The abstract of the document provides an overview of sustained release drug delivery systems. It emphasizes the goal of delivering therapeutic levels of drugs to specific body sites over extended periods. The document discusses the increasing interest in sustained release systems due to high costs of developing new drugs, patent expirations, discovery of new polymers, and improvements in therapeutic efficiency and safety. Various types of matrix tablets, such as hydrophilic, fat-wax, plastic, biodegradable, and mineral matrices, are classified based on their properties and methods of drug release. Advantages, disadvantages, and factors affecting the release from matrix tablets, including physicochemical and biological factors, are also examined. Evaluation tests and criteria for formulating sustained release dosage forms are outlined. The conclusion highlights the benefits of sustained release formulations in enhancing drug efficiency and patient compliance.
.
Matrix tablets, Sustain release polymer, Patient convenience and compliance.
a) Hydrophilic Matrix Tablet:
Drug release rate is typically regulated by using a hydrophilic matrix. The combination of the active ingredient and certain hydrophilic carriers can be compressed directly to create the matrix, or it can be made from a wet granulation that contains the medication and hydrophilic matrix components. In order to initiate the release mechanism and explore several benefits, such as ease of manufacture and superior uniformity of matrix tablets, water is necessary for the hydrophilic matrix. The best option for creating a hydrophilic matrix tablet is to use matrix construction materials with quick polymer hydration capabilities. Because water penetrates polymers quickly, an insufficient polymer hydration rate can lead to early drug diffusion and tablet disintegration. It can be used to create drugs that are soluble in water.
The polymers used in the preparation of hydrophilic matrices are divided into three broad groups as follow:
1) Cellulose derivatives: Methylcellulose 400 and 4000 cps, sodium carboxy methyl cellulose, hydroxyethyl cellulose, and hydroxy propymethyl cellulose (HPMC) 25, 100, 4000, and 15000 cps.
2) Non-cellulose natural or Semi-synthetic polymers
Agar-agar, gum Arabic, alginates, mannose and galactose polysaccharides, chitosan, and modified starches.
3) Acrylic acid polymer:
Carbopol 934 Alginic acid, gelatin, and natural gums are other hydrophilic components that are utilized in the manufacture of matrix tablets.
b) Fat-wax Matrix Tablet:
There are several methods for incorporating drugs into fat wax granulations, including spray drying, mix congealing in an aqueous medium with or without the help of a surfactant, and spray congealing in the air. Using the bulk congealing process, a medication suspension in melted fat wax is let to harden before being ground into granulations for prolonged release. After the active chemicals, waxy materials, and fillers are combined, the combination is compacted using a compactor, heated in a suitable mixture (such as a fluidized-bed and steam-jacketed blender), or granulated using a waxy material solution to create granules. The medication, which is enmeshed in a melt of lipids and wax, is liberated through leaching, hydrolysis, and fat dissolution caused by GI tract pH changes and enzyme action. The rate of drug release and the total amount of drug that can be incorporated into a matrix can both be affected by the addition of different surfactants to the formulation.
c) Plastic Matrix Tablet (Hydrophobic matrices): There has been widespread use of sustained release tablets made of an inert compressed plastic matrix. Because the dissolved medication must diffuse through the capillary network between the compressed polymer particles, release is typically delayed. As long as the plastic material can be ground or granulated to the appropriate particle size to aid in mixing with the drug particle, it is simple to prepare plastic matrix tablets, which contain the active ingredient embedded in a tablet with a coherent and porous skeletal structure. In order to granulate for compression into tablets, the embedding process may be accomplished by,
1) The solid medication is combined with powdered plastic, kneaded in an organic solvent-based solution of the same plastic material or another binding agent, and then granulated.
2) An organic solvent that dissolves the medication in the plastic and turns into granules as the solvent evaporates.
3) Grind the medicine and plastic masses using latex or pseudo latex as the granulating fluid.
Examples include polystyrene, polyvinyl chloride, ethyl cellulose, and cellulose acetate.
d) Biodegradable Matrices: These consist of the polymers which comprised of monomers linked to each other by functional groups and have unstable linkage in the backbone. It is biologically degraded or eroded by enzymes generated by surrounding living cells or by non-enzymatic process into oligomers and monomers that can be metabolized or excreted. Examples are natural polymers such as proteins, polysaccharides and modified natural polymers, synthetic polymers such as aliphatic poly (esters) and poly anhydrides.
e) Mineral Matrices: Mineral matrices consist of polymers which are obtained from various species of seaweeds. Example: Alginic acid which is a hydrophilic carbohydrate obtained from species of brown seaweeds (Phaeophycean) by the use of dilute alkali.
On the basis of porosity of matrix:
Matrix system is also classified according to their porosity.
- Macro-porous Systems:
In such systems the diffusion of drug occurs through pores of matrix which are of size range 0.1 to 1 ?m. This pore size is larger than diffusion molecule size.
- Micro-porous System:
Diffusion in this type of system occurs essentially through pores. For micro porous systems, pore size ranges between 50 – 200 A°, which is slightly larger than diffusion Molecules size.
- Non-porous System:
Non-porous systems have no pores and the molecules diffuse through the network meshes. In this case, only the polymeric phase exists and no pore phase is present.
- Hybrid system:
System in which the drug in matrix of release retarding material is further coated with increase controlling polymer membrane. [10,11,13]
Polymers used in matrix tablet:
- Hydrogels: Poly hydroxy ethylemethylacrylate(PHEMA),Cross-linked polyvinyl alcohol (PVA), Cross-linked polyvinyl pyrrolidone(PVP),Polyethylene-oxide(PEO), Polyacrylamide (PA)
- Soluble polymers: Polyethylene glycol (PEG), Polyvinyl alcohol(PVA), Polyvinylpyrrolidone (PVP), Hydroxypropyl methylcellulose (HPMC)
- Biodegradable polymers: Polylactic acid (PLA), Polyglycolic acid (PGA), Polycaprolactone (PCL), Polyanhydrides, Polyorthoesters
- Non-biodegradable polymers: Polyethylene vinyl acetate (PVA), Polydimethylsiloxane(PDS), Polyether urethane (PEU), Polyvinyl chloride (PVC), Cellulose acetate (CA), Ethyl cellulose (EC)
- Mucoadhesive polymers: Polycarbophil, Sodium carboxy methyl cellulose, Polyacrylic acid, Tragacanth, Methyl cellulose, Xanthan gum, Guar gum, Karaya gum, Locust bean gum.
Advantages of Sustained Release Matrix tablet:
- Patient compliance:
Lack of compliance is mainly observed with chronic disease which required long term treatment, as success of drug therapy depends on the patient ability to comply with the drug treatment. Patient compliance is affected by a various factors, like knowledge of disease process, patient faith in treatment, and understanding of patient related to a strict treatment schedule. Also the complication of therapeutic regimens, the cost of therapy and local or systemic side effect of the dosage form. This problem can be resolved to some extent by administering sustained release drug delivery system.
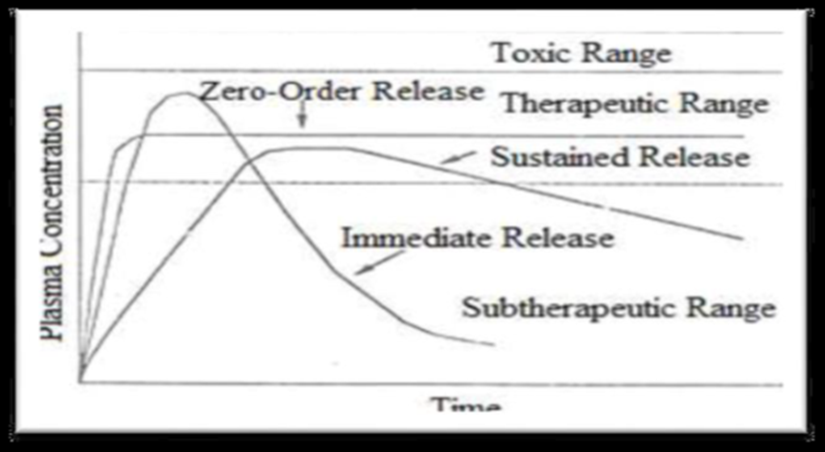
- Reduced 'see-saw' fluctuation:
When a medicine is administered in a standard dose form, the concentration of the drug in the tissue compartments and systemic circulation frequently exhibits a "seesaw" pattern. The drug kinetics, which include the rate of absorption, distribution, elimination, and dosage intervals, primarily determine the magnitudes of these changes. Since recommended dose intervals are rarely shorter than four hours, the "see-saw" pattern is more noticeable only in the case of medications with a biological half-life of less than four hours. The frequency of drug dosing can be significantly decreased with a well-designed sustained release drug delivery system, which can also maintain a constant drug concentration in target tissue cells and blood circulation.
- Total dose reduction:
To treat a diseased condition less amount of total drug is used in Sustained release drug delivery systems. By reducing the total amount of drug, decrease in systemic or local side effects are observed. This would also lead to greater economy.
- Improvement of deficiency in treatment:
Optimal therapy of a disease requires an effective transfer of active drugs to the tissues, organs that need treatment. Very often doses far in excess to those required in the cells have to be administered in order to achieve the necessary therapeutically effective concentration. This unfortunately may lead to undesirable, toxicological and immunological effects in non-target tissue. A sustained release dosage form leads to better management of the acute or chronic disease condition.
- Economy:
The initial unit cost of sustained release products is usually greater than that of conventional dosage form because of the special nature of these compounds but importantly average cost of treatment over an prolong period of time may be less.
Disadvantages of Sustained Release Matrix Tablet
1. Dose dumping: When a formulation is flawed, dose dumping might happen.
2. Less room for dosage modification.
3. The price is higher than for a typical dosage form.
4. Boost the first pass metabolism's potential.
5. Patient education is required for appropriate drug administration.
6. A potential decrease in system availability.
7. Weak connections between in vitro and in vivo
Factor affecting Release from Matrix Tablet:
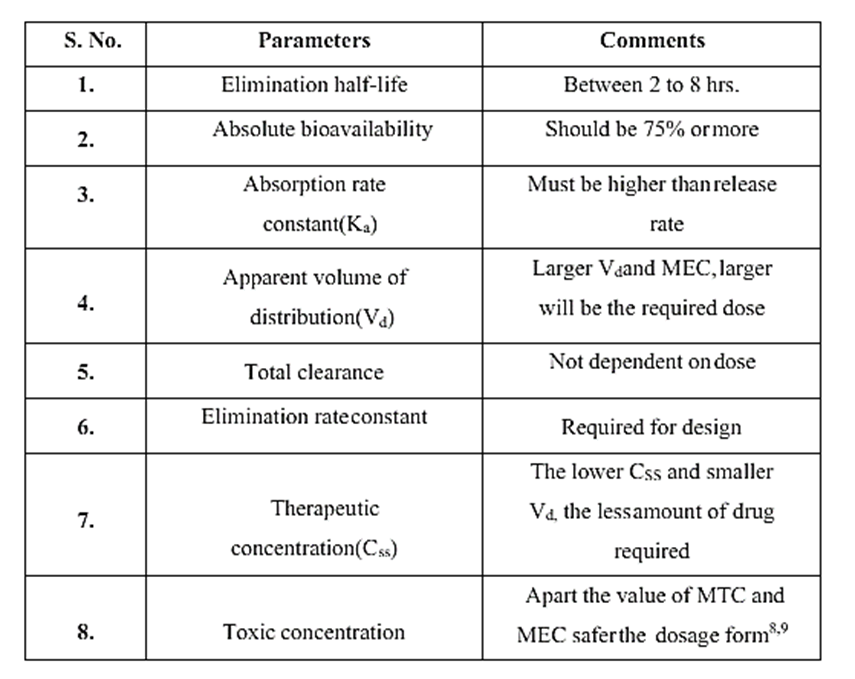
- Physicochemical Factors: Dose size: Generally speaking, a single administration of a standard dosage form should not exceed 500 mg to 1.0 g of medication. Compounds with large dosage sizes are either manufactured into liquid systems or administered in numerous doses. The margin of safety, which entails administering a high dose of a medication with a limited therapeutic range, is another factor to take into account.
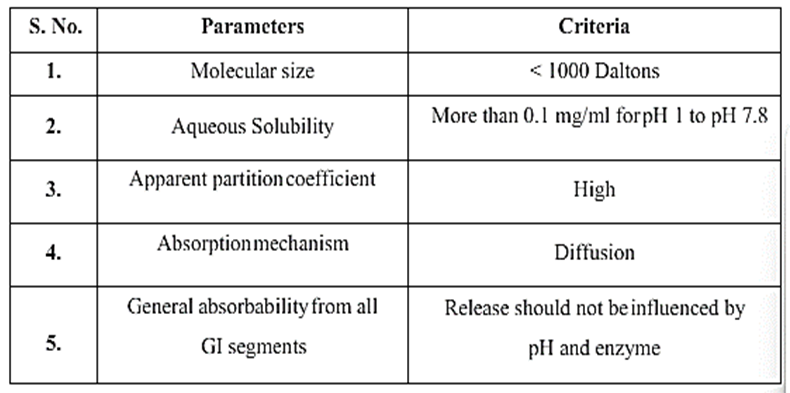
- Ionization, pKa and aqueous solubility: The majority of medications are weak bases or acids. While pharmaceuticals in their original form spread throughout the body, it is advantageous to provide drugs in their original form for drug permeation. Unfortunately, conversion to unaltered form, which is more complicated, will reduce the aqueous solubility. The drug's solubility in aqueous fluids will affect delivery systems that rely on diffusion or dissolution in the same way.
- Stability: The medication loss resulting from acid hydrolysis or metabolism in the gastrointestinal tract is a significant consideration for oral dosing formulations. Highly unstable pharmaceuticals may benefit from controlled drug delivery systems because the medication may be shielded from enzymatic breakdown by being included into a polymeric matrix.
Biological factors affecting release from Matrix Tablet:
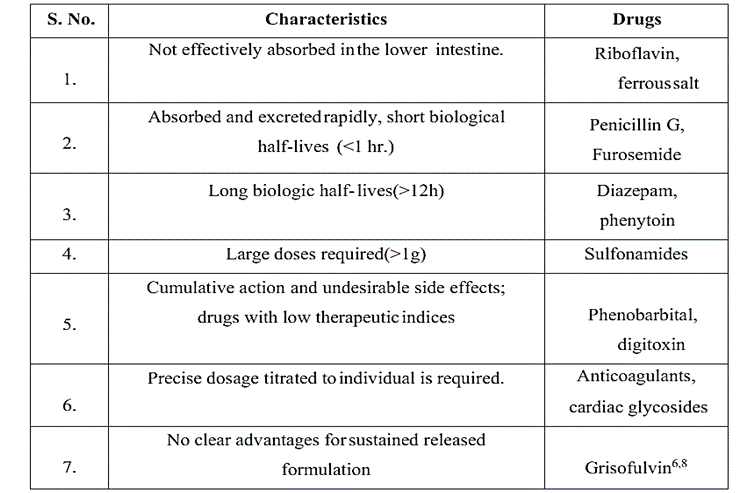
- Biological half-life: Short half-lives make drugs the ideal candidates for formulations with sustained release. Levodopa and other medications with a half-life of less than two hours make bad candidates for sustained release formulation. Digoxin and phenytoin are two examples of medications with longer half-lives than eight hours, which make them poor candidates for sustained release formulations as their effects are already long-lasting.
- Absorption: The goal of creating a sustained release product is to regulate the drug's release rate, which is substantially slower than its absorption rate. The extreme half-life for absorption should be in the range of 3–4 hours if we consider that most medications transit through the GI tract's absorptive sections in 8–12 hours. This means that the dosage form will exit the likely absorptive regions before the drug release is finished.
- Metabolism: Reduced bioavailability of the slowly released dose form as demonstrated by Drugs with slow-releasing dose forms that undergo substantial metabolism in the intestine's tissue or lumen before absorption may have lower bioavailability. A medication with low water solubility may be designed in a dosage form with sustained release. There are a number of methods that can be used to improve the drug's solubility after it has been enhanced, including Sustain Release formulation. However, as the medication is starting to enter the bloodstream, it is possible for it to crystallize. This should be avoided, and precautions should be taken to avoid it.
- Distribution: The apparent volume of distribution is the primary determinant of the medication elimination rate. Drugs with a high apparent volume of distribution, such as chloroquine, are therefore thought to be poor candidates for oral sustained release drug delivery systems because they affect the drug's rate of elimination.
- Protein Binding: All drugs are somewhat bound to plasma and/or tissue proteins, thus unbound drug concentration is more critical to achieve pharmacological reaction than bound drug concentration. Despite the kind of dosage form, protein binding of the drug plays a major impact in its therapeutic action because it increases the biological half-life by widespread binding to plasma. As a result, occasionally an SR drug delivery system is not needed for this kind of medicine.
- Margin of safety: A medicine's safety is typically determined by its therapeutic index; the higher the drug's therapeutic index value, the safer the drug. Generally speaking, oral SR drug delivery systems are not the best option for medications with lower therapeutic indic.
- Molecular size and diffusivity: In many sustained-release systems, drugs have to diffuse across a matrix or membrane that controls the rate of release. Diffusion coefficient, which refers to a drug's capacity to permeate across membranes, is a function of the drug's molecular weight or size, which has a significant impact on the diffusivity value. The diffusing species' molecular weight is represented by the letter "D" in polymers.
Method of Preparation of Matrix Tablet
- Wet Granulation Technique
- Milling and gravitational mixing of drug, polymer and excipients.
- Preparation of binder solution
- Wet massing by addition of binder solution or granulating solvent
- Screening of wet mass.
- Drying of the wet granules.
- Screening of dry granules
- Blending with lubricant and disintegrants to produce "running powder"
- Compression of tablet.
- Dry Granulation Technique
- Milling and gravitational mixing of drug, polymer and excipients
- Compression into slugs or roll compaction
- Milling and screening of slugs and compacted powder
- Mixing with lubricant and disintegrants
- Compression of tablet
- Sintering Technique
- Sintering is defined as the bonding of adjacent particle surfaces in a mass of powder, or in a compact, by the application of heat.
- Conventional sintering involves the heating of a compact at a temperature below the melting point of the solid constituents in a controlled environment under atmospheric pressure.
- The changes in the hardness and disintegration time of tablets stored at elevated temperatures were described as a result of sintering
- The sintering process has been used for the fabrication of sustained release matrix tablets for the stabilization and retardation of the drug release.
Evaluation test for Sustained Release Tablets:
- Weight Variation:
Twenty tablets were weighed individually and then collectively, average weight of the tablets was calculated.
- Hardness:
Hardness test was conducted for tablets from each batch using Monsanto hardness tester and average values were calculated.
- Friability:
The tablets were tested for friability testing using Roche friabilator, which revolves at 25rpm for 4min. Thickness: The thicknesses of tablets were determined using micrometre screw gauge.
- Content Uniformity:
Using UV Visible spectrophotometer found the amount of the drug using the calibration curve method.
In Vitro Dissolution Study:
From the previous explanation, it is evident that sustained-release formulations enhance both the patient's compliance and the effectiveness of the dosage. Drugs can be released from matrix-producing polymers in a controlled way by making matrix tablets. It is simple to adjust release kinetics to meet delivery requirements thanks to preparatory procedures. The significance of these specific excipients in pharmaceutical applications is confirmed by the adaptability of matrix forming polymers to different drug delivery system configurations. When it comes to a range of oral delivery issues, like varying medication plasma levels, limited bioavailability, more frequent dose administration, etc., they are the ideal option. Thus, matrix tablets can alleviate the above-mentioned issues with traditional oral drug distribution.
Criteria to be met by drug proposed to be formulated in Sustained Release dosage forms
Drawing from the preceding discourse, it is evident that sustained-release formulations serve to enhance both the patient's compatibility and the dosage's efficiency. Drugs can be released in a controlled way from matrix producing polymers by making matrix tablets. Release kinetics can be readily adjusted to meet delivery requirements thanks to preparatory methods. Matrix forming polymers' adaptability to different drug delivery system setups validates the significance of these specialized excipients in pharmaceutical applications. For various oral delivery issues, such as varying medication plasma levels, limited bioavailability, more frequent dose administration, etc., they are the ideal option. Therefore, matrix tablets can solve the issues with traditional oral drug delivery mentioned above.


 Jayashree Shivaji Bhamre*
Jayashree Shivaji Bhamre*
 Shraddha Bhavsar
Shraddha Bhavsar
 10.5281/zenodo.13212215
10.5281/zenodo.13212215