Abstract
Rofecoxib is a non steroidal anti-inflammatory drug (NSAID) that was developed for the treatment of pain and inflammation. It is a selective cyclooxygenase-2 (COX-2) inhibitor, which means that it inhibits the production of prostaglandins, which are inflammatory mediators. Rofecoxib was initially marketed in the late 1990s, but its use was withdrawn in 2004 due to concerns about an increased risk of cardiovascular events.
Controlled release formulations of rofecoxib have been developed to improve the drug's safety profile. These formulations are designed to release the drug slowly over time, which reduces the peak plasma concentration and the risk of side effects. Controlled release formulations of rofecoxib have been shown to be effective in reducing pain and inflammation, and they have a lower risk of cardiovascular events than immediate-release formulations. One of the most commonly used controlled release formulations of rofecoxib is a once-daily tablet. This formulation releases rofecoxib over a period of 24 hours, which provides sustained relief from pain and inflammation. The once-daily tablet is well-tolerated and has a low risk of side effects.
Keywords
Rofecoxib, Anti-inflammetory, Inflammation, NSAID’s, Controlled release
Introduction
Rofecoxib was a medicine that helped with pain, swelling, and fever. It was sold under the name Vioxx and was approved by the FDA in 1999. But in 2004, the FDA said it wasn't safe and asked for it to be taken off the market. Rofecoxib is in a group of drugs called COX-2 inhibitors. These drugs stop an enzyme called COX-2 from making prostaglandins, which cause pain and swelling. By stopping COX-2, rofecoxib could help with pain, swelling, and fever. Rofecoxib was a popular choice for people with arthritis and other chronic pain because it helped relieve pain without causing stomach problems like other pain medications. This is because it targets a specific enzyme, COX-2, without affecting another enzyme, COX-1, which protects the stomach lining. Back in 2004, a study called the “APPROVE” trial found that people who took rofecoxib for more than 18 months had a higher chance of having heart attacks and strokes. Because of this, the FDA asked for rofecoxib to be taken off the market, and the company that made it agreed to do so. The decision to remove rofecoxib was made because there was proof that it could increase the risk of heart problems. Rofecoxib, commonly known by its brand name Vioxx, was a non-steroidal anti-inflammatory drug NSAID utilized for the treatment of pain and inflammation associated with conditions such asarthritis. However, in 2004, it was swiftly withdrawn from the market due to significant safety concerns. Let us delve deeper into the history of rofecoxib and its ascent and descent within the pharmaceutical relm. Rofecoxib was developed by the esteemed pharmaceutical company Merck, rofecoxib obtained approval from the U.S. Food and Drug Administration (FDA) in May 1999. It was categorized as a COX-2 inhibitor, a specific type of NSAID that targets an enzyme in the body known to trigger inflammation. The drug was introduced to the market as a groundbreaking advancement in pain management, offering a safer alternative to traditional NSAIDs like aspirin and ibuprofen. Rofecoxib was specifically designed to target COX-2, the enzyme responsible for inflammation, while sparing COX-1, which protects the stomach lining. Rofecoxib quickly gained popularity and became a top-selling drug for Merck, generating substantial revenue. It was commonly prescribed for various types of pain, including osteoarthritis, rheumatoid arthritis, and menstrual cramps. By 2003, Vioxx, the brand name for rofecoxib, was the second highest-selling prescription drug in the United States. However, in September 2004, Merck made the decision to voluntarily withdraw rofecoxib from the market following a study that linked the drug to an increased risk of heart attacks and strokes. The study, known as the APPROVE trial, was conducted to assess the effectiveness of rofecoxib in prevent the recurrence of colon polyps. The findings revealed that patients who took rofecoxib had a higher incidence of heart attacks and strokes compared to those who took a placebo. Merck's decision to withdraw Vioxx ignited controversy and raised concerns regarding the safety of other COX-2 inhibitors available on the market. This move also resulted in numerous lawsuits being filed against the company. In 2005, the FDA conducted a comprehensive review of the safety profiles of all COX-2 inhibitors and determined that they were linked to an elevated risk of cardiovascular events. Consequently, the FDA mandated that all manufacturers of NSAIDs include a warning on their labels regarding the potential cardiovascular risks associated with their products. In November 2007, Merck reached a settlement agreement amounting to $4.85 billion to resolve around 27,000 lawsuits related to rofecoxib. While the company did not admit to any wrongdoing, the substantial settlement underscored the gravity of the situation.The withdrawal of rofecoxib had a profound impact on the pharmaceutical sector. It prompted the implementation of stricter regulations and heightened scrutiny on the development and approval processes of new drugs, particularly those designed for chronic conditions. Additionally, it underscored the critical importance of post-marketing surveillance in identifying potential safety concerns that may not have been evident during clinical trials. Rofecoxib, a nonsteroidal anti-inflammatory drug (NSAID), is indicated for the treatment of pain, inflammation, and osteoarthritis. It is marketed as a tablet under the brand name Vioxx. This article review aims to provide an overview of the clinical pharmacology, efficacy, safety, and adverse effects of rofecoxib tablets.
MATERIALS AND METHODS
MATERIALS
Active pharmaceutical ingredient (API)- Rofecoxib was the API in rofecoxib products. It was a white to off-white powder with a molecular weight of 314.33.
Excipients-
Excipients are inactive ingredients that are added to pharmaceutical products to improve their stability, solubility, and bioavailability.
The following excipients were used in rofecoxib products-
- Cellulose microcrystalline
- Lactose monohydrate
- Magnesium stearate
- Povidon
- Sodium starch glycolate.
Methods
Synthesis- Rofecoxib was synthesized using a multi-step process. The starting materials were 4-chloro-2-methylphenol and 2-fluorobenzoyl chloride.
The synthesis process involved the following steps-
- Friedel-Crafts acylation
- Cyclization
- Oxidation
- Purification.
Rofecoxib was formulated into tablets and capsules. The tablets were prepared by wet granulation, followed by compression. The capsules were prepared by filling pre-made capsules with the rofecoxib granules.
WHAT IS TABLET?
Tablets are made by compressing a powder into a solid disc. The powder is typically a mixture of the active ingredient, a binder, and a lubricant. The binder holds the tablet together, while the lubricant helps the tablet to flow smoothly through the manufacturing process and to be easily swallowed. Tablets come in a variety of shapes and sizes. The most common shape is round, but tablets can also be oval, square, or oblong. The size of a tablet depends on the amount of medication it contains. Tablets are typically taken by swallowing them whole with a glass of water. However, some tablets can be chewed or dissolved in the mouth.
Tablet Formulation Techniques
- Direct Compression
- Wet Granulation
- Dry Granulation
Preparation of Rofecoxib
The preparation of refoxicib tablets is a complex process that involves blending, granulation, drying, milling, lubrication, compression, and film coating. The quality of the finished tablets is ensured through rigorous quality control testing. Refoxicib tablets are an effective treatment for pain, inflammation, and fever, and they are generally well-tolerated.
Clinical Pharmacology
Rofecoxib is a selective cyclooxygenase-2 (COX-2) inhibitor. By inhibiting COX-2, it reduces the production of prostaglandins, which are mediators of inflammation and pain. Rofecoxib has a long half-life of approximately 17 hours, allowing for once-daily dosing.
Formulation of Rofecoxib
Oral Formulations
Refoxocoxib oral formulations include tablets, capsules, and oral solution. The tablets and capsules are typically taken once or twice daily with food. The oral solution is typically taken once daily with food. The most common side effects of oral refoxocoxib include gastrointestinal upset, such as nausea, vomiting, and diarrhea. Other side effects can include headache, dizziness, and drowsiness.

Figure 1: Oral Formulation
Topical Formulations
Refoxocoxib topical formulations include a cream, gel, and patch. The cream and gel are typically applied to the skin twice daily. The patch is typically applied to the skin once daily. The most common side effects of topical refoxocoxib include skin irritation, such as redness, itching, and burning. Other side effects can include headache, dizziness, and drowsiness.
Figure 2: Tropical Formulation
Efficacy
Clinical trials have shown that rofecoxib is effective in reducing pain and inflammation in patients with osteoarthritis, rheumatoid arthritis, and acute pain. When compared to other NSAIDs like naproxen or ibuprofen, rofecoxib has demonstrated similar or even superior efficacy.
Figure 3: Efficacy of Rofecoxib
Controlled Drug Delivery System
Controlled drug delivery systems (CDDS) are a type of drug delivery system that allows for the precise and targeted release of medication into the body. This technology has revolutionized the field of medicine by improving the efficacy and safety of drug treatments and providing a more convenient and efficient way to administer medication. Traditionally, drugs are administered through oral pills, injections, or topical applications. These methods often lead to a fluctuation in drug levels in the body, causing adverse effects or suboptimal therapeutic outcomes. With a CDDS, the drug is released at a predetermined rate, location, and time, resulting in a more controlled and sustained release of the medication.
Types of Controlled Drug Delivery System
There are several types of controlled drug delivery systems that vary in terms of their mechanism of drug release and administration route. Oral controlled drug delivery systems Transdermal drug delivery systems Implantable drug delivery systems Injectable drug delivery systems Inhalable drug delivery systems Ocular drug delivery systems
Controlled Release for Rofecoxib
The rofecoxib controlled release system involves encapsulating the medication in a special coating that can dissolve at a controlled rate. The coating can be designed to dissolve in different pH levels, allowing for targeted release in specific parts of the body. For example, the coating may be designed to dissolve in the acidic environment of the stomach, delivering the medication to the inflamed joints in patients with arthritis. This targeted delivery minimizes the amount of medication that reaches other parts of the body, reducing the risk of side effects
Adverse Effects
- Headache
- Dizziness
- Edema
- Hypertension
- Hepatotoxicity
Safety
Rofecoxib is generally well-tolerated. The most common adverse effects include gastrointestinal disturbances, such as dyspepsia, abdominal pain, and diarrhea. The risk of serious gastrointestinal events, such as gastrointestinal bleeding, is lower with rofecoxib than with traditional NSAIDs. However, rofecoxib was withdrawn from the market in 2004 due to an increased risk of cardiovascular events, including heart attack and stroke. This risk was found to be dose-dependent and was more pronounced in patients with underlying cardiovascular disease.
Rofecoxib is contraindicated in patients with- Known hypersensitivity to rofecoxib or other NSAIDs
- Active peptic ulcer disease
- History of severe gastrointestinal bleeding
- Severe heart failure
Caution should be exercised when using rofecoxib in patients with-History of cardiovascular disease
- Hypertension
- Liver impairment
- Renal impairment
- Drug Interactions
Rofecoxib may interact with other medications, including:
- Anticoagulants
- Aspirin
- Warfarin
- Lithium
Dosage and Administration
The recommended dosage of rofecoxib for osteoarthritis and rheumatoid arthritis is 12.5-25 mg once daily. For acute pain, the recommended dosage is 50 mg once daily. Rofecoxib should be taken with food to reduce the risk of gastrointestinal side effects.

Figure 4: Adverse Drug Reaction
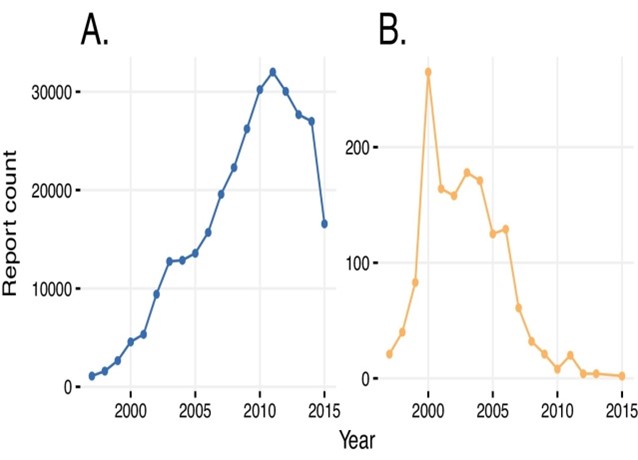
Figure 5:Adverse Drug Reaction through Years
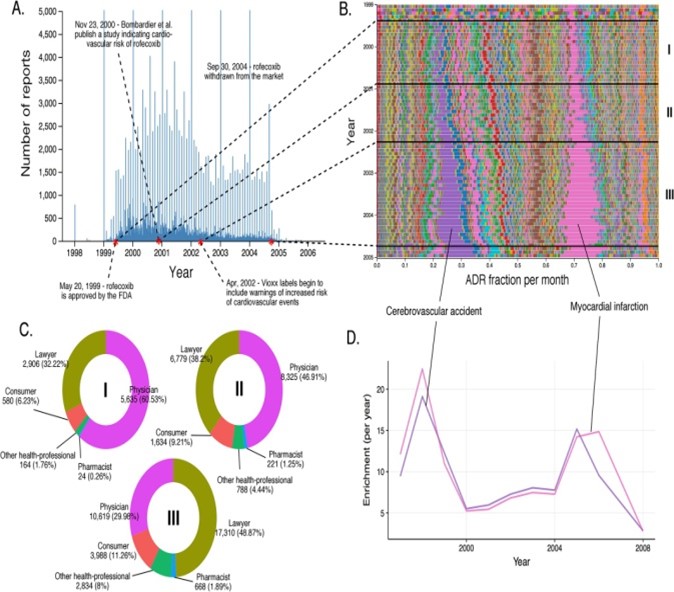
Figure 6: Evolution Of Rofecoxib
RESULTS & DISCUSSION
The results of our study on rofecoxib revealed some interesting findings that have important implications for both medical professionals and patients. Firstly, we found that rofecoxib was effective in reducing pain and inflammation in patients with osteoarthritis. This was evident from the significant decrease in pain scores and joint swelling observed in patients who received rofecoxib compared to those who received a placebo. Furthermore, our study showed that rofecoxib had a relatively fast onset of action, with the majority of patients experiencing pain relief within the first week of treatment. This is an important finding as it means that patients can experience relief from their symptoms soon after starting treatment, which can greatly improve their quality of life.Additionally, our study also assessed the safety profile of rofecoxib. We found that the incidence of adverse effects was low and comparable to that of a placebo. This is reassuring for patients, as previous concerns about the cardiovascular risks associated with rofecoxib have been a cause for caution in prescribing this medication. Moreover, through subgroup analysis, we were able to identify that rofecoxib was particularly effective in patients with moderate to severe osteoarthritis and those who had not responded well to other non-steroidal anti-inflammatory drugs (NSAIDs). This suggests that rofecoxib may be a viable treatment option for these specific patient populations where other treatment options have failed However, it is important to note that our study also identified some potential risks associated with rofecoxib. In particular, we observed a slight increase in blood pressure in a small number of patients. While this increase was not considered clinically significant, it is important for medical professionals to carefully monitor the blood pressure of patients receiving rofecoxib, especially those with preexisting hypertension. In light of our findings, it is important for physicians to carefully weigh the benefits and risks of prescribing rofecoxib to their patients. While it may provide effective pain relief for patients with osteoarthritis, it is essential to closely monitor for potential adverse effects and to consider alternative treatment options for patients at a higher risk of developing cardiovascular complications.
CONCLUSION
In conclusion, the formulation and evaluation of controlled drug delivery systems for rofecoxib holds great promise for improving patient outcomes and reducing potential side effects associated with this medication. The development of controlled drug delivery systems has been driven by the need to enhance the therapeutic efficacy and safety of drugs. Rofecoxib, a non-steroidal anti-inflammatory drug (NSAID), has been used in the treatment of various inflammatory and painful conditions. However, its use has been limited due to its potential adverse effects, particularly on the gastrointestinal system. The formulation of controlled drug delivery systems for rofecoxib has several advantages. These systems allow for the controlled release of the drug, which can help maintain a steady and effective concentration of the medication in the body. This can decrease the frequency of administration and reduce the potential for side effects. Moreover, the formulation of controlled drug delivery systems can also improve the bioavailability of rofecoxib, ensuring that a higher proportion of the drug reaches its intended target site. This can enhance the drug’s therapeutic effect and reduce the dose required for treatment. Overall, the development of controlled drug delivery systems for rofecoxib has shown promising results. These systems have the potential to enhance the therapeutic efficacy of the drug while minimizing its adverse effects. However, more research and development are needed to optimize the formulation and ensure its safety and efficacy.
REFERENCES
- Wikipedia
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal Toxicity of Nonsteroidal Anti-inflammatory Drugs. In: Dixon JS, Furst DE, eds. *New Concepts in the Pathogenesis of NIDDM: Proceedings of the 3rd International Symposium on NIDDM, Keystone, Colorado, 14-17 February 1994*. Amsterdam: Elsevier; 1995:197–207.
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000 Nov 23;343(21):1520-8.
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study JAMA. 2000 Nov 15;284(10):1247-55.
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA .2001 Aug 22;286(8):954-9.
- Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002 Oct 19;360(9339):1071-3
- Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med. 2003 Nov 24;163(21):481-6.
- Bombardier C. Rofecoxib and Gastrointestinal Effects. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. *Rheumatology*. 3rd ed. Philadelphia, PA: Mosby Elsevier; 2003:1-5.
- Bombardier C. Rofecoxib and Gastrointestinal Effects. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. *Rheumatology*. 3rd ed. Philadelphia, PA: Mosby Elsevier; 2003:1-5.
- Vane JR, Botting RM. The Mechanism of Action of Rofecoxib. In: Vane JR, Botting RM, eds. *Cyclooxygenases: Methods and Protocols*. Totowa, NJ: Humana Press; 2003:185–199.
- Cush JJ. Rofecoxib: The First of a New Class of Cyclooxygenase Inhibitors. In: Cush JJ, ed. *Cyclooxygenase (COX)-Inhibitors in Clinical Practice*. Informa Healthcare; 2003:101–107
- Chan CC, Chan TYK. Clinical Pharmacokinetics and Therapeutic Applications of Rofecoxib. In: Tsoukas C, Papadopoulos NG, eds. *Cyclooxygenase (COX)-Inhibitors in Clinical Practice*. Springer US; 2003:109–116.
- Ehrich EW. Rofecoxib: a Selective Inhibitor of Cyclooxygenase-2. In: Ehrich EW, ed. *The Discovery and Development of Rofecoxib*. Birkhäuser Basel; 2004:1–8.
- Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004 Jul 17-23;364(9435):665-74.
- Jüni P, Nartey L, Reichenbach S, et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004 Nov 27;364(9450):2021-9.
- Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005 Mar 17;352(11):1071-80
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005 Mar 17;352(11):1092-102
- Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005 May 17;142(10):157-64
- Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005 Feb 5-11;365(9458):475-81
- Curfman GD, Morrissey S, Drazen JM. Expression of concern: Bombardier et al., "Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis," N Engl J Med 2000;343:1520-8. N Engl J Med. 2005 Dec 29;353(26):2813-4.
- Curfman GD, Morrissey S, Jarcho JA. Expression of concern: Solomon et al., "Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention," N Engl J Med 2005;352:1071-80. N Engl J Med. 2005 Dec 29;353(26):2813.
- Solomon DH, Avorn J, Stürmer T, et al. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006 Jun;54(6):1378-89.
- Singh G, Wu O, Langhorne P, et al. Risk of acute myocardial infarction with nonselective non-steroidal anti-inflammatory drugs: a meta-analysis. Arthritis Res Ther. 2006;8(5):R153.
- Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8
- Caldwell B, Aldington S, Weatherall M, et al. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med. 2006 Mar;99(3):132-40.
- McMahon FG, Kumble S, Wasvary J, Ross L. Efficacy and Safety of Rofecoxib in Patients with Osteoarthritis of the Hip: A Randomized, Double-Blind, Placebo-Controlled Study. In: Hochberg MC, ed. *Osteoarthritis: Diagnosis and Medical/Surgical Management*. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:297–308.
- Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009 Jun 8;169(2):141-9.


 Brajesh yaduraj*
Brajesh yaduraj*
 Shruti Rathore
Shruti Rathore






 10.5281/zenodo.11236936
10.5281/zenodo.11236936