Abstract
Chromatographic separation techniques are essential tools in analytical chemistry, utilized for the isolation, identification, and quantification of complex mixtures. This review provides an overview of various chromatographic methods, including gas chromatography (GC), liquid chromatography (LC), and their advanced variations such as high-performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC). Each technique’s principles, advantages, and limitations are discussed, along with their applications across diverse fields, including pharmaceuticals, environmental analysis, and food safety. Recent advancements in stationary phase materials and detection technologies are highlighted, showcasing the evolution of chromatographic techniques toward improved efficiency and resolution. The review emphasizes the importance of selecting appropriate methods tailored to specific analytical challenges, underscoring the ongoing innovations that continue to enhance the capabilities of chromatographic separations.
Keywords
Chromatography, Separation techniques , Gas chromatography (GC), Liquid chromatography (LC),High-performance liquid chromatography (HPLC), Supercritical fluid chromatography (SFC),Thin-layer chromatography (TLC), Pharmaceutical analysis, Environmental monitoring.
Introduction
Chromatography is a fundamental analytical technique used to separate, identify, and quantify components in complex mixtures. Since its inception by Mikhail Tswett in 1890, who utilized paper chromatography to isolate plant pigments, the field has significantly advanced, giving rise to a multitude of chromatographic methods tailored for various applications.
The principle of chromatography relies on the differential distribution of compounds between a stationary phase and a mobile phase. As the mixture moves through the stationary phase, components interact differently based on their chemical and physical properties, leading to separation. This versatility allows chromatography to be applied in numerous fields, including pharmaceuticals, environmental science, food safety, and biotechnology. The growing complexity of samples and the demand for high-throughput analysis have driven innovations in chromatographic techniques. Methods such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) have become standards in laboratories due to their sensitivity and accuracy. Furthermore, advancements in stationary phase materials and detection technologies have enhanced the resolution and efficiency of separations. This review aims to provide an overview of various chromatographic techniques, their principles, applications, and recent advancements. By highlighting the importance of these methods in analytical chemistry, we aim to inform researchers and practitioners about the best practices and innovations in chromatographic separation.
Chromatography is a crucial analytical technique employed for the separation, identification, and quantification of components in complex mixtures. Since its development by Mikhail Tswett in 1890, who first used paper chromatography to separate plant pigments, the field has expanded to include a diverse range of methods tailored for specific applications.
At its core, chromatography operates on the principle of differential partitioning between a stationary phase and a mobile phase. As a sample mixture is introduced, its components interact variably with these phases, resulting in their separation based on various factors such as polarity, size, and affinity. This makes chromatography an indispensable tool across multiple disciplines, including pharmaceuticals, environmental monitoring, food safety, and clinical analysis. The demand for precise and rapid analytical methods has spurred advancements in chromatographic techniques. High-performance liquid chromatography (HPLC), gas chromatography (GC), and newer methods like supercritical fluid chromatography (SFC) have emerged as key techniques in laboratories due to their high sensitivity, accuracy, and versatility. Recent innovations in stationary phase technology and detection systems have further improved separation efficiency and resolution.
Type of chromatography:
- Thin Layer Chromatography
- High Performance Thin Layer Chromatography
- Paper Chromatography
- Column Chromatography
- Adsorption Chromatography
- Partition Chromatography
- Ion Exchange Chromatography
- High Performance Liquid Chromatography
- Gas Chromatography
- Supercritical Fluid Chromatography
1) Thin Layer Chromatography: Thin layer chromatography, or TLC is a method for analyzing mixtures by separating the compounds in the mixture. TLC can be used to help determine the number of components in a mixture, the identity of compounds, and the purity of a compound by observing the appearance of a product or the disappearance of a reactant, it can also be used to monitor the progress of a reaction.TLC is a sensitive technique – microgram (0.000001 g) quantities can be analyzed by TLC- and it takes little time for an analysis (about 5-10 minutes)
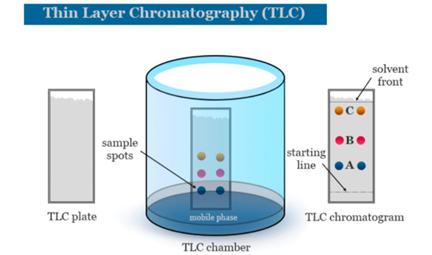
- Principle: Thin layer chromatography (TLC) is a technique used to identify the components/compounds present in a mixture by separating them using a thin stationary phase (silica gel) supported on an inert substrate and a mobile phase (solvent)
- Application:
- Identification of compounds like acids, alcohols, proteins, alkaloids, amines, antibiotics etc.
- To evaluate reaction process by assessment of intermediates, reaction course etc.
- To purify samples i.e. for purification process
- To keep a check on the performance of other separation processes
- Detecting pesticides and insecticides in water and food supplies.
- Determining the dye content in fibers for legal purposes
- Advantage:
- The components are separated in very little time as the components will elute out very quickly.
- The solvents for the TLC plate can be changes.
- Disadvantages:-
- In this method the plate length is limited and hence separation takes place only up to certain length.
- The separation takes place in an open system
2) High Performance Thin Layer Chromatography: Chromatography is a physical process of separation in which the components to be separated are distributed between 2 immiscible phases-a stationary phase which has a large surface area and mobile phase which is in constant motion through the stationary phase.
- Principle: Separation my result due to adsorption or partition or by both phenomenon depending upon the nature of adsorbents used on plates and solvents system used for development.
- Application:
- Food Analysis: quality control, additives, pesticides, stability testing.
- Clinical Application: metabolism studies, drug screening, stability testing etc.
- Industrial Application: process development and optimization, In-process check, validation etc.
- HPTLC’s simple and sensitive method help in therapeutic drug monitoring that aids in the clinical management of patient therapy
- Advantage:
- Ability to analyze multicomponent crude samples
- The separation process is easy to follow especially with colored compounds.
- Disadvantages:
- Lack of standardization and reproducibility
- Costly & requires large quantities of expensive organics
3) Paper Chromatography: Paper chromatography is an analytical chemistry technique for separating and identifying color mixtures.In paper chromatography, substances are distributed between stationary phase and a mobile phase. Stationary phase is usually a piece of filter paper and mobile phase is the colors that travels up the stationary phase. Components of the samples will separate readily according to how strongly they absorb on the stationary phase vs. how readily they dissolve in the mobile phase.
Principle: The principal of separation is mainly partition rather then absorption cellulose layers in filter paper contains moisture which acts as stationary phase. Organic solvents or buffers are used as mobile phases, instead of water as stationary phase other organic solvents can be used by suitable modification.

- Application:
- Paper chromatography is specially used for separation of mixture having polar and non-polar compounds.
- For separation of amino acids.
- It is used to determine organic compound biochemical in urine etc.
- Sometime used for evolution of inorganic compound like salt and complex.
- Effectively separating free amino acids in human serum
- Evaluating reaction kinetics makes it a valuable tool in synthetic chemistry
- Advantage:
- Paper chromatography requires very less quantitative material.
- Paper chromatography is cheaper compared to other chromatography methods.
- Disadvantages:
- Volatile substances cannot be separated using paper chromatography techniques.
- Paper chromatography cannot be compatible with large amounts of sample.
- Quantitative analysis is not useful in paper chromatography.
- Paper chromatography cannot be separated complex mixture.
4) Column Chromatography: Column chromatography can be used to separate the components in a mixture.The stationary phase is a solid, or a solid that has been thinly coated in a viscous liquid and packed into a glass column.The sample is applied carefully to the top of the packing and a solvent, which acts as the mobile phase, is dripped slowly onto the column from the reservoir above.A tap at the bottom of the column allows the solvent, which is called the eluent, to leave the bottom of the column at the same rate as it enters it at the other end.
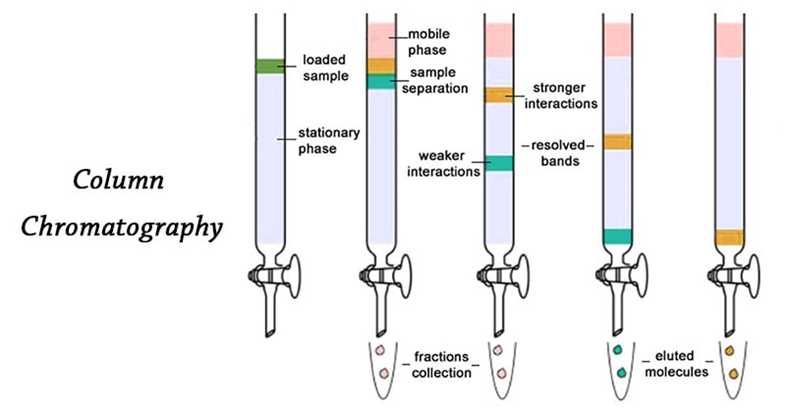
- Principle: The principle of column chromatography is that different compounds move through a column at different rates due to their differential adsorption to the column’s adsorbent.
- Application:
- Separation of mixture of compound.
- Removal of impurities or purification process.
- Isolation of active constituents.
- Isolation of metabolites from biological fluids.
- Separation of Diastereomers.
- Separation of Tautomeric Mixtures
- Advantage:
- Any type of mixture can be separated
- Any quantity of mixture can be separated
- Wider choice of Mobile Phase
- Automation is possible
- Disadvantages:
- Time consuming
- More amount of Mobile Phase are required
- Automation makes the techniques more complicated & expensive
5) Adsorption Chromatography: Adsorption chromatography is one of the oldest chromatographic techniques, utilizing a mobile phase—liquid or gas—that interacts with a stationary solid phase. The separation of solutes occurs due to their differing affinities for the stationary phase; compounds that adsorb more strongly move more slowly, while those that adsorb less move faster. Common forms of this technique include column chromatography, gas-solid chromatography, thin-layer chromatography, and high-performance liquid chromatography (HPLC). Each method has unique applications and advantages, allowing for effective separation and analysis of complex mixtures.
- Principle: It is differential adsorption of solute on the surface of stationary phase.Certain solid material have ability to hold molecule at their surface
- Application:
- Separation of relatively non-polar, water insoluble compounds
- Separation of isomers
- Purification of antibody fragments
- Advantage:
- Easy to use: Adsorption chromatography is relatively simple and inexpensive.
- Versatile: It can be used for many types of samples, including polar and non-polar compounds.
- Disadvantages:
- Not suitable for base labile compounds.
- Cause rearrangement and ring expansion of unsaturated compounds.
- React chemically with acidic compounds.
6) Partition Chromatography: In partition chromatography, a thin liquid layer coats a solid support, creating an equilibrium between the solute in the mobile phase and the stationary liquid. This technique separates compounds based on their partitioning behavior between the two phases. Types of partition chromatography include high-performance liquid chromatography (HPLC), paper chromatography, gas-liquid chromatography, thin-layer chromatography (TLC), and partition column chromatography. Each of these methods is suited for different applications and offers varying degrees of resolution and efficiency in separating complex mixtures.
- Principle: The principle of partition chromatography is the separation of a mixture’s components based on how they partition between a liquid mobile phase and a stationary phase
- Application:
- Removal of detergents from protein solutions
- Separation of mycotoxins, bile acids, and steroids
- Elimination of insecticides, phenols, and pesticides
- Determination of trace metal concentrations in water-based solutions.
7) Ion Exchange Chromatography : Ion-exchange chromatography leverages the ionic charges of the twenty common amino acids, which serve as the building blocks of proteins. Amino acids possess positively or negatively charged side groups (R groups), influencing the overall charge of the protein. Proteins with a net positive charge are classified as “basic,” while those with a net negative charge are considered “acidic.” This technique allows proteins to bind to a support with the opposite charge, facilitating separation based on their ionic properties. Ion-exchange chromatography is particularly valuable for protein purification, enabling the identification and isolation of different chemical families acidic, basic, and neutralwithin a sample.
- Principle: The principle involved in the chromatography is the attraction between oppositely charged particles.Many biological materials such as amino acids and proteins, have ionisable groups and they carry a net positive or negative charge can be utilized in separating mixtures of such compounds.
- Application:
- Softening of hard water
- Demineralization of water
- To analyze base composition of nucleic acid
- To concentrate the metal ions in the sample
- Advantage:
- Cost effective
- Low maintenance cost
- Efficient technique
- Quick separation
- Disadvantages:
- Nature and properties of ion exchange resins
- Nature of exchanging ions
8) High Performance Liquid Chromatography : HPLC is a form of liquid chromatography used to separate compounds that are dissolved in solution.HPLC instruments consist of a reservoir of mobile phase, a pump, an injector, a separation column, and a detector.Compounds are separated by injecting a sample mixture onto the column. The different component in the mixture pass through the column at differentiates due to differences in their partition behavior between the mobile phase and the stationary phase.
- Principle: High Performance Liquid Chromatography [HPLC] is principle is based on adsorption as well as partition chromatography is depending on the nature of stationary phase, if stationary phase is solid principle is based on adsorption chromatography and if stationary phase is liquid principle is based on partition chromatography.
- Application:
- Qualitative analysis
- Checking the impurity of a compound
- Presence of impurities
- Quantitative analysis
- Isolation and identification of drugs
- Advantage:
- Seapartion of voltile and non- voltile components.
- Thermally unstable compounds isolated.
- Quick analysis
- Disadvantages:
- It can be costly, complex to operate and doesn’t work for all samples.
- Need a skill to run the instruments
9) Gas Chromatography: Gas chromatography – “It is a process of separating component(s) from the given crude drug by using a gaseous mobile phase.” It involves a sample being vaporized and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid.
- Principle: gas chromatography (gas liquid chromatography) runs on principle of partitioning of volatile samples with gaseous mobile phase and liquid stationary phase.
- Application:
- Analysis of drug products like antibiotics, antivirals, anesthetics, sedatives.
- Analysis of dairy products like milk, butter, for detection of milk sugars, fatty acids.
- Gas chromatography for food quality evaluation.
- Advantage:
- Highly selective
- Highly sensitive for the detection of compounds like halogens, quinones, peroxides, nitrites, etc.
- It is non-destructive
- More sensitive than TCD and FID.
- Disadvantages:
- Least sensitive to compounds whose molecules have negligible affinity for electrons.
- Carrier gas used should be of pure form like pure nitrogen.
10) Supercritical Fluid Chromatography: Supercritical fluid extraction and Supercritical fluid chromatography are techniques which use supercritical fluids as solvent for both extraction and separation respectively.The properties such as density, viscosity and diffusion constant of the supercritical fluids are intermediate between those of a substance in gaseous and liquid state.This helps in efficient extraction and chromatographic separation compared to other techniques.
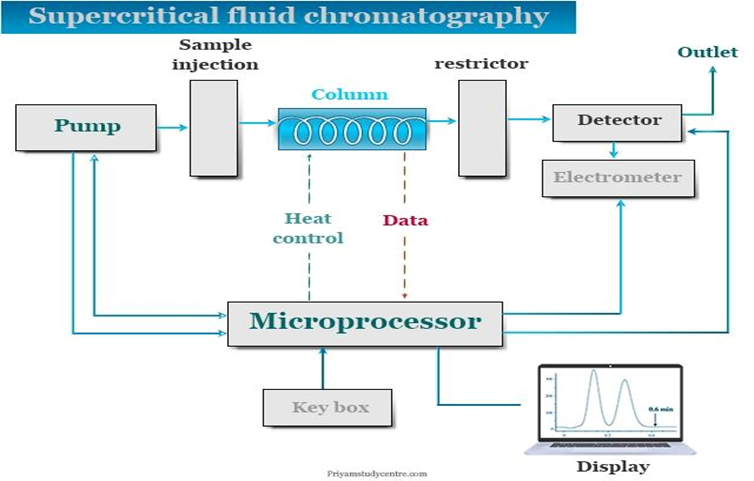
- Principle: The basic principle of SFE and SFC is almost same. Both techniques use supercritical fluids as the solvent for extraction and separation.
- Application:
- SFC is used in industry primarily for separation of chiral molecules.
- SFC now commonly used for chiral separation and purification in the pharmaceutical industry.
- Advantage:
- Applications in quality control and sample
- Minimizes use of organic solvents
- Detection down to ppb or µg (depending on choice of detector)
- Non-destructive method for isolation or purification of compounds
- Disadvantages:
- SFC is pressure operating conditions. High-pressure vessels are expensive and bulky.
- Maintaining pressure in SFC is difficult
REFERENCES
- Cloonan, S. M., & O’Leary, J. (2020). Introduction to Chromatography: Theory and Practice. Academic Press.
- Scopes, R. K. (1994). Protein Purification: Principles, High-Resolution Methods, and Applications. Springer.
- Snyder, L. R., Kirkland, J. J., & Dolan, J. W. (2010). Introduction to Modern Liquid Chromatography. John Wiley & Sons.
- Baker, R. (2002). Introduction to Supercritical Fluid Chromatography. American Chemical Society.
- Katz, E. (2015). Ion-Exchange Chromatography: Principles and Applications. Wiley-Blackwell.
- Fried, B., & Gibbons, R. (2006). Affinity Chromatography: Principles and Methods. Wiley-Interscience.
- McMaster, J. (2014). Thin Layer Chromatography: A Laboratory Handbook. Springer.
- Harris, D. C. (2015). Quantitative Chemical Analysis. W.H. Freeman and Company.
- Roh, Y., & Kim, H. (2018). “Recent Advances in High-Performance Liquid Chromatography”. Analytical Chemistry, 90(1), 254-265
- Poole, C. F., & Poole, S. K. (2012). Handbook of Supercritical Fluids. Academic Press.
- T. Yugandharudu, M. Surendra, T. Viswasanthi. A Review on Analytical Method Development and Method Validation. International Journal of Pharmaceutical Research & Analysis. Vol 2 / Issue 1 / 2012 / 32-48.
- Navjot kaur, Ritika. A review on thin layer chromatography: forensic analytical technique, Pramana Research Journal, ISSN NO: 2249-2976
- Mahesh Attimarad, K. K. Mueen Ahmed, Bandar E. Aldhubaib, and Sree Harsha, High-performance Thin layer chromatography: A powerful analytical technique in pharmaceutical drug discovery, Pharm Methods. 2011 Apr-Jun; 2(2): 71–75.doi: 10.4103/2229-4708.84436
- Snyder, L. R., Kirkland, J. J., & Dolan, J. W. (2010). Introduction to Modern Liquid Chromatography. John Wiley & Sons.
- Scopes, R. K. (1994). Protein Purification: Principles, High-Resolution Methods, and Applications. Springer.
- Baker, R. (2002). Introduction to Supercritical Fluid Chromatography. American Chemical Society.
- Fried, B., & Gibbons, R. (2006). Affinity Chromatography: Principles and Methods. Wiley-Interscience.
- Katz, E. (2015). Ion-Exchange Chromatography: Principles and Applications. Wiley-Blackwell.
- Vivek Tukaram Shinde, R.S. Garad, Santosh Jain, Kamble Manoj, Bansode Badal, Column
- Chromatography, International Journal of Creative Research Thoughts (IJCRT) www.ijcrt.org, 2023 IJCRT | Volume 11, Issue 5 May 2023 | ISSN: 2320-2882
- Balammal G1 and Saravana Kumar A2. A review on basic chromatographic techniques. Indian Journal Of Pharmaceutical Science & Research, Vol 4 | Issue 4 | 2014 | 221-238.
- Jaha Sultana Mohammed. A brief review on Ion Exchange Chromatography. Vol. 5, Issue
- https://www.researchgate.net/publication/322368583_chromatography_and_HPLC_principles


 Swati Appasaheb Pachore * 1
Swati Appasaheb Pachore * 1
 Anushka Anil shinde 2
Anushka Anil shinde 2
 Laware sandip Gangadhar 3
Laware sandip Gangadhar 3




 10.5281/zenodo.13935296
10.5281/zenodo.13935296