Abstract
Microencapsulation is a process by which very tiny droplets or particles of liquid or solid material are surrounded or coated with a continuous film of polymeric material. In order to improve bioa-vailability, stability, control release, and target delivery of active pharmaceutical ingredients (APIs), as well as to mask their bitter taste, to increase their efficacy, and to minimize their side ef-fects, a variety of microencapsulation technologies have been widely used in the pharmaceutical industry. Commonly used microencapsulation technologies are emulsion, coacervation, centrifugal extrusion, spray drying, sol-gel encapsulation, solvent evaporation, pan coating, supercritical fluid encapsulation and polymerization. The main objective of this article is taking a look at microencap-sulation as a novel drug delivery system. This approach has been used in amazing fields like phar-maceutical, agriculture, textile, food, printing and defence.
Keywords
Microencapsulation, coating material, core material, solvent evaporation.
Introduction
Microencapsulation is a process by which very tiny droplets or particles of liquid or solid material are surrounded or coated with a continuous film of polymeric material.1 The product obtained by this process is called as microparticles, microcapsule. Particles having diameter between 1 to 1000µm are known as microparticles or microcapsules or microspheres. Particles larger than 1000µm are known as macro-particles.11 Generally micro-particles consists of two components are:
a) Core material
b) Coating or wall or Shell material.
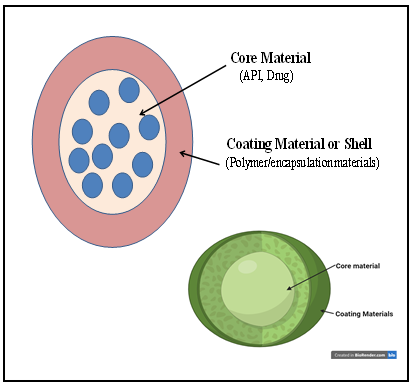
Fig1. Coating and core materials of microencapsulation
Controlled Drug delivery systems involve a multidisciplinary scientific approach and contribute to health care. They have many advantages compared to conventional dosage forms, including better efficacy, less toxicity, and better patient compliance. These systems often use polymers as carrier systems for drugs. Among all the dosage forms prepared with polymers, micro and nanoparticles are of high importance due to their ability to accumulate in the target areas of the body. It is a process by which solid, liquid, or gaseous materials are coated with a polymer, leading to small capsules. The properties of microparticles depend on the selection of polymers which may be hydrophilic, hydrophobic, or a combination of both. Many polymers like polyvinyl pyrrolidone, methylcellulose, cellulose nitrate, silicones, lipids, waxes like paraffin, carnauba, stearic acid, stearyl alcohol have been used as coating materials. 19
Microparticles:
“Microparticles” refers to the particles having the diameter range of 1 to 1000µm irrespective of the precise exterior and/or interior structures.
Microspheres:
“Microspheres” particularly refers to the spherically shaped microparticles within the broad category of microparticles and it consists of micrometric reservoir systems. 20
Microcapsules:
Microcapsules refers to microparticles having a core surrounded by the coat or wall materials distinctly different from that of the core or pay-lood or nucleus, which may be solid, liquid, or even gas and it consists of micrometric Matrix systems.
Microcapsules can be classified on three types (Fig.)
i). Mononuclear: Containing the shell around the core
ii). Polynuclear: Having many cores enclosed with in shell.
iii). Matrix type: Distributed homogeneously into the shell material.

Fig 2. Classification of microcapsules
Fundamental Consideration:

Advantages, Disadvantages, And Applications
ADVANTAGES
i) Taste masking masks the bitter taste of the drugs
ii) Environmental protection protects the drug molecules against oxygen, light, and moisture
iii) To produce targeted drug delivery.
iv) Conversion of liquids to pseudo solids or free-flowing powders.
v) Sustains or prolongs the drug release and also enhances the solubility of poorly soluble drugs.
vi) To increase of bioavailability.
vii) To improve patients compliance.
DISADVANTAGES
i) Expensive techniques.
ii) This causes reduction in shelf life of hygroscopic agents.
iii) Requires skilled labor
iv) Reproducibility of the microparticles is less compared to conventional dosage forms
v) The change in process parameters like temperature, pH, and solvents may affect the drug stability
Applications
i) Protecting the drug molecules from moisture, oxygen, interactions with other additives in the formulation, and other atmospheric effects
ii) To mask the unpleasant taste of some drugs
iii) To reduce GIT irritation
iv) Liquids can be converted to pseudo-solids for easy storage and handling
v) Microencapsulation is used to protect flavors, colors, nutrients and to control the release of active ingredients.
Techniques Of Microencapsulations
Microencapsulation techniques are of two types, namely chemical and physical. The physical methods are subdivided into physicochemical and physicomechanical methods.22 The methods of microencapsulation are given in figure.
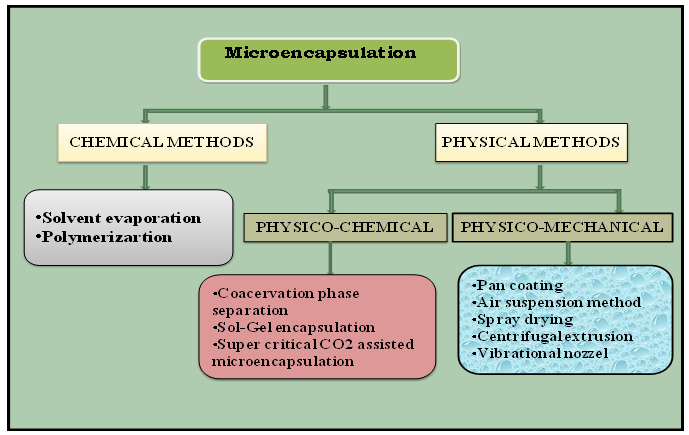
Fig 4. Different techniques of microencapsulation
A. Chemical Methods
1. Solvent evaporation
Solvent evaporation is based on the evaporation of the emulsions internal phase by agitation. The coating material (polymer) is dissolved in a volatile organic solvent, and the drug is dissolved or dispersed in the polymer solution to obtain an emulsion, suspension, or solution. Then the organic phase is emulsified in a dispersed phase under agitation.24 The dispersed phase used in this step is a non-solvent of the polymer, immiscible with the organic solvent, and contains an emulsifying agent. After the emulsion has stabilized, agitation is continued, and the solvent diffuses through the continuous phase and evaporates, leaving behind the solid microspheres. These microspheres are recovered by filtration and washed, dried.25 The process of solvent evaporation is given in figure 4.
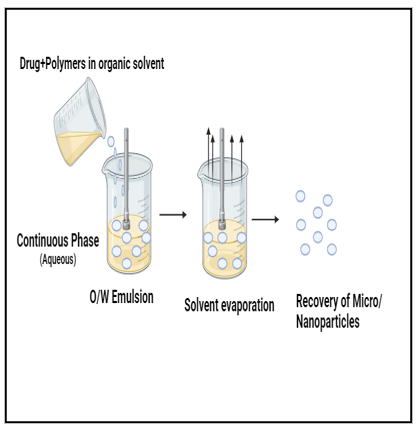
Fig 4. Solvent evaporation method
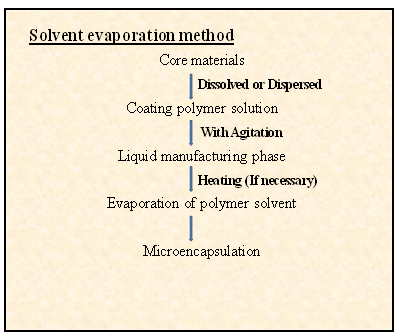
Fig 5. General steps in microcapsulation solvent method
2. Polymerization
This is new techniques utilizes polymerization to form protective microcapsules. The core material supporting phase is usually a liquid or gas, and therefore polymerization reaction occur at liquid-liquid, liquid-gas, solid-liquid, or solid-gas interface. The microencapsulation by polymerization is done in two ways: In situ and interfacial polymerization.2,3

Fig 6. General steps in microencapsulation polymerization method
a) In-situ polymerization
This method involves the direct polymerization of a single monomer on the particle or liquid droplet surface. In the in situ polymerization, the liquid to be encapsulated is dispersed in a stirred tank with water. The speed of the stirrer can control the size of the liquid droplets. Then monomers are added, which on chemical reaction (polymerization), forms polymers. The polymerization reaction is induced by pH change (adding acid or base) or temperature change and can be accelerated by a catalyst. The formed polymer gets deposited on the surface of the droplets and encapsulates the droplets. These encapsulated particles are then collected by filtration, washed, and stored.

Fig 7. In-situ polymerization
a) Interfacial Polymerization
Interfacial polymerization is a techniques in which polymerization of two monomers, one oil soluble and other water soluble, takes place and a polymer is formed at the interface of two immiscible substances. This techniques is mainly used for the encapsulation of liquids rather than solids because penetration of monomers to polymerization zone is much easy from the liquid state rather than the solids. In interfacial polymerization occurs at the interface of two immiscible solutions. Monomers are mixed along with the core materials in organic solvents. This is added to the continuous phase, which is immiscible with the organic phase used. This results in the formation of an emulsion. Polymerization can occur due to pH change, temperature change, or catalysts. The monomers at the oil and water interface react and form a polymer membrane entrapping the drug inside it. These particles are then separated from the continuous phase to remove excess stabilizers, catalysts, and surfactants.

Fig 8. Interfacial Polymerization method
Physico-Chemical Methods
1) Coacervation phase separation:
When appositely charged hydrophilic colloids are mixed and formed a separate colloids layer which is called as coacervate. This phenomenon in which separation takes place on mixing two dispersion is called coacervation.4
Eg. Gelatin is positively charged and acacia having negatively charged particle.
Coacervation phase separation includes two methods are:
i) Simple coacervation:- It involves the use of single polymer such as gelatin or ethyl cellulose.
ii) Complex coacervation:- It involve the two appositely charged polymer in aqueous solution. Eg. Gelatin and acacia gum.
There are three steps involves involved in the coacervation phase separation they are:
- Formation of three immiscible phases
- Coating deposition
- Coating rigidization
The first step involves forming three immiscible phases: liquid vehicle phase, coat material phase and core material phase. The core material dispersed in coat solution (Polymer solution).5 The polymer in coat solution is formed by changing pH and temperature catalyst and polymer-polymer interactions. This polymer deposited on the core materials in the second step. The polymer will be absorbed on the coat materials at the interface of liquid vehicle and core materials. The polymer coat is made rigid by cross-linking, thermal, desolvation methos in the third step.6 The coacervation phase separation is given in figure

Fig 9. Coacervation phase separation
2) Supercritical CO? assisted microencapsulation
Compressed CO? in a liquid or supercritical state is non-toxic, non-flammable, and is used as a solvent in this process. By lowering the pressure, CO? is easily separated from the polymers.7 For any substance to achieve a supercritical state, the temperature and pressure should be above that substances critical temperature and pressure. For CO?, the critical temperature and pressure are 31°C and 74 bar, respectively. There are three steps in this method.8
i) In the first step, the polymers are exposed to supercritical CO2 for some time.
ii) A solution of CO2 and additives (drugs and excipients) is added in the second step. Then the additives are transferred into the polymer from CO2.
iii) In the final step, CO2 is released, and the additive is trapped inside the polymer particles. The transfer of additives into the polymer particles is enhanced by exposure to supercritical CO2.
3) Sol-Gel encapsulation
.Lipophilic substances can be trapped inside spherical amorphous silicon dioxide using this method. At first, the drug is solubilized in silicon phases like tetramethoxy and tetraethoxysilanes (TMOS and TEOS). Then this solution is made into an oil-in-water (O/W) emulsion.9 At the oil and water interface, the hydrolysis of silicon takes place. This hydrolyzed silicon, after condensation, forms silica and leads to the formation of a hard silica shell around the drug substances.10
Physico-Mechanical Methods
1) Pan coating
Pan coating is a physical method of preparing microencapsulation and It can only be used for particles greater than 600 microns in diameter. Pan coating is the oldest method of obtaining small, coated particles or tablets and is very widely used in the pharmaceutical industry. In pan coating, while the coating is being applied, the particles are tumbled in a pan. A pipe stretches into the pan for even heat distribution while the pan is rotating. Figure shows the process of polymer coating on the particle.12, 13

Fig 10. Pan coating method
2) Air suspension method
The coating materials are sprayed over the fine core materials suspended in a vertical air current in this microencapsulation method. The solution of the coating materials, after evaporation, forms an encapsulating layer on the solid core materials.14 The process is repeated to achieve the required film thickness. The particle size of the core materials used in this process is usually large. This methods has improved flexibility and control over the pan coating method.15

Fig 11. Air suspension method
3) Spray drying
In spray drying, the fine atomized liquid droplets contact hot gases and get dried, leading to the formation of dried particles. These dried particles are then collected in a cyclone separator or filter bag. For particle formation and drying, spray drying is the most widely used process and is suitable for the continuous production of powders, agglomerates, and granulates from the liquid.16 Liquids like solutions, pumpable suspensions, and emulsions can be included in the spray dryer feed. Flowability, particle size, bulk density, and mechanical strength of the final powders can be significantly controlled due to the versatility of the spray drying process.17
4) Centrifugal Extrusion
In this process, the liquid is extruded from the nozzles using centrifugal force. The orifices of the nozzles are located on the outer circumference of the rotating cylinder. The liquid core material is pumped through the inner orifice, while the coating materials are pumped through the external orifice. This forms a co-extruded röd of liquid core materials surrounded by coating materials. This extruded rod breaks and forms droplets, which on further drying, includes capsules. The size of the capsule is affected by the speed of the rotating cylinder.
5) Vibrational nozzle
In this method, encapsulation of core materials is done by laminar flow of liquid through a nozzle and vibrations of the nozzle. Uniform droplets are obtained when the vibrations are in resonance with the Rayleigh instability. The solidification of the core can be achieved by gelation (sol-gel) or adding an external binder in the liquids. The droplets' size ranges from 100-5000 µm, which can be controlled by the size of the nozzle orifice and vibrations of the nozzle. The vibrational nozzle equipment used in the industries and research will have capacities ranging from 1-10,000 kg/hr, and the temperature ranges from 20-1500 °C.18
CONCLUSION:
Different properties of the microcapsules and their methods have been discussed in this critical review. Effects of different techniques of microcapsule production, type of the core, and the wall material and the storage conditions were also discussed. The capsule protects the active ingredient from its surrounding environment until an appropriate time. it can be concluded that the properties of the microcapsules differ according to the different techniques used, type of core material, and the type of shell material used and on the basis of these properties only, packaging and storage conditions are decided. So we need in future to use different technology for preparing and encapsulating core material and improve the efficacy of drug molecules.
REFERENCES
- Dubey, R., 2009. Microencapsulation tech-nology and applications. Defence Science Journal, 59(1), p.82.
- H?ncal, A.A. and Ka?, H.S., 2000. Microen-capsulation technology: interfacial polymeri-zation method. Handbook of Pharmaceutical controlled release technology, p.271.
- Khan, M.G., Gauttam, V., Chandel, H.S., Ali, A. and Tariq, K., 2017. Development of microencapsulation: A review of literature. International journal of scientific study, 5(4), pp.264-268.
- Kas, H. S. and Oner, L., 2000. Microencap-sulation using coacervation/ phase separation: An overview of the technique and applications (pp. 301-328). Marcel Dekker, Inc.: New York, NY, USA.
- Bakan, J.A., 2019. Microencapsulation us-ing coacervation /phase separation techniques. In Controlled release technologies (pp. 83-105). CRC Press.
- Samejima, M., Hirata, G. and Koida, Y., 1982. Studies on microcapsules. I. Role and effect of coacervation-inducing agents in the microencapsulation of ascorbic acid by a phase separation method. Chemical and Pharmaceutical Bulletin, 30(8), pp.2894-2899
- Soh, S.H. and Lee, L.Y., 2019. Microencap-sulation and nanoencapsulation using super-critical fluid (SCF) techniques. Pharmaceu-tics, 11(1), p.21.
- Chen, A.Z., Li, Y., Chau, F.T., Lau, T.Y., Hu, J.Y., Zhao, Z. and Mok, D.K.W., 2009. Microencapsulation of puerarin nanoparticles by poly (L-lactide) in a supercritical CO2 pro-cess. Acta Biomaterialia, 5(8), pp.2913-2919.
- Subbaiah, B.V., Krishnamoorthy, B. and Muthukumaran, M., Available through Online Review Article www. ijptonline. Com
- Wang, L.Y., Tsai, P.S. and Yang, Y.M., 2006. Preparation of silica microspheres en-capsulating phase-change material by sol-gel method in O/W emulsion. Journal of microen-capsulation, 23(1), pp.3-14.
- Jyothi, S.S., Seethadevi, A., Prabha, K.S., Muthuprasanna, P. and Pavitra, P., 2012. Mi-croencapsulation: a review. Int. J. Pharm. Bi-ol. Sci, 3(2), pp.509-531.
- Tarun, G. and Murthy, R.S.R., 2011. Pa-tented microencapsulation techniques and its application. Journal of Pharmacy Research, 4(7), pp.2097-2102.
- Jyothi, S.S., Seethadevi, A., Prabha, K.S., Muthuprasanna, P. and Pavitra, P., 2012. Mi-croencapsulation: a review. Int. J. Pharm. Bi-ol. Sci, 3(2), pp.509-531.
- Garg, A., Chhipa, K. and Kumar, L., 2018. Microencapsulation techniques in pharmaceu-tical formulation. European Journal of Phar-maceutical and Medical Research, 5(3), pp.199-206.
- Mishra, D.K., Jain, A.K. and Jain, P.K., 2013. A review on various techniques of mi-croencapsulation. Int J Pharm Chem Sci, 2(2), pp.962-968.
- Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A. and Saurel, R., 2007. Applica-tions of spray-drying in microencapsulation of food ingredients: An overview. Food research international, 40(9), pp.1107-1121.
- Rigon, R.T. and Zapata Noreña, C.P., 2016. Microencapsulation by spray-drying of bioactive compounds extracted from black-berry (Rubus fruticosus). Journal of food sci-ence and technology, 53, pp.1515-1524.
- Whelehan, M. and Marison, I.W., 2011. Microencapsulation using vibrating technolo-gy. Journal of microencapsulation, 28(8), pp.669-688
- Nordstierna, L., Abdalla, A.A., Nordin, M. and Nydén, M., 2010. Comparison of release behaviour from microcapsules and micro-spheres. Progress in Organic Coatings, 69(1), pp.49-51.
- Lengyel, M., Kállai-Szabó, N., Antal, V., Laki, A.J. and Antal, I., 2019. Microparticles, microspheres, and microcapsules for ad-vanced drug delivery. Scientia Pharmaceutica, 87(3), p.20.
- Ozkan, G., Franco, P., De Marco, I., Xiao, J. and Capanoglu, E., 2019. A review of mi-croencapsulation methods for food antioxi-dants: Principles, advantages, drawbacks and applications. Food chemistry, 272, pp.494-506.
- Mishra, D.K., Jain, A.K. and Jain, P.K., 2013. A review on various techniques of mi-croencapsulation. Int J Pharm Chem Sci, 2(2), pp.962-968.
- Dubey, R., 2009. Microencapsulation technology and applications. Defence Science Journal, 59(1), p.82.
- Li, M., Rouaud, O. and Poncelet, D., 2008. Microencapsulation by solvent evaporation: State of the art for process engineering ap-proaches. International Journal of pharmaceu-tics, 363(1-2), pp.26-39.
- Tiwari, S. and Verma, P., 2011. Microen-capsulation technique by solvent evaporation method (Study of effect of process variables). International journal of pharmacy & life sci-ences, 2(8).


 Rakesh Bhute*
Rakesh Bhute*
 Twinkal Revatkar
Twinkal Revatkar
 Ashish Lande
Ashish Lande












 10.5281/zenodo.14811012
10.5281/zenodo.14811012