Abstract
This review explores the potential applications of liposomes as a drug delivery system, focusing on their structure, formulation methods, advantages, limitations, and recent breakthroughs. It also discusses the diverse range of drugs and therapeutic agents that can be encapsulated within liposomes and their clinical applications in targeting specific diseases. The review provides an in-depth analysis of liposome-based drug delivery.
Keywords
Liposome; Controlled Release; Targeted Drug Delivery; Novel Delivery.
Introduction
Liposomes, derived from the Greek words 'Lipos' meaning fat and 'Soma' meaning body, are spherical concentric vesicles that enclose a water droplet, particularly artificially used to carry drugs into tissue membranes. These round sac phospholipid molecules, which are nanoparticles (100nm in size), have potential therapeutic properties and are used in various fields such as drug delivery, cosmetics, and biological membrane structure. Liposomes are a tiny bubble with a membrane composed of a phospholipid bilayer, typically made of phospholipids like phosphatidylet- hanolamine and phosphatidylcholine. These phospholipids are amphiphilic with a hydrophilic polar head and a hydrophobic hydrocarbon tail. Their discovery by Bangham in 1961 led to the development of liposomes as a potential carrier for various drugs.

Figure 1 Basic Liposome Structure
Structure of liposomes:
Phospholipids
- Naturally occurring phospholipids used in liposome:
- Phosphatidylethanolamine
- Phosphatidylcholine
- Phsphatidylserine
- Synthetic phospholipids used in the liposomes are:
- Dioleoyl phosphatidylcholine
- Disteroyl phosphatidylcholine
- Dioleoyl phosphatidylethanolamine
Cholesterol
Liposomes, derived from the Greek words 'Lipos' meaning fat and 'Soma' meaning body, are spherical concentric vesicles that enclose a water droplet, particularly artificially used to carry drugs into tissue membranes. These round sac phospholipid molecules, which are nanoparticles (100nm in size), have potential therapeutic properties and are used in various fields such as drug delivery, cosmetics, and biological membrane structure. Liposomes are a tiny bubble with a membrane composed of a phospholipid bilayer, typically made of phospholipids like phosphatidylet-hanolamine and phosphatidylcholine. These phospholipids are amphiphilic with a hydrophilic polar head and a hydrophobic hydrocarbon tail. Their discovery by Bangham in 1961 led to the development of liposomes as a potential carrier for various drugs.
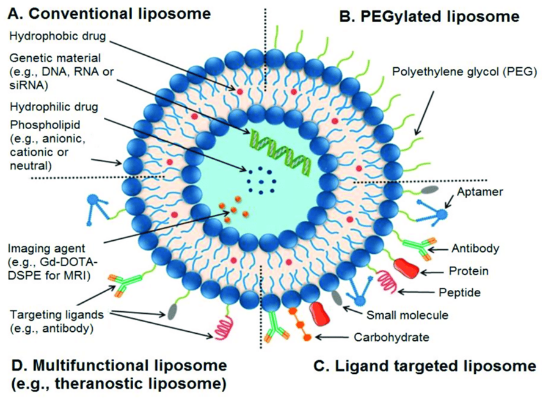
Figure 2 An illustration of liposome and its structural components
Advantages of Liposomes:
- Liposomes significantly enhance vaccine development by enhancing the stability and delivery of antigens, thus enhancing immune responses.
- Liposomes enable targeted drug delivery to specific tissues or cells, minimizing exposure to healthy tissues and reducing side effects.
- Liposomes can provide sustained therapeutic effects by gradually releasing drugs over time, reducing the need for frequent dosing.
- Liposomes enhance the bioavailability and solubility of water-soluble drugs, a crucial factor in drug effectiveness.
- Protection of Sensitive Compounds: Liposomes can protect sensitive drugs or bioactive compounds from degradation due to environmental factors, such as enzymes, pH changes, or oxidation.
- They can be customized in terms of size, composition, and surface modifications to enhance their performance for specific drugs or therapeutic applications.
- Researchers can modify the properties of liposomes, including size, charge, and surface functionality, to suit specific applications.
- Immunogenicity: Liposomes can enhance the immunogenicity of vaccines, resulting in a stronger and more specific immune response.
- Diagnostic Applications: Liposomes are used in diagnostic assays for drug screening, disease detection, and other diagnostic purposes.
- Biocompatibility: Liposomes are generally well-tolerated by the body, making them suitable for various medical and cosmetic applications.
Disadvantages of Liposomes:
- Liposome production can be challenging and costly, potentially limiting their widespread use in large-scale pharmaceutical manufacturing.
- Uniformity: Achieving uniformity in liposome size and composition can be difficult, affecting their performance and reproducibility.
- Compatibility Issues: Some drugs may not be suitable for encapsulation in liposomes due to compatibility issues, limiting the range of drugs that can benefit from liposomal delivery.
- Regulatory Approval: Obtaining regulatory approval for liposomal drug products can be a complex and time consuming process, adding to the development timeline and cost
- Niche Applications: Liposomes may not be suitable for all drug delivery needs, and alternative delivery systems may be preferred in certain cases.
- Short Circulation Half-Life: Liposomes can be rapidly cleared from the bloodstream by the body's immune system, limiting their time window for drug delivery.
- Storage Stability: Liposomes can be prone to instability during storage, leading to aggregation, leakage of encapsulated substances, or changes in size and structure
- Expense: Producing liposomal formulations can be costly, which may lead to higher drug prices for liposome based therapies.
- The intricate nature of liposomal formulation development necessitates specialized expertise, potentially limiting the accessibility of these products to researchers and manufacturers.
- Liposomes' biodegradability may vary depending on their composition, raising environmental concerns due to their potential for non-reversible degradation.
Classification of Liposomes:

Figure 3 Classification of Liposomes
Mechanism of formation of Liposomes:
Liposome performs their motion by four distinct Mechanism-
- Endocytosis – This take location via phagocytic cells of reticuloendothelial system together with neutrophils.
- Adsorption – It occurs to the cellular surface through non precise electrostatic forces or by using interplay with cell surface additives.
- Fusion- It takes place by means of the insertion of liposomal bilayer into plasma membrane with continuous release of liposomal content into the cytoplasm.
- Lipid exchange- on this transfer of liposomal lipids to the cellular membrane without association of liposomal contents.
Method of preparation
The methods can be classified broadly into two categories: mechanical dispersion methodsand solvent dispersion methods.
1. Mechanical Dispersion Methods
a) Thin-Film Hydration (Bangham Method)
This is the most common and simple method of preparing liposomes.
Process:
1. Dissolve phospholipids in a volatile organic solvent (e.g., chloroform or methanol).
2. Remove the solvent by rotary evaporation to form a thin lipid film on the walls of a round-bottom flask.
3. Hydrate the lipid film by adding an aqueous buffer (e.g., PBS) with gentle agitation, which leads to the formation of multilamellar vesicles (MLVs).
4. Subject the MLVs to further processing (e.g., sonication, extrusion) to obtain smaller unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs).
Applications
Widely used for basic research, encapsulation of both hydrophilic and hydrophobic drugs.
b) Sonication
This method reduces the size of liposomes (MLVs) prepared by the thin-film hydration method.
Process:
- The MLV suspension is sonicated using either a probe-type or bath-type sonicator.
2. The mechanical energy breaks the larger vesicles into smaller SUVs (20-100 nm).
Applications:
Suitable for forming small liposomes, but sonication can lead to degradation of phospholipids and encapsulated drugs.
c) Extrusion
Extrusion is used to achieve uniform liposome size by forcing them through polycarbonate membranes with specific pore sizes.
Process:
1. Pass the MLV suspension through membranes under pressure.
2. This produces LUVs with a more uniform size distribution.
Applications:
Preferred for producing large, uniform liposomes for drug delivery.
2. Solvent Dispersion Methods
a) Ethanol Injection Method
In this method, lipids dissolved in ethanol are injected rapidly into an aqueous solution.
Process:
1. Dissolve lipids in ethanol.
2. Inject the ethanol solution into an aqueous phase under rapid stirring.
3. The lipid molecules self-assemble into
liposomes due to the sudden change in solvent
polarity.
Applications:
Simple and quick, but the presence of residual ethanol can be a limitation.
b) Reverse-Phase Evaporation Method (REV)
This method is useful for encapsulating a large volume of aqueous solution into liposomes.
Process:
1. Dissolve phospholipids in an organic solvent (e.g., ether).
2. Add an aqueous phase and form a water-in-oil emulsion by sonication.
3. Evaporate the organic solvent under reduced pressure, which causes the emulsion to collapse into liposomes.
Applications:
Useful for encapsulating large aqueous volumes and proteins.
c) Solvent-Spherule Method
This method involves dissolving lipids in an organic solvent, which is then emulsified into an aqueous solution.
Process:
1. Lipids are dissolved in an organic solvent such as chloroform.
2. The lipid solution is then emulsified into an aqueous solution.
3. Upon removal of the organic solvent, liposomes are formed.
3. Detergent Removal Methods
This method involves the formation of liposomes by the removal of detergents that solubilize lipids.
a) Dialysis
Process:
1. Phospholipids are first dissolved in a detergent solution (e.g., Triton X-100).
2. The detergent-lipid micelle solution is placed in a dialysis bag and dialyzed against a detergent-free buffer.
3. As the detergent is gradually removed, liposomes form spontaneously.
Applications:
Suitable for producing high-quality liposomes without shear stress.
Marketed formulations of Liposomes:
|
Brand Name
|
Drug
|
Indication
|
Type of Liposome
|
|
|
|
Breast cancer, Ovarian cancer, AIDS-related Kaposi's sarcoma
|
PEGylated Liposome
|
|
|
Amphotericin B
|
Fungal infections, Leishmaniasis
|
Liposomal Amphotericin B
|
|
DepoDur
|
Morphine sulfate
|
Post-operative pain management
|
DepoFoam technology (multi-vesicular)
|
|
Marqibo
|
Vincristine sulfate
|
Acute lymphoblastic leukemia (ALL)
|
Sphingomyelin/cholesterol-based liposome
|
|
Onivyde
|
IRinotecan
|
Metastatic pancreatic cancer
|
Liposomal formulation
|
|
Vyxeos
|
Daunorubicin and Cytarabine
|
Acute myeloid leukemia (AML)
|
Dual-drug liposome formulation
|
|
Visudyne
|
Verteporfin
|
Age-related macular degeneration (AMD), Pathologic myopia
|
Liposomal formulation
|
|
Myocet
|
|
Metastatic breast cancer
|
Non-PEGylated liposomal doxorubicin
|
|
DepoCyt
|
Cytarabine
|
Lymphomatous meningitis
|
Sustained-release liposomal formulation
|
|
MEPACT
|
Mifamurtide
|
Non-metastatic osteosarcoma
|
Liposomal formulation
|
|
Inflexal V
|
Influenza vaccine
|
|
|
Liposomal adjuvant vaccine
|
|
Evaluation of Liposomes:
1. Particle Size and Size Distribution
Particle size is crucial as it influences the circulation time, tissue distribution, and cellular uptake of liposomes.
- Dynamic Light Scattering (DLS): Measures the hydrodynamic diameter of liposomes in solution and provides a size distribution profile.
- Electron Microscopy (TEM/SEM): Provides direct visualization of liposome size and shape.
- Nanoparticle Tracking Analysis (NTA): Measures the size and number of individual particles based on Brownian motion.
- importance: Small liposomes (<100>
2. Zeta Potential (Surface Charge)
Zeta potential measures the surface charge of liposomes and is critical for predicting the stability of the formulation.
- Zeta Potential Analyzer: Uses electrophoretic light scattering to measure the charge on the surface of liposomes.
- Importance: A highly positive or negative zeta potential (? ±30 mV) indicates good electrostatic stability and prevents aggregation due to repulsion forces. Neutral or slightly charged liposomes may aggregate over time.
3. Encapsulation Efficiency (EE%)
Encapsulation efficiency evaluates the percentage of drug that is successfully encapsulated within the liposome.
- Ultracentrifugation/Dialysis: Separates free drug from liposome-encapsulated drug.
- HPLC or UV Spectroscopy: Used to quantify the amount of encapsulated drug after separation.
- Importance: High encapsulation efficiency is desirable for drug delivery to minimize wastage and enhance therapeutic efficacy.
4. Drug Release Profile
Evaluating the release profile of the drug from liposomes is crucial to understand the kinetics and ensure controlled delivery.
- In vitro Release Studies: Liposomes are incubated in conditions mimicking physiological environments (pH, temperature) and sampled over time to measure drug release.
- HPLC or UV Spectroscopy: Quantifies drug release at specific time points.
- Importance: The release profile must be controlled to prevent burst release and ensure sustained delivery at the target site.
5. Morphology and Lamellarity
The morphology and number of bilayers (lamellarity) affect encapsulation, release, and the interaction of liposomes with biological membranes.
- Cryo-TEM: Visualizes liposome structure in near-native conditions, including lamellarity.
- Freeze-Fracture Electron Microscopy: Provides detailed images of liposome bilayers.
- Importance: Multilamellar vesicles (MLVs) offer different drug release kinetics compared to unilamellar vesicles (SUVs), influencing therapeutic action.
6. Stability
Stability studies assess the physical and chemical integrity of liposomes over time under various conditions.
- Size and Zeta Potential Monitoring: Monitors changes in liposome size or charge to detect aggregation or instability.
- Oxidation and Hydrolysis Testing: Measures degradation of lipids, especially oxidation of unsaturated fatty acids.
- Temperature Stability Studies: Liposomes are subjected to different temperatures to assess shelf-life.
- Importance: Stability is critical for ensuring that liposomes retain their therapeutic properties during storage and transport.
7. Pharmacokinetics and Biodistribution
Evaluating the pharmacokinetics and biodistribution of liposomes is crucial to understand their in vivo behavior, including circulation time, tissue targeting, and clearance mechanisms.
- Fluorescence or Radioactive Labeling: Liposomes are labeled with fluorescent or radioactive markers to track their distribution in animal models.
- Blood Sampling and Tissue Analysis: Quantifies the amount of liposomes in the bloodstream and various organs over time.
- Importance: PEGylated liposomes, for example, have prolonged circulation times due to reduced recognition by the reticuloendothelial system (RES).
Application for Liposomes
Drug Delivery:
· Liposomes are commonly used as drug delivery vehicles to encapsulate and deliver both hydrophobic and hydrophilic drugs.
· They can improve drug solubility, stability, and bioavailability.
· Liposomal drug formulations can target specific tissues or cells, reducing systemic side effects.
Vaccines:
· Liposomes are used as adjuvants or carriers for vaccines to enhance immunogenicity.
· They can improve antigen delivery to immune cells, leading to a stronger immune response.
Cosmetics and Skincare:
· Liposomes are utilized in cosmetics and skincare products for controlled release of active ingredients, such as vitamins and antioxidants.
· They can enhance the penetration of ingredients into the skin, improving their efficacy.
Gene Delivery
· Liposomes can be used to deliver genetic material, including DNA and RNA, for gene therapy applications.
· They protect and facilitate the transport of genetic cargo into target cells.
Diagnostics:
· Liposomes can serve as carriers for contrast agents in medical imaging, such as magnetic resonance imaging (MRI) and ultrasound.
· They enable targeted imaging of specific tissues or cells.
Cancer Therapy
· Liposomal formulations of chemotherapy drugs, like Doxil (liposomal doxorubicin), are used to treat cancer.
· They can improve drug circulation time and reduce damage to healthy tissues.
Food Technology
· Liposomes are applied in the food industry for encapsulating and protecting sensitive ingredients, such as vitamins, flavors, and antioxidants.
· They can improve the stability and bioavailability of these additives in food products.
Biotechnology
· Liposomes are used in research and biotechnology applications for drug screening and delivery to cells in vitro.
· They are valuable tools for studying cell membrane interactions and drug transport mechanisms.
Transdermal Drug Delivery:
· Liposomal formulations can be applied topically to deliver drugs through the skin.
· They offer controlled release and can avoid the first-pass metabolism in the liver.
Personal Care Products
· Liposomes are employed in personal care products such as sunscreens and moisturizers to enhance the delivery of active ingredients.
Veterinary Medicine
· Liposomes are used in veterinary medicine for drug delivery to animals, similar to their applications in human medicine.
Environmental Remediation:
· Liposomes can be utilized for the controlled release of remediation agents in environmental cleanup efforts.
Intracellular Delivery:
· Liposomes are valuable tools in research for delivering molecules into specific organelles within cells.
Nutraceuticals
· Liposomes are used to enhance the bioavailability of nutraceutical compounds in dietary supplements.
Wound Healing
· Liposomal formulations can be applied to wound dressings to promote the controlled release of wound-healing agents.

Figure 4 Application for Liposomes
Recent Approaches in Liposome Research
Below are some of the prominent approaches:
1. Targeted Liposomal Delivery Systems
- Ligand-conjugated liposomes: Recent studies have focused on attaching ligands (e.g., antibodies, peptides, or small molecules) to the surface of liposomes to target specific cells or tissues. This approach enhances drug accumulation at the desired site, minimizing off-target effects. For example, HER2-targeting liposomes for breast cancer treatment are being developed using trastuzumab as a ligand.
- pH-sensitive liposomes: These are designed to release their payload in response to the acidic environment of tumors or intracellular compartments (such as endosomes and lysosomes). Such liposomes remain stable in the bloodstream but release their contents once they encounter a lower pH.
- Stimuli-Responsive Liposomes
•Thermo-sensitive liposomes: Liposomes that release their payload in response to increased temperature have gained traction. These formulations can be used in combination with hyperthermia (heat therapy) to trigger localized drug release at tumour sites
•Magnetic liposomes: Incorporating magnetic nanoparticles into liposomes allows for drug delivery under the guidance of an external magnetic field. This approach helps concentrate the therapeutic agent at the disease site while minimizing systemic exposure.
•Ultrasound-responsive liposomes: These formulations allow for controlled drug release using ultrasound waves, which can non-invasively trigger liposomal drug release in specific tissues.
3. Immunoliposomes
Immunoliposomes, which are antibody-conjugated liposomes, are designed for targeted drug delivery to cancer cells or other disease-specific sites. By attaching monoclonal antibodies to the surface, these liposomes can specifically bind to antigens overexpressed in certain diseases, particularly cancers.
Example: Anti-CD19 immunoliposomes for targeted therapy of B-cell malignancies.
4. Liposomal Vaccines
Liposomes are now being explored as carriers for vaccines. They offer protection of the antigen, ensure slow release, and enhance immune responses. Some COVID-19 vaccine candidates have utilized liposomal technology to deliver mRNA effectively.
Example: The development of liposomal mRNA vaccines for infectious diseases such as COVID-19, which leverage lipid nanoparticles for encapsulating mRNA, as seen with the Pfizer-BioNTech and Moderna vaccines.
5. Liposomal Gene Therapy
Liposomes are being utilized to deliver gene-editing tools like CRISPR-Cas9 to target specific genes in diseases. Cationic liposomes, which carry a positive charge, are especially effective in encapsulating and delivering negatively charged nucleic acids (like DNA or RNA) to cells.
CONCLUSION
Liposomes are an innovative drug delivery system with potential applications in pharmaceuticals. Research has shown their ability to overcome challenges from traditional methods, enhancing therapeutic efficacy and safety. Despite challenges, continued innovation in liposomal technologies holds great promise for the future of drug delivery in the pharmaceutical industry. Liposomes offer a versatile approach to drug delivery, improving efficacy, reducing side effects, and enabling precise therapy targeting. Further advancements in liposomal technology are expected to expand their use in various medical applications.
REFERENCES
- 777


 Aarti Nimse *
Aarti Nimse *
 Dr. Sachin Kale
Dr. Sachin Kale
 Rutuja Giram
Rutuja Giram
 Ashvini Kakad
Ashvini Kakad




 10.5281/zenodo.14582120
10.5281/zenodo.14582120