Abstract
Parkinson’s disease is one of progressive disorder that is caused by generation of nerve cells in the part of the brain called the substantia nigra, which controls movement. These nerve cells die or become impaired, losing the ability to produce an important chemical called dopamine. Some studies shown that symptoms of parkinson’s disease develop in patients with an 80 percent or greater loss of dopamine- producing cells in the substantia nigra. Normally dopamine operates in a delicate balance with other neurotransmitters to help coordinate the millions of nerve and muscle cells involved in movement. The symptoms occur when dopamine doesn’t produce this balance may have disrupted results in tremor (trembling in hands, arms, legs and jaw) rigidity, slowness of movement and impaired balance and cordination. The main cause of parkinson’s essentially remains unknown. It involves oxidative damage, environmental toxins, genetic factors and accelerated aging. A 74- year old female patient took tab. cilacar for 16 years back continuing same medicine till now for managing hypertension and she had h/o parkinsonism, diabetes and dyslipidemia for 14 years back and she was admitted to a private hospital was taking the treatment for further evaluation and diagnosed on Fronto-temporal dementia associated with parkinsonism (FTDP-17) still she was continuing the Anti- hypertensive drugs without any change in dose and its frequency further it may leads to nerve cell damage and she had a s/h/o left supracondylar displaced fracture with complaints of pain and edema on left elbow, walking difficulty and have done k-wire fixation on 1month. Recently she was admitted with complaints of vomiting since 1 day and decreased appetite for 3 days. Her report shows level of Total count, urea, FBS, ESR and CRP was found to be elevated and potassium was found to be declined. Her urine routine shows albumin was trace and pus cells was found to be plenty. She was initially treated with stat medicines and after managed with antibiotics, anti-emetic agents, proton pump inhibitors and own medicines. Here, we reporting the case of cilnidipine induced by dementia associated parkinsonism which was used to treat for hypertension.
Keywords
Dementia associated parkinsonism, calcium channel blocker agents, hypertension, role of inflammatory response and treatment patterns.
Introduction
Cilnidipine belongs to a class of calcium antagonist or calcium channel blocker to treat the high pressure in patients with essential hypertension. In contrast to cilnidipine this drug is one of the calcium channel blockers (CCBs) and is reported to block two different calcium channels, L- type calcium channels in vascular smooth muscle, which exerts an antihypertensive effect similar to the L- type channel blockers, such as amlodipine and N- type calcium channels in sympathetic nerve endings, which suppresses increases in sympathetic activity in response, such as vasodilation induced by blockade of L- type calcium channels in vascular smooth muscle. In this manner, cilnidipine is suggested to be an effective calcium channel blocker in combination with inhibitors of the renin- angiotensin system as an anti-hypertensive medication used to inhibit chronic kidney disease progression and cardiovascular complications. The therapeutic effects of L/N – type calcium channel blockers cilnidipine with other CCB’s o the ambulatory B and HR profile and on cardiorenal function in hypertensive patients (1).
Parkinson's disease (PD) is a common neurodegenerative disorder which underlying mechanism leading to dopaminergic neuron death remains elusive and current therapies remain purely symptomatic. Increasing evidence has suggested that L-type calcium channels and the central renin-angiotensin system play a role in PD. The age-dependent reliance on L-type calcium channel in dopaminergic neurons contributes to increased intracellular oxidative stress. Angiotensin II, the effector peptide of the central renin-angiotensin system (RAS) in substantia nigra, is a pro-inflammatory compound that can activate the oxidative cascades with resulting neuronal death. These in vitro studies form the bases of hypothesis that antihypertensive agents, especially angiotensin receptor blockers (ARBs), inhibitors of angiotensin converting enzyme (ACEIs), and calcium channel blockers (CCBs), may have possible neuroprotective effects in PD (2).
EPIDEMIOLOGY
Recent population-based cohort studies suggest that PD is associated with several cardiovascular risk factors, such as diabetes mellitus and hypertension. Data from one population-based cohort study of Finland shows that, as compared with normotensive subjects, women with hypertension are associated with a 60% increased risk of PD. Therefore, the role of antihypertensive drugs in risk of PD is worth to be explored. Few epidemiologic studies have examined the association between antihypertensive agents use and PD with inconsistent results. One recently published cohort study demonstrated that use of one subclass of CCBs that targets L-type calcium channels is associated with decreased PD incidence and mortality The possible reasons that studies comparing the risk of PD between CCB users and non-users have different results may come from the age of the study participants, definition of drug exposure, and criteria for PD diagnosis. Furthermore, the effect of other classes of antihypertensive drugs on the development of PD is largely unknown (3,4).
Case Report
A 74- year old female patient had chief complaints of vomiting since 1 day and decreased appetite since 3 days and she was taking Tab. Cilacar (cilnidipine 5mg p/o 1-0-1) for 16 years back continuing same medicine till now for managing hypertension and she had h/o parkinsonism with increased drowsiness she took Tab. Syndopa (Levodopa 100mg+ Carbidopa 10mg) p/o ½ - ½ - ½ and Tab. Qutipin (Quitipin 25mg p/o HS) for diabetes she took Tab. Oxra (Dapagliflozin 10mg p/o 0-1-0) and dyslipidemia Tab. Atorva (Atorvastatin 10mg p/o0-0-1) for 14 years back and she was admitted to a private hospital was taking the treatment for further evaluation and diagnosed on Fronto-temporal dementia associated with parkinsonism (FTDP-17) still she was continuing the Anti- hypertensive drugs without any change in dose and its frequency further it may leads to nerve cell damage and she had a s/h/o left supracondylar displaced fracture with complaints of pain and edema on left elbow, walking difficulty and have done k-wire fixation on 1month. Recently she was admitted with complaints of vomiting since 1 day and decreased appetite for 3 days. Her report shows level of Total count, urea, FBS, ESR and CRP was found to be elevated and potassium was found to be declined. Her urine routine shows albumin was trace and pus cells was found to be plenty and she came for hyponatremia for conservative management. She was diagnosed with hyponatremia, covid-19. The day she was admitted on 29/11/22 and discharged on 6/12/22
She was initially treated with stat medicines of Inj. Pantop (Pantoprazole) 40mg IV at 10.45pm, Inj. Emeset (Ondansetron) 4mg IV 10.45pm and Inj. Renerve plus 100ml NS IV 11pm and after managed with antibiotics of Inj. Izone, S (cefoperazone+ sulbactam 1.5gm IV 1-0-1), anti-emetic agents Inj. Emeset (ondansetron 4mg IV 1-0-1), proton pump inhibitors Inj. Panto (pantoprazole 40mg IV 1-0-1), Ciplox eye drops (ciprofloxacin P/E 2°-2°-2°), syp, potassium chloride 1ml p/o 1-1-1), cap. Becozules /o 1-0-0), Tab. Aldactone (Aldactone 50mg p/o 0-1-0), Critipro powder 2tsp p/o 1-1-1) and own medicines Tab. Cilacar (cilnidipine 5mg p/o 1-0-1), Tab. Syndopa (Levodopa 100mg+ Carbidopa 10mg p/o ½ - ½ - ½), Tab. Qutipin (Quitipin 25mg p/o HS), Tab. Oxra (Dapagliflozin 10mg p/o 0-1-0) and Tab. Atorva (Atorvastatin 10mg p/o 0-0-1). Her USG abdomen and pelvis shows in liver- parenchyma shows fatty changes, both left and right kidney- mild hydronephrosis noted, urinary bladder- grossly distended with volume 92/cc. Echogenic foci sediments noted within wall appears thickened 4mm. This impression shows grade I fatty liver, bilateral mild hydronephrosis- due to over distended urinary bladder and chronic cystitis. Final diagnosis it was reported as klebsilla pneumonia.
In this patient, during the time of admission period his vitals was checked according to follows as: Body temperature: 98.1°F (98.6°F), pulse: 82bpm (72bpm), respiratory rate: 24/min (28/min), blood pressure: 150/90 mmHg (140/90mmhg), spo2: 98% (95-100%). Her urine routine examination report shows albumin- faint trace, sugar- nil, pus cells- plenty, RBC’S- 1-2, EC- 2-3.
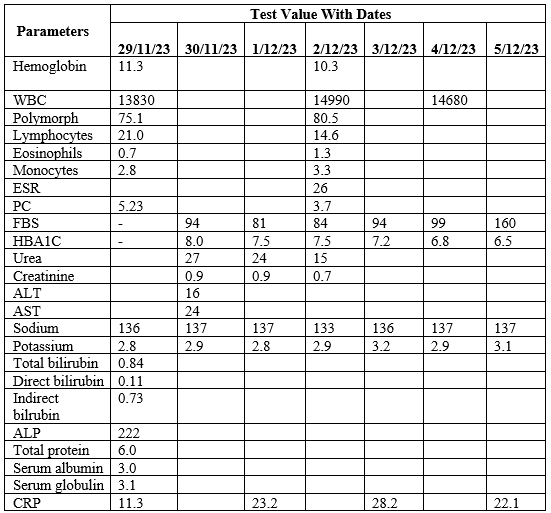
According to laboratory investigation report due to initial period (Table 1).
DISCUSSION
Our study shows that use of CCBs in hypertensive patients has a dose-dependent decreased association with PD compared to use of BBs. This potentially beneficial effect was most obvious with the use of central-acting dihydropyridine CCBs. Additionally, although there was no association between the use of ACEIs or ARBs with risk of PD, a potentially decreased association was found for high cumulative use of these two classes of antihypertensive agents (5).
Although in vitro evidence has suggested that the increased reliance on L-type -calcium channels in dopaminergic neurons with advanced age accelerate the degeneration process, studies related to use of CCBs on reducing the incidence of PD are inconsistent. Three studies comparing the risk of PD between CCBs users and non-users showed that current long term use of dihydropyridine CCBs, especially central-acting ones, was associated with a reduced risk of PD. However, another study demonstrated that current, but not past, dihydropyridine CCBs use was associated with a reduced risk of PD, even including peripherally-acting amlodipine and non-dihydropyridine CCBs. Our study, which compared the risk of PD between difference classes of antihypertensive drugs and non-brain penetrating beta-blockers, reinforced the role of centrally acting dihydropyridine CCBs, especially Felodipine, in decreasing the incidence of PD in hypertensive patients. The potential beneficial effects of central-acting dihydropyridine CCBs in PD may come from the unique property of this class of CCBs specifically target L-type calcium channels and have a much higher concentration in the brain than peripheral acting ones. Although the non-dihydropyridine CCBs verapamil and diltiazem can also transverse the blood-brain barrier, neither is known to bind to L-type Ca+2 channels (6.7). Further studies are needed to explore the potential disease-modifying effect of CCBs in PD disease course. These observations form a basis of studies to test whether central-acting dihydropyridine CCBs would slow down disease progression or even neuroprotection in susceptible subjects. However, one recent trial failed to show isradipine, a central-acting dihydropyridine CCB, in delaying parkinsonism progression. In addition to felodipine, our results showed that among dihydropyrine CCB, a further decreased association was also observed for higher cumulative doses of amlodipine. Similar to our findings, one recent nationwide cohort study also found that the beneficial effects of dihydropyridine CCB on the risk of PD were most obvious for amlodipine and felodipine. Given that amlodipine dose not cross blood-brain barrier as readily as felodipine, we believe that amlodipine may have some other effects, rather than antagonist effects on calcium channels in the central nervous system. Further studies are needed to confirm the disease-modifying effect of central-acting CCBs in PD disease course (8.9).
In our study, we also observed that high cumulative doses of ACEIs and ARBs were also associated with a decreased incidence of PD compared to beta-blockers, which beneficial effects was not present at low cumulative doses. Previous animal studies have suggested that ACEIs and ARBs may be neuroprotective due to their antioxidant properties. Supportively, one double-blind placebo-controlled trial have shown that four weeks of treatment of perindopril, an ACEI, produced an improvement in the motor response to levodopa among patients with moderately severe PD. Our results further support the notion that the dopaminergic systems interact with RAS in nigral-basal ganglia circuits.
However, our study has several limitations. First, the information regarding the blood pressure levels, which have been shown to be associated with risk of PD, was not available in the claims dataset. Further longitudinal study including serial measures of blood pressures over time is needed to clarify the interrelated roles of blood pressure level, use of anti-hypertensive agents, and PD. Second, vascular parkinsonism is a potential co-morbidity in patients with hypertension. Nonetheless, we tried to validate our diagnosis by excluding participants who had cerebrovascular diseases before PD diagnosis to avoid including vascular parkinsonism (10,11). We also attempted to validate our findings by using more stringent diagnostic criteria in the sensitivity analysis. Third, we could not exclude the possibility that some hypertensive patients already had subclinical or early PD at study entry. However, misclassification of the non-differential outcome in all antihypertensive agent groups would bias the study results toward the null hypothesis. In addition, the results were similar when we excluded patients who were diagnosed with PD within one year of study entry. In addition, smoking is known to decrease risk of PD. We were unable to directly control for smoking due to lack of data. Instead, we adjusted for COPD, since it may serve as a proxy for heavy smoking. Other confounding factors, such as the consumption of tea or coffee, other lifestyle-related factors and parameters of vascular function, such as brachial-ankle pulse wave velocity and extent of carotid artery atherosclerosis were not included in the study (12).
CONCLUSION
The strength of our study was its relatively homogeneous population and the comparison of PD risk between different classes of antihypertensive medications. This nationally representative cohort involved a large sample size. Data collection from the NHI pharmacy database rather than self-reported questionnaires reduced the misclassification of exposure. Furthermore, covariates including underlying diseases, especially diabetes mellitus, medication use, and healthcare utilization prior to initiation of antihypertensives were taken into consideration. We made adjustments for the use of medications potentially affecting PD risk, such as NSAIDs, anti-diabetic agents, statins, and neuroleptic agents (13). We observed that use of centrally-acting dihydropyridine CCBs and higher doses of ACEIs and ARBs had a decreased association with PD compared to that of beta-blockers in hypertensive patients. Further long-term follow up studies are needed to confirm the potential use of antihypertensive agents in PD management. Finally, PD patients with hand tremor could possibly be misdiagnosed with essential tremor and treated with beta-blockers. However, it is unlikely that the observed association between antihypertensives and PD was due to the use of beta-blockers as a reference group, as the reverse association was observed for dihydropyridine but not non-dihydropyridine CCBs in our population (14,15).
REFERENCE
- Forno LS (1996) Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 55: 259–272.
- Jenner P (2003) Oxidative stress in Parkinson's disease. Ann Neurol (Suppl 3): S26–36.
- Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol 7: 97–109.
- Ebrahimi-Fakhari D, Wahlster L, McLean PJ (2012) Protein degradation pathways in Parkinson's disease: curse or blessing. Acta Neuropathol 124: 153–172.
- Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J (2007) Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 30: 842–847.
- Qiu C, Hu G, Kivipelto M, Laatikainen T, Antikainen R, et al. (2011) Association of blood pressure and hypertension with the risk of Parkinson disease: National FINRISK Study. Hypertension 57: 1094–1100.
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, et al. (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086.
- Surmeier DJ (2007) Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol 6: 933–938.
- Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M, et al. (1996) 1-Methyl-4-phenyl- 1,2,3,6- tetrahydropyridine induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res 741: 185–196.
- Wright JW, Harding JW (2012) Importance of the brain Angiotensin system in Parkinson's disease. Parkinsons Dis 2012;
- Louis ED, Benito-Leon J, Bermejo-Pareja F (2009) Antihypertensive agents and risk of Parkinson's disease, essential tremor and dementia: a population-based prospective study (NEDICES). Neuroepidemiology 33: 286–292.
- Muñoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, et al. (2006) Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology 51: 112–120.
- Reardon KA, Mendelsohn FA, Chai SY, Horne MK (2000) The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson's disease. Aust N Z J Med 30: 48–53.
- Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, et al. (2007) Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol Neurodegener 2: 1.
- Garrido-Gil P, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL (2012) Involvement of PPAR-gamma in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson's disease. J Neuroinflammation 9: 38.


 Dhanya Dharman*
Dhanya Dharman*

 10.5281/zenodo.12667011
10.5281/zenodo.12667011