Zinc oxide nano-particles (ZnO NPs) are one of the best metal oxide nano-particles with wide role in many industries and research institutes. Numerous methods of synthesis have been adopted in the production of Zinc oxide nano-particles so as to meet its high demand. The environmental implications and economic challenges attached to most of the means of Zinc oxide nano-particles synthesis have resulted in the quest for other alternatives with environmental and economic benefits. Interestingly, the biological method of synthesis using plant sources have been found appropriate for the production of Zinc oxide nano-particles dues to its numerous health, environmental, economic, and medicinal benefits. The distinctive features of Zinc oxide nano-particles synthesized using plant extracts enhanced its application in agriculture for the production of fertilizers, pesticides, and fumigants. In the field of medicine and pharmacy, phytosynthesized Zinc oxide nano-particles have gained remarkable usage in the production of disinfectant, antifungal, anticancer, antioxidant, anti-inflammatory and anti-diabetics agents. Despite the enlisted benefits of biosynthesized Zinc oxide nano-particles, the difficulties associated with the elucidation of formation mechanism and reactions still remain unraveled. This review described the summary of the recent advances in the synthesis, mechanism routes, characterization techniques, and applications of biosynthesized Zinc oxide nano-particles in agriculture, medicine, and textile industries.
Green synthesis, Zinc oxide, Nano-particles, Plant extracts and biomedical applications
Metal oxide nano-particles Specially Zinc oxide nano-particles shown wide range of applications in various fields such as pharmacy, agriculture, catalysis, medicine, textile, heavy industry dealing with consumer goods, and antimicrobial assay [1–5]. In particular, ZnO nano-particles applications in antimicrobial therapy, medicinal purpose, coating, pesticides, sunscreen materials, cosmetics, agriculture, and antimicrobial agent are remarkable [6, 7]. The excellent antimicrobial potentials of Zinc oxide nano-particles have aided its numerous applications in shampoos, surgical tapes, antiseptic creams and calamine lotions. Zinc oxide nano-particles has been reported to demonstrate broad spectrum against bacterial growth at low concentration [8]. Zinc oxide nano-particles earn its remarkable antimicrobial application and biosafety features in medicinal applications having been approved by the United State Food and Drug Administration. The use of Zinc oxide nano-particles in electronics, rubber fabrication, biosensors, transducers, pharmaceutical, and biomedical field have been increasing rapidly in this era [9]. Biomolecules and secondary metabolites present in plant extracts, such as tannins, flavanones, saponins, polyphenols, alkaloids, and terpenoids, have been reported to be responsible for the effective reduction of zinc precursors; the effectiveness of plant extracts in reduction of zinc precursors was higher than microorganism when compared [10].
Previous study has shown that the morphological identities of nanoparticles and Zinc oxide nano-particles influence their various application; the following techniques, such as UV visible spectroscopy, scanning tunneling microscope, atomic force microscope, scanning electron microscope, dynamic light scattering, transmission electron microscope, differential scanning calorimetry, Fourier transmission infrared spectroscopy, energy dispersive x-ray, and particle size analysis, had been used in characterization of Zinc oxide nano-particles to determine their various properties [15]. In this review, we provide an elaborate scientific information on recent development in the synthesis and characterization techniques of Zinc oxide nano-particles using plant materials. Also, recent advancement in the applications of phytosynthesized Zinc oxide nano-particles was also well presented.
Significance of Green Synthesis of zinc oxide nanoparticles
Due to its advantages over conventional chemical and physical synthesis methods, both environmentally and financially, the green production of zinc oxide (ZnO) nanoparticles has attracted a lot of attention. The following are some salient points emphasizing the importance of green synthesis
There are several significant environmental benefits when comparing the green synthesis of zinc oxide (ZnO) nanoparticles to traditional chemical and physical synthesis techniques. These benefits come from using biological materials and environmentally friendly processes, which reduce their harmful effects on the environment and promote sustainability.
Naturally occurring biological entities such as bacteria, fungi, algae, and plant extracts are used in the green synthesis process to create ZnO nanoparticles. These biological materials take the place of the hazardous chemicals and solvents that are typically used in conventional synthesis by functioning as stabilizing and reducing agents. This reduction in dangerous substances helps to lessen the influence on the environment as well as the risk to ecosystems and public health.
High pressures, high temperatures, and energy-intensive processes are often required in traditional synthesis approaches. Conversely, green synthesis consumes a great deal less energy because it frequently occurs at ambient temperature and pressure. This energy efficiency not only lessens the impact on the environment but also improves the synthesis process's sustainability and economic viability.
Compared to conventional methods, green synthesis techniques usually produce less waste and byproducts. The reaction route is made simpler and cleaner by the use of biological materials, increasing the quality of the ZnO nanoparticles and lowering the quantity of waste that needs to be handled or disposed of. Cutting back on trash is crucial to conserving resources and lessening environmental impact.
- Biodegradability and environmental friendliness:
Microbial cultures and plant extracts are examples of biological agents used in green synthesis that are both biodegradable and environmentally benign. This is in sharp contrast to the synthetic chemicals used in traditional techniques, which may be permanently harmful to the environment. The ability of the biological agents to break down ensures that they won't accumulate in the ecosystem, reducing the long-term ecological risks.
Green synthesis processes often make use of abundant, sustainably harvested biological ingredients. For example, plant extracts can be produced from agricultural waste or inedible plant components, promoting a circular economy. Because it is different from the scarce and sometimes uncommon raw components needed for conventional synthesis, this helps save natural resources.
- Economic and Practical Benefits:
The green synthesis of zinc oxide (ZnO) nanoparticles is a more advantageous option than standard synthesis procedures because of its many practical and economical benefits. These benefits are significant for both industrial applications and environmentally responsible activities.
Most green synthesis methods make use of inexpensive, readily available biological components such as plant extracts, bacteria, fungus, and algae. Since these resources are easily farmed or regularly generated from agricultural waste, the total cost of raw materials is reduced. Green synthesis procedures also generally use ambient temperature and pressure, which further reduces energy usage and associated costs. This reduces the cost of ZnO nanoparticle production, particularly for large-scale industrial uses.
The ecologically benign synthesis of ZnO nanoparticles usually makes use of straightforward procedures that don't call for sophisticated machinery or drawn-out purifying steps. To speed up the synthesis process, plant extracts, for example, can be utilized as reducing and stabilizing agents by simultaneously lowering metal ions and inhibiting nanoparticle agglomeration. Its simplicity allows it to be more widely adopted at a reduced operating cost and with more scalability across a range of industries.
Green synthesis techniques are scalable by nature due to the easy availability of biological materials and their simplicity. Processes that employ plant extracts or microbial cultures can easily be scaled up by increasing the volume of the biological medium, all without requiring significant modifications to the synthesis approach. This scalability is crucial for meeting the demand for ZnO nanoparticles in sectors such environmental remediation, medicines, and cosmetics.
- Enhanced Safety and Reduced Environmental Impact:
Conventional methods of generating ZnO nanoparticles often necessitate the use of toxic chemicals and result in harmful byproducts, which calls for stringent safety measures and waste disposal protocols. Green synthesis, on the other hand, uses non-toxic, biodegradable components, which results in safer working conditions and a less environmental impact. In addition to satisfying consumer and regulatory demands for more environmentally friendly products, this reduces the costs associated with adhering to health and safety laws [25].
Biological chemicals used in green synthesis can give ZnO nanoparticles additional functional properties by serving as natural capping agents. The stability and dispersibility of the nanoparticles are enhanced by these biological capping agents, which is beneficial for a range of applications. Time and money are saved when there is less need for extra processing and modification due to increased stability.
One of the key advantages of green synthesis is the improved biocompatibility of ZnO nanoparticles. The ability of a material to interact with biological systems without having an adverse effect is referred to as "biocompatibility". Using natural biological agents, such as plant extracts, bacteria, fungi, and algae, during the production process is crucial to improving the biocompatibility of ZnO nanoparticles.
Because the green manufacturing method doesn't employ any dangerous chemicals, the nanoparticles it creates are safer for biological applications. For instance, it has been shown that ZnO nanoparticles produced using plant extracts have a lower potential for injury than those produced using conventional chemical methods.
Biological components are often used as organic capping agents in green synthesis, encasing the nanoparticles in a coating that is biocompatible. These coatings can reduce the likelihood that the body will respond adversely by strengthening the bond between the nanoparticles and biological tissues.
Due to their enhanced biocompatibility, green-synthesised ZnO nanoparticles are widely used in biomedical applications such drug delivery, wound healing, and antibacterial agents. Their ability to be safely incorporated into biological systems makes them excellent for these uses [28].
Synthesis of Zinc oxide nano-particles
Chemical Methods
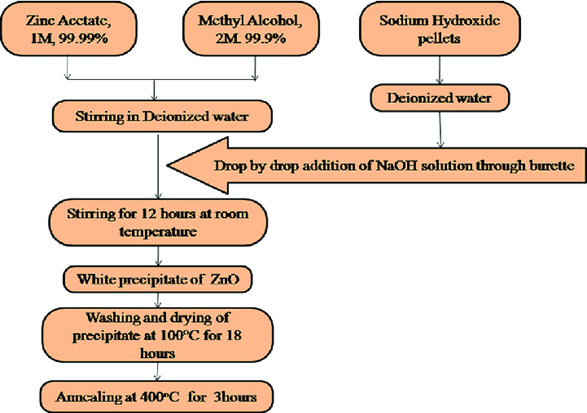
Micro-emulsion, chemical reduction, precipitation, hydrothermal techniques, and sol-gel are the most common chemical methods of synthesizing Zinc oxide nano-particles [16]. Among these methods, the sol-gel synthesis is the most commonly used method, which uses chemical reagent and zinc precursor salt for the regulation of the solution pH and prevent precipitation of Zn(OH)2. Thereafter, the solution is treated thermally under high temperatures to obtain Zinc oxide nano-particles [17, 18]. Stabilizers like citrates or polyvinylpyrrolidone are usually added during synthesis to control the morphological properties and to prevent agglomeration of Zinc oxide nano-particles[19]. The concentration of zinc precursor and other reagents used during synthesis have been reported to significantly affect the shape and size Zinc oxide nano-particlesproduced. Variation in concentration of zinc precursor and reagent has been utilized in obtaining Zinc oxide nano-particleswith sizes in the range of nanometers to micrometer [20]. However, the chemical method suffers some limitations because they require high energy, toxic reagents, and expensive equipment. Findings have shown that traces of toxic reagent used during synthesis are detected in synthesized nanoparticles which could be hazardous and also limit its applications [21]. The procedures for the chemical methods of Zinc oxide nano-particles synthesis is showed in Figure. 1.


 Shruti S. Jadhav*
Shruti S. Jadhav*
 Dr. Mohini A. Salunke
Dr. Mohini A. Salunke
 Mohammad Zishan Ibrahim
Mohammad Zishan Ibrahim

 10.5281/zenodo.13151621
10.5281/zenodo.13151621