016). Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Science Translational Medicine, 8(331), 331ra39-331ra39. [DOI: 10.1126/scitranslmed. aad3236.
- Patel, A., Sant, S., & Lalwani, G. (2019). Recent advances in carbon based nanosystems for cancer theranostics. Biomaterials Science, 7(3), 1000-1020. [DOI: 10.1039/c8bm01404.
- Kauffman, D. R., Star, A., & Naumov, A. V. (2021). Recent advances in carbon nanotube-based sensors and electronics. Nano Convergence, 8(1), 1-23. [DOI: 10.1186/s40580-021-00276.
- Taghdisi SM, Lavaee P, Ramezani M, Abnous K. Reversible targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes, Eur J Pharm Biopharm. 2011; 77(2):200-206.
- Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications, Nat protocols 2009; 4:1372-1382.
- Pan B, Cui D, Xu P, Ozkan C, Feng G, Ozkan M et al. Synthesis and characterization of polyamidoamine dendrimer-coated multi-walled carbon nanotubes and their application in gene delivery systems Nanotechnology 2009; 20(12):125101
- Xing D, Ou Z, Wu B, Zhou F, Wang H, Tang Y. Functional single-walled carbon nanotubes based on an integrin ? ? monoclonal antibody for higher efficient cancer cell targeting Nanotech 2009; 20(3):23-31.
- Li R, Wu R, Zhao L, Hu Z, Guo S, Pan X et al. Folate and iron difunctionalized multiwall carbon nanotubes as dual-targeted drug nanocarrier to cancer cells Carbon 2011; 49(5):1797-180 .
- Podesta JE, Al-Jamal KT, Herrero MA, Tian B, Ali-Boucetta H, Hegde V et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model Small 2009; 5(10):1176-1185.
- Cifuentes-Rius A, de Pablo A, Ramos-Pérez V, Borrós S. Tailoring carbon nanotubes surface for gene delivery applications, Plasma Processes and Polymers 2014; 11(7):704-713


 Sandhya Jaiswal*
Sandhya Jaiswal*
 Samiksha sahu
Samiksha sahu






 10.5281/zenodo.11519993
10.5281/zenodo.11519993