Transdermal drug delivery systems may be absorbed directly into the systemic circulation and do not require liver metabolism before working, they have emerged as one of the most effective controlled drug release systems in topical formulation. To improve the transdermal drug delivery system's efficacy in regulating drug release, polymers are incorporated into the formulation. The TDDS's use of polymers has slowed the drug's controlled release rate from the patch. Combining polymers has increased the medication release's effectiveness. A thorough explanation of the application of polymers in the transdermal medication delivery system is provided in this article. Additionally, polymers aid in the medicine's skin-sticking properties and encourage a greater degree of drug release from the dosage form. Recent advances have led to a rise in the quantity and kind of polymers utilised in TDDS. The usage of synthetic, semisynthetic, and natural polymers in TDDS is discussed in this article. Natural polymers have been found to have a greater influence on TDDS and to have fewer side effects, such as allergy and irritation. As a result, patient compliance has increased and medication release has been more effectively achieved. Artificial and semisynthetic order to effectively manage the release of the medication from the patches, polymers have been created and used extensively in TDDS patches. A transdermal patch is a medicated adhesive patch that is applied above the skin to release a predetermined amount of medication into the bloodstream at a predetermined rate through the skin.
Stratum corneum, Polymers, Controlled release, Hydrogels, TDDS.
a) Natural polymers: Natural polymers are derived from either plants or animals. We refer to them as animal and plant polymers. Examples include: cellulose, jute, leather, silk, wool, R&A, DNA, and natural rubber.
b) Semisynthetic polymers: These are polymers made from natural fibres that are generated through a straightforward chemical process to enhance their physical characteristics, such as tensile strength and lastrus nature.
For example, viscous rayon, cupra ammonium silk, and acetate rayon.
c) Synthetic fibres: In a lab, synthetic fibres are created by polymerising basic chemical molecules.
For example, nylon, terylene, polyethene, polystyrene, nylon, PVC, backlite, Teflon, Orion, and synthetic rubber.
2. The classifications based on the structure are three types of polymers as follows:-
a) Linear polymers: These polymers consist of a long, straight chain formed by the links between the monomers. There are no side chains in these chains. Their molecules have a high melting temperature, tensile strength, and density due to their close packing.
For example, polyesters, Nylons, PVC, and polyethene.
b) Branched polymers: They have a straight long chain with different side chains. Their molecules are irregularly packed hence they have low density, tensile strength and melting point.
Ex. Polypropylene (side chain -CH3), amylopectin and glycogen
c) Network or crosslinked polymers: In these monomeric units are linked together to constitute a three dimensional network. The links involved are called cross links. They are hard, rigid and brittle due to their network structure.
Ex. Bakelite, Maia mine, formaldehyde resins, vulcanized rubber etc.
3. The classifications based on polymerization process are two types as follows:-
a) Addition polymers: These are polymers created by continuously adding monomers without removing the byproducts in between. These polymers are integral multiples of the monomer unit because they contain all of the monomer atoms.
Examples include PVC, Teflon, polyethene, and polypropylene. Typically, alkenes and their derivatives serve as the monomeric units.
b) Condensation polymers: These are created when two monomers are combined and tiny molecules like NH3, alcohol, or water are removed. Their molecules are linked by amide and ester bonds. They do not have an integral multiple of monomer units in their molecular mass.
Ex: Polyesters, polyurethanes, and polyamides (Nylons)
4. The classification based on molecular forces:-
a) Elastomers: These are polymers in which the weakest attractive forces maintain the integrity of the polymer chains. They consist of sparsely cross-linked, randomly coiled molecular chains. The polymer stretches when the stain is applied, then returns to its original place when the force is released. These polymers, often known as elastomers, are elastic.
For example, vulcanised rubber and neoprene
a) Fibers: They have high intermolecular attractive force like H-bonding. They have high tensile strength and used in textile industries.
Ex. Nylon-6, Nylon-66, and Terylene
c) Thermoplastic polymers: These are the polymers having intermolecular forces between elastomers and fibers. They are easily molded in desired shapes by heating and subsequent cooling at room temperature. They may be linear or branched chain polymers. They are soft in hot and hard on coding.
Ex. Polythene, Polystyrene, PVC
d) Thermosetting polymers: When heated, these polymers become rigid and infusible. These do not remould when heated under pressure and do not become pliable. These polymers are cross-linked and are not reusable.
For example, Bakelite
5. The classification based on the homogeneity of Polymers: Pectin homopolymers and copolymers.
a) Homopolymers consists of only one type of repeating unit.
b) Copolymers are polymers consisting of more than one type of repeating unit.
6. The classification based on growth polymerization, implies two types:
a) Chain growth polymerisation: In this type of polymerisation, molecules are added across the double bond at the reactive end of the expanding chain. Growth chain polymerisation is a common process for alkenes and their derivatives. For example, polyethene
b) Step growth polymerisation: This kind of polymerisation uses a sequence of independent reactions to cause step-wise intermolecular condensation. Losses of simple molecules like NH3, H2O, and HCl occur during this process. When a monomer has many functional groups, it is feasible. It continues by forming trimmers, tetramers, dimers, etc. For example, Dacron.
7. The classification based on bio-stability:
a) Biodegradable: Once the active agent has been released, the biodegradable polymer breaks down naturally in the body through biological processes, without the need to remove a drug delivery system. The majority of biodegradable polymers are made to hydrolyse into increasingly smaller, physiologically acceptable molecules when the polymer chains break down. Additionally WO ss He Water cannot dissolve biodegradable polymers because they are hydrophobic. On the other hand, they experience hydrolysis and fragment into smaller pieces. Despite not being soluble in water, they can be broken down by the body. The degradation products are commonly employed in controlled medication delivery because they are biocompatible. For example, polyanhydrides, polyorthoesters, polylactides (PLA), polyglycolides (PGA), and poly(lactide-co-glycolides) (PLGA).
a) Non-biodegradable: Since these polymers don’t erode or break down, they must be cleared out of the system as soon as the medicine has completely released from them. Example: Polymethacrylates
HISTORY
• Pharmaceutical and medical product compositions frequently include polymers. Polymers have long been used in the medical industry. For decades, natural polymers and synthetic polymers have been utilised in herbal medicines; nonetheless, the issue is complex. The initial research in the 1960s focused on the use of polymers as wound dressings, injectable or implanted deporters, and blood plasma expanders. In 1975, Helmut Ringsdorf presented the first design for polymers with pharmacological effects.
• The first polymer-drug conjugation to treat cancer was tested in a clinical setting in 1994. It was a Doxorubicin copolymer, or HPMA (N 2-hydroxy propyl meth) acrylamide).
• Five years after the first therapeutic nanoparticles were approved as a treatment for metastatic breast cancer, two polymer-protein conjugates were introduced to the market: PEG-GCSF and PEG-interferon-u, an antiviral medication used to treat chronic hepatitis C and hepatitis B (3).
• Advantage:
1.Controlled medication release: The release rate of pharmaceuticals may be precisely regulated thanks to polymers, which produces therapeutic benefits that are both maintained and controlled while lowering the frequency of dosage requirements.
2.Enhanced solubility and stability of poorly soluble drugs can be achieved using polymers, hence improving their bioavailability and efficacy.
3. Tailored delivery: polymers allow for the administration of drugs to particular cells, tissues, or organs in a tailored manner, reducing adverse effects and optimising therapeutic results
4.Lessened toxicity: some medications’ toxicity can be decreased by encasing them in polymers. Enhancing their security profile.
5.Minimisation of dosage frequency: The development of an extended-release polymer can lessen the frequency of management, enhancing adherence of patients
6.Benefits of the transdermal method include the avoidance of gastrointestinal absorption issues and hepatic pass effect, dosage reduction, and dosage interval.
7. Increases patience compliance and lengthens the activity’s duration.
8. Prompt removal from the skin
9. Using topical patches is a non-invasive, painless technique to send drugs straight to the body.
10. These systems enable self-administration.
11. Systemic medication interactions are decreased.
12. It provides a longer action duration.
• Disadvantages:
1.High cost: creating a polymer-based simulation can be expensive, particularly when working with new polymers or intricate delivery networks.
2. Complex formulation: Creating drug delivery systems based on polymers can be difficult and call for specific expertise in material science and polymer chemistry.
3.Difficulties with regulations Obtaining regulatory approval for innovative polymer-based drug delivery systems can present difficulties because of worries about long-term impacts, safety, and biocompatibility.
4. Potential innsnogenicity: Certain polymers, particularly those obtained from natural sources, may cause immunological reactions in certain people.
5. Variable release profiles: It might be difficult to achieve constant and predictable medication release rates from polymer matrixes because of things like polymer breakdown and physiological state fluctuation.
•Physiology of skin:
The human skin is the largest and most accessible organ in the body. It has been utilised as a location for pharmaceutical drug administration.
Transdermal delivery is the process of delivering a medication via healthy skin to the systemic circulation.

Fig: Structure and layers of the skin
? Pathways for transdermal drug delivery systems:
Drug penetration into the skin can happen in a variety of ways when it is administered to the skin’s surface.
?Membrane penetration controlled system.
?Matrix diffusion controlled systems.
?Adhesive dispersion type system.
?Micro reservoir type diffusion system.
Drugs may enter through the appendages or the stratum corneum.
There are two potential pathways for penetration into the stratum corneum:
- Penetration through corneocytes and Transcellular route.
2. Permeation through the gaps between the cells.
The Transdermal Drug Delivery System (TDDS) uses the following polymers:
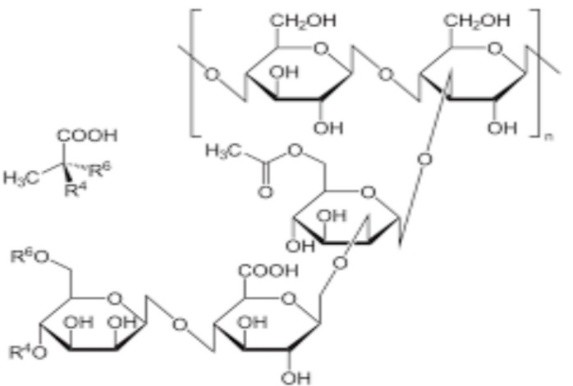
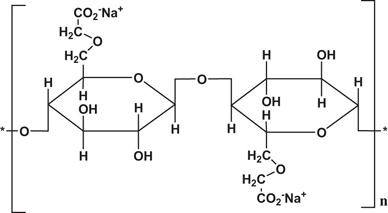
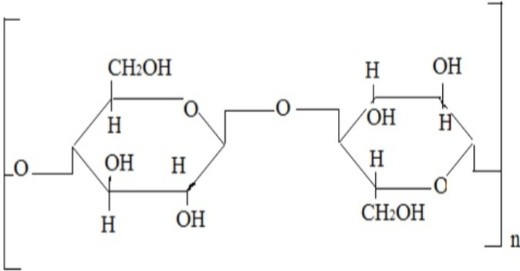
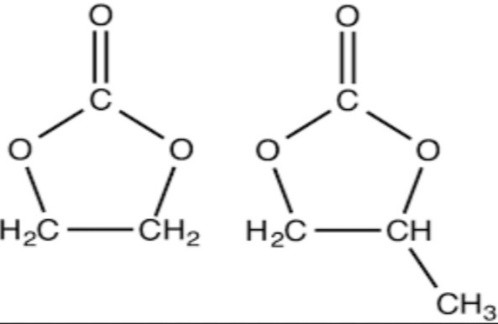
Natural Polymers for Transdermal Medication Administration: Predefined drug delivery rates can be attained by the use of natural polymers. Since natural polymers are hanically polysaccharides, they have low toxicity, are biodegradable, and are highly friendly with humans. For example: Chitosan, Sodium CMC, Xanthan Gum, and Sodium Alginate. Natural rubber.
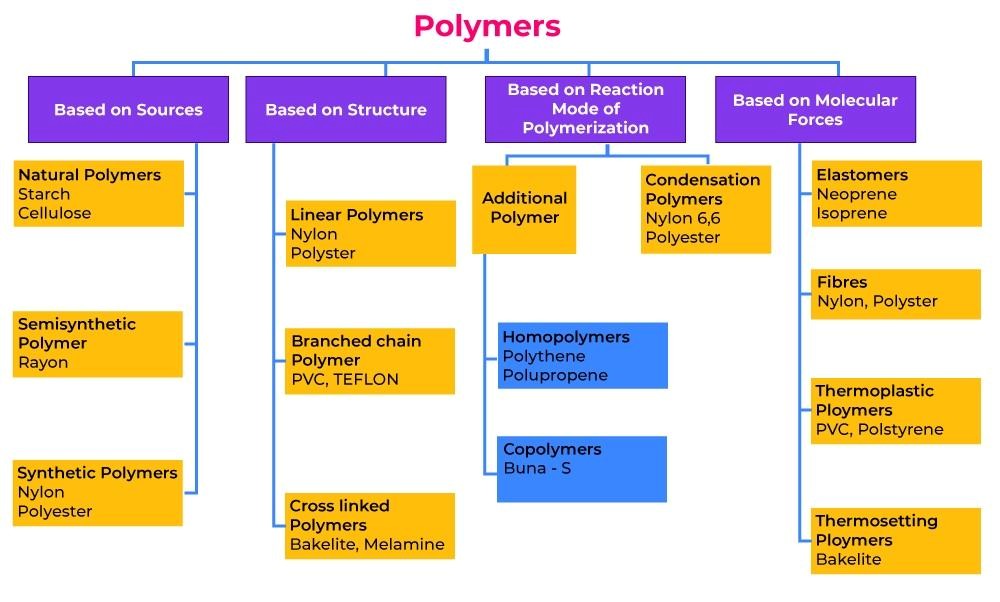
1. Xanthan Gum:
The fermentation of Xanthomonas campetrix, which is present in the plant’s leaves and other green sections, produces xanthan gum.
° It is a polymer with a high molecular weight that contains sugars such as d-glucose and d-mammose.
° XG has high stability in both acidic and alkaline medium.
° The rate of release of drug can be controlled by changing the pH of the release medium.
° The regulated release of medications in TDDS is enhanced and increased when XG is combined with other polysaccharides.
° XG possesses excellent muco adhesive properties, which are crucial for formulations like TDDS.
° Extended drug release of 98.f65% over a 12-hour period is demonstrated with XG-based patches.
° Utilising clove oil, PEG-400, and tween 80 in the formulation of the Topical nanoemalgel, XG has employed it.


 Sayali Jadhav*
Sayali Jadhav*
 Ulka Mote
Ulka Mote














 10.5281/zenodo.14216276
10.5281/zenodo.14216276