Abstract
Galantamine hydrobromide, a reversible cholinesterase inhibitor, is widely used for treating Alzheimer's disease. However, its therapeutic usefulness is limited by its poor absorption and solubility.This study aimed to develop and optimize a galantamine hydrobromide preparation to enhance its bioavailability.A ionotropic gelation method was employed to prepare galantamine hydrobromide hydrogels(microspheres),which involves polyelctrolytes and oppositely charged ion solution. Hydroxypropyl methylcellulose (HPMC) is used as an additional component which improves gel strength and stability . The effects of formulation variables, including drug-to-carrier ratio, solvent composition, and evaporation temperature, on the preparation's physicochemical properties ,swelling studies and in vitro dissolution were studied. The optimized preparation exhibited a significant increase in galantamine hydrobromide's solubility and dissolution rate, resulting in enhanced bioavailability
Keywords
Galantamine, Microspheres, Preparation, Analytical methods
Introduction
One medication for AD that has been approved by the Food and Drug Administration (FDA) is galantamine hydrobromide (GH).It is a neuroactive medication that can slow down the effects of AD and cross the blood-brain barrier. GH is classified as an acetylcholinesterase inhibitor (AChEI). Acetylcholinesterase (AChE) is a degradative enzyme found in the brain that is blocked by the drug. Because it is not degraded into inactive molecules, the neurotransmitter acetylcholine (ACh) performs its cognitive tasks in the brain. A tertiary alkaloid called galantamine hydrobromide has been identified from a variety of plant species, including Lycoris and Narcissus. By preventing the acetylcholinesterase enzyme from doing its job, it helps treat Alzheimer's disease. This prevents the breakdown of the neurotransmitter acetylcholine. Acetylcholine can therefore continue to exist and perform its role as a neurotransmitter that crosses synapses to convey messages. Benefits of galantamine hydrobromide include reduced muscarinic side effects, accelerated recovery from respiratory depression, blood-brain barrier penetration, nicotinic acetylcholine receptor binding, and improved microglial amyloid-beta peptide phagocytosis.A synthetic, highly cross-linked, hydrophilic polymer is called Carbopol[1].When its concentration is greater than 0.50%, a translucent and smooth gel is produced[2]. The acidic carbopol can be neutralized by adding triethanolamine to the polymer solution[3]. In addition to serving as plasticizers in formulations to encourage drug mobility over the skin, penetration enhancers are utilized to increase and improve the medication's permeability across the skin barrier. The primary goal of this study is to create an appropriate gel-type drug reservoir for a transdermal patch administration method. Physiochemical testing and in vitro drug release investigations were used to assess the gel. 6H-Benzofuro with Galantamine Hydrobromide (GH) [3a,3,2-ef][2]. The tertiary alkaloid benzazepin-6-ol,4a,5,9,10,11,12-hexahydro-3methoxy-11-methyl-hydrobromide is a reversible, competitive acetylcholinesterase inhibitor that is prescribed to treat the symptoms of Alzheimer's disease and other types of dementia by enhancing patients' cognition, function, and day-to-day activities[4,5,6,7].One of the fundamental effects of the medication in these conditions is the reduction of acetylcholine deficiency in the patient’s brains.
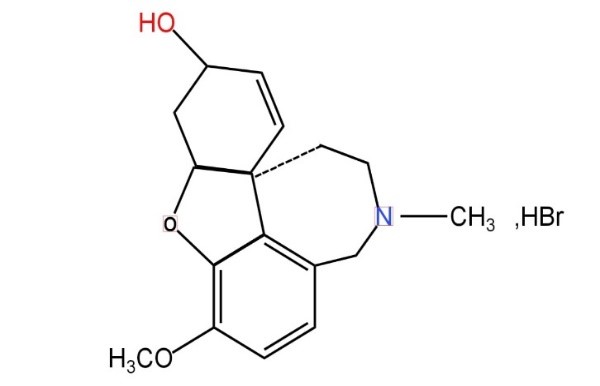
Structure of Galantamine Hydrobromide
MECHANISM OF ACTION:
Acetylcholinesterase inhibition that is reversible and allosteric modulation of the nicotinic receptor were the two mechanisms of action that galantamine exhibited in both in vitro and animal experiments. Since it has been shown that positive activation of the nicotinic receptor enhances cognitive performance, galantamine's dual mode of action may provide an extra advantage over medications that only work on acetylcholinesterase. In addition, galantamine regulates the release of other neurotransmitters such as glutamate, ?-aminobutyric acid, and serotonin; consequently, it may reduce the behavioral symptoms of dementia by modifying other neurotransmitter systems. Galantamine has been shown to lessen drug-induced (i.e., scopolamine) cognitive loss in animal learning and memory models[4,8,9].
Microspheres:
Solid, nearly spherical particles with a diameter of 1–1000 ?m are known as microspheres, and may include dispersed drugs in particular solutions or microcrystalline forms. Often, the terms microsphere and microcapsule are used interchangeably[10] .Since they are not microcarriers, they have the advantage of being local, while nanoparticles travel throughout the 100 nm range that the lymph conveys into the interstitium.

Fig:Microsphere
Benefits of Microspheres:
1.Decrease in size can boost the efficacy of the poorly soluble substance by increasing its surface area
2.Maintaining a consistent level of drugs in the body that can enhance adherence to patients
3.Lowering dosage and danger
4. Polymers used in drug packaging shield the medication from enzymatic cleavage and make it appropriate for drug delivery system
5. Higher patient compliance is a result of shorter dosage times.
Types Of Microspheres:-
- Microspheres with bioadhesion
- Microspheres that are magnetic
- Microspheres that float
- Microspheres that are radioactive
- Microspheres made of polymers[11,12]
METHOD OF PREPARATION:
Materials Required
We bought galantamine hydrobromide from Xi’an Yiyang Bio-Tech, located in Xi’an City, China. In Waltham, Massachusetts, USA, Fisher Scientific sold Carbopol 940. We purchased sodium hydroxide, monopotassium phosphate, disodium monohydrogen phosphate, potassium chloride, sodium chloride, propylene glycol, and triethanolamine from Merck (Darmstadt, Germany).We purchased acetonitrile and trifluoroacetic acid from Avantor Performance Materials in Center Valley, Pennsylvania, USA. All of the compounds employed were analytical or reagent grade, and no additional purification was necessary[13].
Polymers Required
1.HPMC(Hydroxy Propyl Methyl Cellulose)
The empirical formula for hydroxypropyl methylcellulose is C8H15O8-(C10H18O6)n-C8H15O8, and its molecular weight is around 86000. Methyl cellulose and hydroxypropyl ether are semisynthetic components of the product's composition. HPMC is a white or creamy-white, granular, fibrous powder that has no flavor or odor. In water, it can dissolve. Methocel is a range of hydroxypropyl methyl cellulose goods. It comes in three series: E, F, and K. Each series offers a range of product models. K series for release resistance and hydrophilic gel matrix of sustained-controlled preparation; E and F series for adhesion promoter and release resistance of eye ophthalmic preparation, suspending agent and thickener of liquid preparation, and adhesive of tablet and granule; E series for thin film coatings, coating for tablets, and closed core[14,15].
2.Carbopol
The synthetic polymer carbopol is generally regarded as innocuous and does not significantly interact chemically with the components of a gel formulation. Because of its superior muco-adhesion activity, it is mainly utilized as a gelling agent. The PH of 7.4, which is the physiological PH of saliva, allows it to retain its characteristics while reducing irritation to the oral mucosa[16].
3.Ethyl Cellulose
Pharmaceuticals have made extensive use of the class of organo-soluble thermoplastics known as ethyl cellulose. Only a very tiny percentage of water-insoluble excipient polymers are authorized and recognized worldwide for use in pharmaceutical applications, including ethyl cellulose compounds. Depending on the amount of ethoxyl present, ethyl cellulose is soluble in different amounts in specific organic solvents but nearly insoluble in glycerin, propylene glycol, and water[17].
PROCEDURE
Mucoadhesive microspheres of Galantamine were prepared using ionotrophic gelation method at 1:1, 1:0.9:0.1, of Drug: Sodium alginate: polymer. The cross linking polymer sodium alginate and sustained release polymer (Carbopol, HPMC K 100 M, and Ethyl Cellulose ) were soaked in the water for 12 hours. The pure drug such as Galantamine was dissolved in 15 ml of water and mixed with the above polymer mixture solution. The above solution was added drop wise using 22 gauge syringe to the 15 ?Cl2. The formed microspheres were allowed for 30 minutes in the above solution under stirring condition for the completion of reaction and for the formation of spherical microspheres. The prepared microspheres were filtered, washed with distilled water and finally dried at 40OC. The dried microspheres were stored in air tight glass vials. The prepared microspheres were evaluated for various physico chemical properties such as drug content, entrapment efficiency, particle size, in vitro dissolution, release kinetics study, microscopic study and FTIR study[18] .
Preparation Of Galantamine Microspheres:
Mucoadhesive microspheres of Galantamine were prepared using ionotrophic gelation method at 1:1, 1:0.9:0.1, of Drug: Sodium alginate: polymer. The cross linking polymer sodium alginate and sustained release polymer (Carbopol, HPMC K 100 M, and Ethyl Cellulose ). (F-1 to F-4). The microspheres were prepared by keeping the sodium alginate concentration constant. 250 mg of the drug was used in each formulation. The sodium alginate was used as cross-linking agent and 250 mg was used. The sustained release polymer was changed as per the formulation as mentioned above[19].The prepared microspheres were evaluated for entrapment efficiency and % yield. The formulations of the prepared microspheres were summarized in the table below.
Formulation And Physico Chemical Properties Of The Prepared Galantamine Microspheres:
|
S.NO
|
INGREDIENTS
|
F-1
|
F-2
|
F-3
|
F-4
|
|
1.
|
Galantamine
|
250
|
250
|
250
|
250
|
|
2.
|
Na alginate
|
250
|
225
|
225
|
225
|
|
3.
|
HPMC K 1OO C
|
-
|
25
|
-
|
-
|
|
4.
|
Ethyl Cellulose
|
-
|
-
|
25
|
-
|
|
5.
|
Carbopol
|
-
|
-
|
-
|
25
|
|
6.
|
% Yield
|
67
|
71
|
70
|
69
|
|
7.
|
Average Particle size µm
|
670
|
720
|
720
|
720
|
|
8.
|
Drug content (Theoretical )_%
|
16
|
16
|
16
|
16
|
|
9.
|
Drug content ( Practical )%
|
11.60
|
11.18
|
10.88
|
9.60
|
|
10.
|
% Entrapment efficiency
|
72.5
|
69.9
|
68
|
60
|
|
11.
|
Theoretical amount to be taken for 16 mg of Galantamine
|
32
|
32
|
32
|
32
|
|
12.
|
Actual amount of microspheres to be taken for 1 Dose of Galantamine
|
44
|
45
|
47
|
64
|
Drug loaded micro capsules (100 mg) were powdered and suspended in Water. Then the contents suspended in the water were kept for sonication (Power sonic 505, HWASHIN technology co) for about 20 minutes and shaking using mechanical shaker (ORBITEX, Scigenics biotech) for about 20 minutes for the complete extraction of drug from the microcapsules. The resultant solution was filtered through 0.45 µm membrane filter (MILLIPORE). Drug content was determined by UV- visible spectrophotometer (Labindia,) at 280 nm. The theoretical drug content of the prepared microspheres was found between 16 mg depends up on the polymer proportion. The actual drug content was found between 11.6 to 9.6 %. The highest drug content was observed for the formulations prepared with HPMC K 100 M. The entrapment efficiency of the prepared Galantamine microspheres was found between 60 to 72.5 %[20] .The average particle size was observed 650 µm and the particle size was increased with increase in the polymer proportion and was found 800 µm. The particle size distribution and the entrapment efficiency was summarized in the FIG-1&2.

FIG-1: Particle size of the Galantamine microspheres
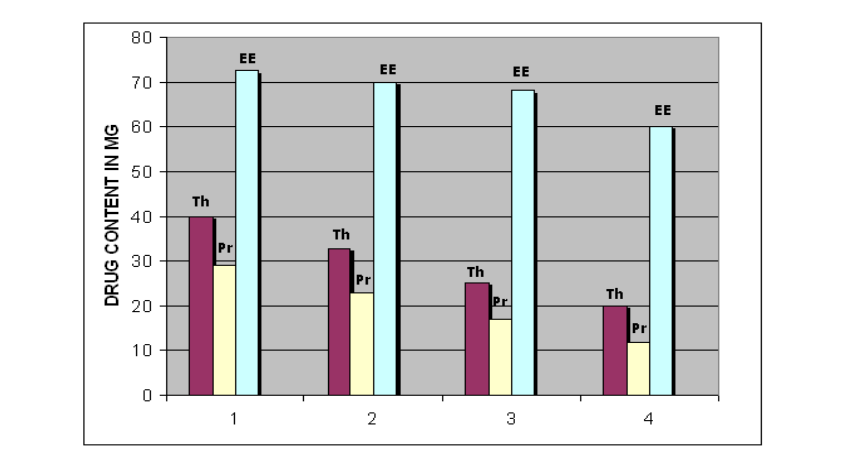
FIG-2: Entrapment efficiency of the Galantamine microspheres
Physicochemical Properties:
Dose size:
An oral product with a dose size larger than 0.5 grams is not a good fit for a sustained release system since adding a sustaining dose and perhaps a sustaining mechanism would typically result in an excessively high volume product.
Ionization, Pka and Aqueous Solubility:
For conventional dosage forms, the drug can typically dissolve completely in the stomach and then be absorbed in the alkaline pH of the intestine; for sustained release formulations, a large portion of the drug will arrive in the small intestine in solid form; therefore, the solubility of the drug is likely to change several orders of magnitude during its release. The pH Partition hypothesis merely states that the unchanged form of a drug species will be preferentially absorbed through many body tissues. It is crucial to pay attention to the relationship between the compound's PKa and its absorptive environment. Since the drug's dissolution will limit the release of these compounds over the period of a dose form in the GIT, they are inherently regulated. According to reports, 0.1 mg/ml is the minimum solubility requirement for a medicine to be manufactured in a sustained release system (Fincher et al., 1968). Diffusion systems will therefore be a bad option for medications that are only weakly soluble since the concentration in solution will below.
Partition coefficient:
Compounds having a comparatively high partition coefficient are mostly fat soluble and readily pass through membranes, leading to high bioavailability. Poor bioavailability will come from compounds with a very low partition coefficient having trouble passing through membranes. Additionally, diffusion across polymer membranes is equal to partitioning effects.
ANALYTICAL METHODS:
Methods for quantification of Galantamine hydrobromide substance
According to the USP, galantamine hydrobromide is analyzed by HPLC using the external standard method and the chromatographic system for permissible content of related substances. The European Pharmacopoeia states that the substance is determined by acid-base neutralization potentiometric titration with 0.1 M sodium hydroxide solution [21,22]. We have developed HPLC techniques using UV and mass-detection for the measurement of galantamine hydrobromide material. Regarding HPLC techniques that use UV detection at ? = 225 nm[23] , ? = 232 nm, and ? = 289 nm[24] .
Methods for analysis of Galantamine hydrobromide in medicinal products
According to USP, HPLC and a UV test at ? = 289 nm are used to identify galantamine hydrobromide in tablets, and a gradient HPLC method is used to ascertain the allowable content of related substances [25,26] .
Methods for analysis of Galantamine hydrobromide in biological fluids
The following techniques have been developed for the detection of galantamine hydrobromide in biological fluids:
1) HPLC techniques using UV [27], fluorescence [28], and mass detection [29]
2) capillary zone electrophoresis (serum, urine) [30]
3) micellar electrokinetic chromatography [31]
4) fluorimetry [32]
CONCLUSION:
The preparation of microspheres using Ionotropic gelation method comes under targeted drug delivery which is having many benefits as it improves bio availability by enhancing solubility and provides sustain release of galantamine hydrobromide and when this drug is formulated as microsoheres it improves the efficacy of drug and minimizes side effects. Formulating the drug as microspheres increases patient compliance and reduces the dosing frequency.Carbopol shows more efficiency when compared to other polymers used in this formulation. Formulations containing Hpmc had found to have more drug content.The ability to encapsulate both hydrophilic and hydrophobic drugs within microspheres makes this method particularly versatile. The use of microspheres in combination with other drug delivery systems, such as nanoparticles and liposomes, may also be explored. While the ionotropic gelation method offers several advantages, there are also challenges and limitations associated with its use.
ACKNOWLEDGEMENT:
My heartfelt appreciation goes out to each and every author and contributor whose work has been cited in Formulation and Analysis of Galantamine Hydrobromide microspheres. Their innovative concepts and perceptive research have substantially enhanced this review. I also want to express my gratitude to the editors and peer reviewers for their insightful criticism and helpful recommendations, which have raised the caliber of this work. We would especially like to thank my institution for providing the tools and assistance needed to complete this task.
REFERENCES
- Jana S, Manna S, Nayak AK, Sen KK, Basu SK. Carbopol gel containing chitosan-egg albumin nanoparticles for transdermal aceclofenac delivery. Colloids and surfaces B: Biointerfaces. 2014 Feb 1;114:36-44.
- Roberts GP, Barnes HA. New measurements of the flow-curves for Carbopol dispersions without slip artefacts. Rheologica acta. 2001 Sep;40(5):499-503.
- Islam MT, Rodriguez-Hornedo N, Ciotti S, Ackermann C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharmaceutical research. 2004 Jul;21:1192-9.
- Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs. 2000 Nov;60:1095-122.
- Corey JB. Galantamine: a review of its use in Alzheimer's disease and vascular dementia. International journal of clinical practice. 2003 Apr;57(3):219-23.
- Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clinical pharmacokinetics. 2002 Aug;41:719-39.
- Evans JG, Wilcock G, Birks J. Evidence-based pharmacotherapy of Alzheimer's disease. International Journal of Neuropsychopharmacology. 2004 Sep 1;7(3):351-69.
- Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs. 2000 Nov;60:1095-122.
- Robinson DM, Plosker GL. Galantamine extended release. CNS drugs. 2006 Aug;20:673-81.
- Farlow MR. Clinical pharmacokinetics of galantamine. Clinical pharmacokinetics. 2003 Dec;42:1383-92.
- Reddy BV, Krishnaveni K. Formulation and evaluation of efavirenz microspheres. Der Pharmacia letters. 2015;7(6):1-9.
- Ando S, Putnam D, Pack DW, Langer R. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. Journal of pharmaceutical sciences. 1999 Jan;88(1):126-30.
- Saralidze K, Koole LH, Knetsch ML. Polymeric microspheres for medical applications. Materials. 2010 Jun 7;3(6):3537-64.
- Yen WF, Basri M, Ahmad M, Ismail M. Research Article Formulation and Evaluation of Galantamine Gel as Drug Reservoir in Transdermal Patch Delivery System.
- Sahoo CK, Rao SR, Sudhakar M. HPMC a biomedical polymer in pharmaceutical dosage forms. J. Chem. Pharm. Sci. 2015 Jan;8:875-81.
- Gangane PS, Sapkal SB, Welankiwar AS, Magar PS, Bhusari DV. once a daily tablet formulation and in vitro evaluation of hpmc based intra gastric floating tablet of levofloxacin. Research Journal of Pharmacy and Technology. 2015 Apr 1;8(4):395.
- Lin YP, Chen WC, Cheng CM, Shen CJ. Vaginal pH value for clinical diagnosis and treatment of common vaginitis. Diagnostics. 2021 Oct 27;11(11):1996.
- Harika K, Sunitha K, Maheshwar K, Rao M. Basic concepts of cellulose polymers-a comprehensive review. Archives of Pharmacy Practice. 2012;3(3-2012):202-16.
- Sachan NK, Singh B, Rao KR. Controlled drug delivery through microencapsulation. Malaysian J Pharm Sci. 2006;4(1):65-81.
- Rahman MA, Ahuja A, Baboota S, Bali V, Saigal N, Ali J. Recent advances in pelletization technique for oral drug delivery: a review. Current drug delivery. 2009 Jan 1;6(1):122-9.
- OKADA S, NAKAHARA H, ISAKA H. Adsorption of drugs on microcrystalline cellulose suspended in aqueous solutions. Chemical and pharmaceutical bulletin. 1987 Feb 25;35(2):761-8.
- Tsvetkova DD, Ivanova SA, Obreshkova DP, Petkova VB, Atanasov P, Yordanova-Laleva PD, Pashev AS. METHODS FOR ANALYSIS OF GALANTAMINE HYDROBROMIDE.
- Tsvetkova DD, Ivanova SA, Obreshkova DP, Petkova VB, Atanasov P, Yordanova-Laleva PD, Pashev AS. METHODS FOR ANALYSIS OF GALANTAMINE HYDROBROMIDE.
- Deshpande GR, Roy AK, Rao NS, Rao BM, Rudraprasad Reddy J. Rapid screening of volatile ion-pair reagents using UHPLC and robust analytical method development using DoE for an acetyl cholinesterase inhibitor: galantamine HBr. Chromatographia. 2011 Apr;73:639-48.
- Zhi CM, Cang Y. Determination of Galantamine hydrobromide by HPLC. J. Appl. Chem. Ind. 2007;3(1):927-30.
- Tsvetkova DD, Ivanova SA, Obreshkova DP, Petkova VB, Atanasov P, Yordanova-Laleva PD, Pashev AS. METHODS FOR ANALYSIS OF GALANTAMINE HYDROBROMIDE.
- Claessens HA, Van Thiel M, Westra P, Soeterboek AM. High-performance liquid chromatographic determination of galanthamine, a long-acting anticholinesterase drug, in serum, urine and bile. Journal of Chromatography B: Biomedical Sciences and Applications. 1983 Jan 1;275:345-53.
- Tsvetkova DD, Ivanova SA, Obreshkova DP, Petkova VB, Atanasov P, Yordanova-Laleva PD, Pashev AS. METHODS FOR ANALYSIS OF GALANTAMINE HYDROBROMIDE.
- Maláková J, Nobilis M, Svoboda Z, Lísa M, Hol?apek M, Kv?tina J, Klimeš J, Pali?ka V. High-performance liquid chromatographic method with UV photodiode-array, fluorescence and mass spectrometric detection for simultaneous determination of galantamine and its phase I metabolites in biological samples. Journal of Chromatography B. 2007 Jun 15;853(1-2):265-74.
- Tsvetkova DD, Ivanova SA, Obreshkova DP, Petkova VB, Atanasov P, Yordanova-Laleva PD, Pashev AS. METHODS FOR ANALYSIS OF GALANTAMINE HYDROBROMIDE.
- Hsieh YH, Yang YH, Yeh HH, Lin PC, Chen SH. Simultaneous determination of galantamine, rivastigmine and NAP 226?90 in plasma by MEKC and its application in Alzheimer's disease. Electrophoresis. 2009 Feb;30(4):644-53.
- Culzoni MJ, Aucelio RQ, Escandar GM. Spectrofluorimetry in organized media coupled to second-order multivariate calibration for the determination of galantamine in the presence of uncalibrated interferences. Talanta. 2010 Jun 30;82(1):325-32.


 Kurukuri Easha madhuri *
Kurukuri Easha madhuri *
 Kadali Anusha
Kadali Anusha
 Kalyan Srinivas Yatham
Kalyan Srinivas Yatham




 10.5281/zenodo.14787326
10.5281/zenodo.14787326