Abstract
Objective:
The study's objective is to evaluate and compare the oral Montelukast sodium tablet 10 mg quality control standards of the different brands of pharma companies which are mostly prescribed in the surrounding area by medical practitioners.
Method:
All four brands are named as A, B, C and D respectively taken. In vitro quality control test like Physical appearance, Thickness test, Weight variation test, Hardness test, Friability test, In vitro Disintegration test, In vitro Dissolution test using USP Paddle II method and analyzed by UV Spectrophotometer of each brands are performed.
Result & discussion:
To determine the quality of the tablets, the quality control standards of four distinct brands of Montelukast tablets were analysed and tested. Quality control tests are carried out to ensure that brands A, B, C, and D meet specified characteristics. These tests include weight variation, hardness, thickness, friability, disintegration, and dissolution. The Brand A & Brand B are film coated tablet, comparing with their dissolution profile it was that Brand B(99.2%) has shown good drug release rather than Brand A(96.24%) but both are complying their standards of drug release according to Indian Pharmacopeia. Brand C & Brand D are Un-coated tablet, comparing with their dissolution profile it was that Brand D (99.52%) has shown good drug release rather than Brand C (96.46%) but both are complying their standards of drug release according to Indian Pharmacopeia.
Conclusion:
The pharmaceutical industries produce Montelukast tablets that meet Indian Pharmacopoeia standards and are generally of uniform quality, with minimal difference amongst them.
Keywords
Montelukast sodium, Quality control testing, Comparative Analysis, Dissolution study, % Drug release etc.
Introduction
Montelukast sodium is a medication primarily used to manage asthma and allergic rhinitis (hay fever). It belongs to a class of drugs known as leukotriene receptor antagonists which function by blocking leukotriene’s, substances in the body that contribute to inflammation and allergic reactions. Montelukast sodium is available in tablet, chewable tablet, and granule forms1. It is often prescribed as a maintenance treatment to control asthma symptoms and to prevent asthma attacks triggered by exercise or allergens. Additionally, it can alleviate symptoms such as sneezing, runny nose, and nasal congestion associated with allergic rhinitis2. Montelukast sodium is typically taken orally once daily, with or without food, as directed by a healthcare professional. It's crucial to follow the prescribed dosage and usage instructions for optimal effectiveness and safety. The FDA has approved the oral dose of Montelukast, which comes in film-coated, chewable, and oral granule forms. It is used to treat chronic asthma, prevent exercise-induced bronchoconstriction, and for prophylaxis. It is also authorized to treat the symptoms of both seasonal and ongoing allergic rhinitis3. One medicine used in the maintenance treatment of asthma is Montelukast, which is marketed under several brand names, for this purpose, it is typically less recommended than inhaled corticosteroids. When an acute asthma attack occurs, it is ineffective; other uses include protracted hives and allergic rhinitis. Treatment for allergic rhinitis is basic .It is mostly prescribed in India for the allergic and asthmatic conditions and breathing problems .It helps in breathing easier by reducing inflammation in the respiratory
tract4. The IUPAC name for Montelukast sodium is: Sodium (R)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2yl)phenyl)propylthio)methyl)cyclopropyl)acetate. The molecular formula is C35H36CINO3-S. The molecular weight of Montelukast sodium is approximately 608.18 g/mol. It belongs to class Leukotriene receptor antagonist (CysLT1 receptor blocker). The Montelukast sodium molecule's structure is represent in Fig 15-6:

Fig 1: Structure of Montelukast sodium.
OBJECTIVE OF STUDY
In the pharmaceutical sector, complete product quality assurance is necessary to prevent the use of products that do not adhere to the standards and guidelines outlined in the Indian Pharmacopoeias. There is a possibility that mistakes will be made during the production process, thus it is important to manage this possibility. In order to assure the quality of the products, extensive quality control tests must be conducted. Pharmaceutical quality control places an intense focus on evaluating the product in a variety of ways to identify potential defects in manufacturing. The study's objective is to evaluate and compare the oral Montelukast sodium tablet 10 mg quality control standards of the different brands of pharma companies which are mostly prescribed in the surrounding area by medical practitioners.
MATERIAL AND METHODS
Materials
For comparative evaluation of Montelukast sodium tablets (10 mg) 4 tablet brands of different phrama companies are purchased from the local market i.e. Montu 10, Montek 10, Montemac 10, Montelet 10 and randomly coded A, B, C and D respectively. List of different brands of Montelukast tablets and their type are shown in Table 1.

Table 1: List of different brands of Montelukast tablets & their Type
Chemicals
The chemicals used in study as per the monograph of Montelukast Tablets as follows
- Sodium lauryl sulphate (SLS) – Media for Disintegration & Dissolution study
Instruments
List the instruments utilised for this investigation are in Table 2.

Table 2: Instruments used during the comparative study
Methods
The four brands, labeled A, B, C, and D respectively, underwent quality control tests outlined as follows during the study.
- Physical Appearance
- Weight variation test
- Thickness test
- Hardness test
- Friability test
- Disintegration test
- Preparation of Standard Calibration curve
- Dissolution test performed with the USP Paddle II technique and examined with a UV Spectrophotometer.
- Physical Appearance
This test of physical appearance was performed by visual inspection. In that color, odor, surface texture, shapes etc.7
- Weight variation test
To determine whether the tablet weights are uniform, a weight variation test is conducted. The number of tablet particles that contain the indicated amount of the medicinal component is the tablet's weight. The weights of the pills are frequently tested after they are made to make sure they are the desired weight. Twenty tablets were taken and accurately weighed. The standard weight of a single tablet was then calculated as the average weight. Every tablet's weight was recorded individually, and it was noted if each tablets fell within the range or not 8.The standard limit of the weight variation are shown in Table 3.

Table 3: Standard specifications of weight variation tests
- Thickness
The thicknesses of the tablet of different brands are determined by the digital vernier calliper at the middle position of Tablet 8.
4. Hardness test
Using a hardness tester, this test is conducted to determine the strength of tablets. Tablets that are too soft may crumble in transit. Excessively firm tablets may also cause an issue since they might harm teeth and take longer for the body to break down. For the goals of both quality control and research and development of new formulations, an acceptable hardness and tablet strength testing are required. The sliding scale of the hardness tester was initially calibrated at zero. The tablet was then placed in digital hardness tester. To measure the tablet's hardness, the tester's spring and screw thread were twisted until the tablet broke. The range between the 4 to 8 kg /cm2 according to I.P 9.
5. Friability test
Friability tests are carried out to determine the extent to which tablets will tolerate coating, packaging, transportation, and other mechanical handling conditions. Friability is the ability of a tablet to break, crumble, or fracture when compressed. When handling or storing, this tendency is often limited to uncoated tablets and surfaces. The initial weight was determined by weighing ten tablets. After that, the tablets were placed within compartment first of the friability tester's drum and the count was adjusted to 100 for four minutes while the device rotated at 25 rpm. Subsequently, an updated weight was obtained for the tablets, which was used to determine the final weight. It was determined what the loss percentage was. I.P states that a weight reduction % shouldn't be higher than 1 .
6. In-Vitro Disintegration Test
The purpose of the disintegration test is to check if, under the experimental conditions, tablets or capsules dissolve in a liquid medium within the allotted time. The disintegration tester was first put together. Each 1000 ml beaker was filled with 600ml of distilled water. At 37°C, the temperature was kept constant. The tablet was inserted into each of the six tubes. The switch button was pressed, and the duration of time was noted for the tablet to break down. Disintegration is said to have occurred when there are no longer any residues visible in the disintegration chamber If there are, they are composed of a mushy mass without a discernibly hard, swollen core; or 45 Merely broken pieces of the shell or coating (tablets) can adhere to the lower surface of the disc 11 .
Limit ¬-:
- Uncoated Tablets: 15 min or as per individual monograph.
- Film Coated Tablets: 30 min or as per individual monograph.
7. Standard calibration curve
Preparation of stock solution
10 mg of Montelukast sodium was accurately weighed with the help of weighing balance and poured into 100 ml volumetric flask. Drug was dissolved in 100 ml of 0.5 % Sodium lauryl sulphate and volume was made up to 100 ml in volumetric flask. Prepare the then prepared the 5 µg/ml, 10µg/ml, 15 µg/ml, 20 µg/ml, 25 µg/ml, 30 µg/ml, with diluted by Phosphate buffer 6.8. And the absorbance was recorded 12.
8. Dissolution Study
The dissolution test is an essential in vitro technique in the formulation and development of pharmaceutical dosage forms. It determines the percentage of drug release over time, indicating the manufacturing process's reproducibility and, in certain conditions, its ability to predict in vivo drug release.
- Apparatus No. 1
- Medium – 900 ml of 0.5 %w/v solution of sodium lauryl sulphate in water.
- Speed and time- 50 rpm and 30 minutes.
- Withdraw a suitable volume of the medium and filter.
- Determination by UV spectrophotometer at 240 nm 12-14.
- Developing the 0.5% SLS solution
The resulting mixture performed as a dissolving agent. It used 5g of sodium lauryl sulphate for every litre of distilled water. Without shaking, sodium lauryl sulphate was added gradually to the distilled water. It required some time to break down entirely and form a transparent liquid.
RESULTS
- Physical Appearance
The color, surface, shape of tablets is determined by visual inspection. The physical appearances of the different brands of Montelukast sodium are shown in Table 4.

Table 4: Physical characteristics of Montelukast tablets
- Weight Variation test
The individual weight of the was measured by the analytical balance. The Percentage weights variation of Montelukast tablets are shown in Table 5. The Average weights of the individual brands are shown in Fig 2

Table 5: Calculations and results of weight variation

Fig. 2: Weight variation of diff. brands
- Thickness
Thickness of the Different brands of Montelukast sodium Tablets (10 mg) are determined by the digital vernier caliper and results are mentioned in Table 6.

Table 6: Individual thickness of tablets and results
The graphical presentations of the average thickness of individual brands are shown in Fig 3.

Fig. 3: Thickness of diff. brands
- Hardness
Hardness of the Different brands of Montelukast sodium Tablets (10 mg) are determined by the digital Hardness tester and results are mentioned in Table 7.

Table 7: Hardness of tablets and results
The graphical presentations of the average Hardness of individual brands are shown in Fig 4.

Fig. 4: Hardness of diff. brands of tablets
- Friability Test
Friability of the Different brands of Montelukast sodium Tablets (10 mg) are determined by the Roche Friability instruments for 4 min.at 25 rpm speed and results are mentioned in Table 8.

Table 8: Friability of tablets
The graphical presentations of the Friability of individual brands are shown in Fig 5.

Fig. 5: Friability of diff. brands
- In Vitro Disintegration Test
Disintegration time of the Different brands of Montelukast sodium Tablets (10 mg) are determined by the Disintegration instruments at 37 ±5 ?c and results are mentioned in Table 9.

Table 9: Disintegration time and results
The graphical presentations of the average Disintegration time of individual brands are shown in Fig 6.
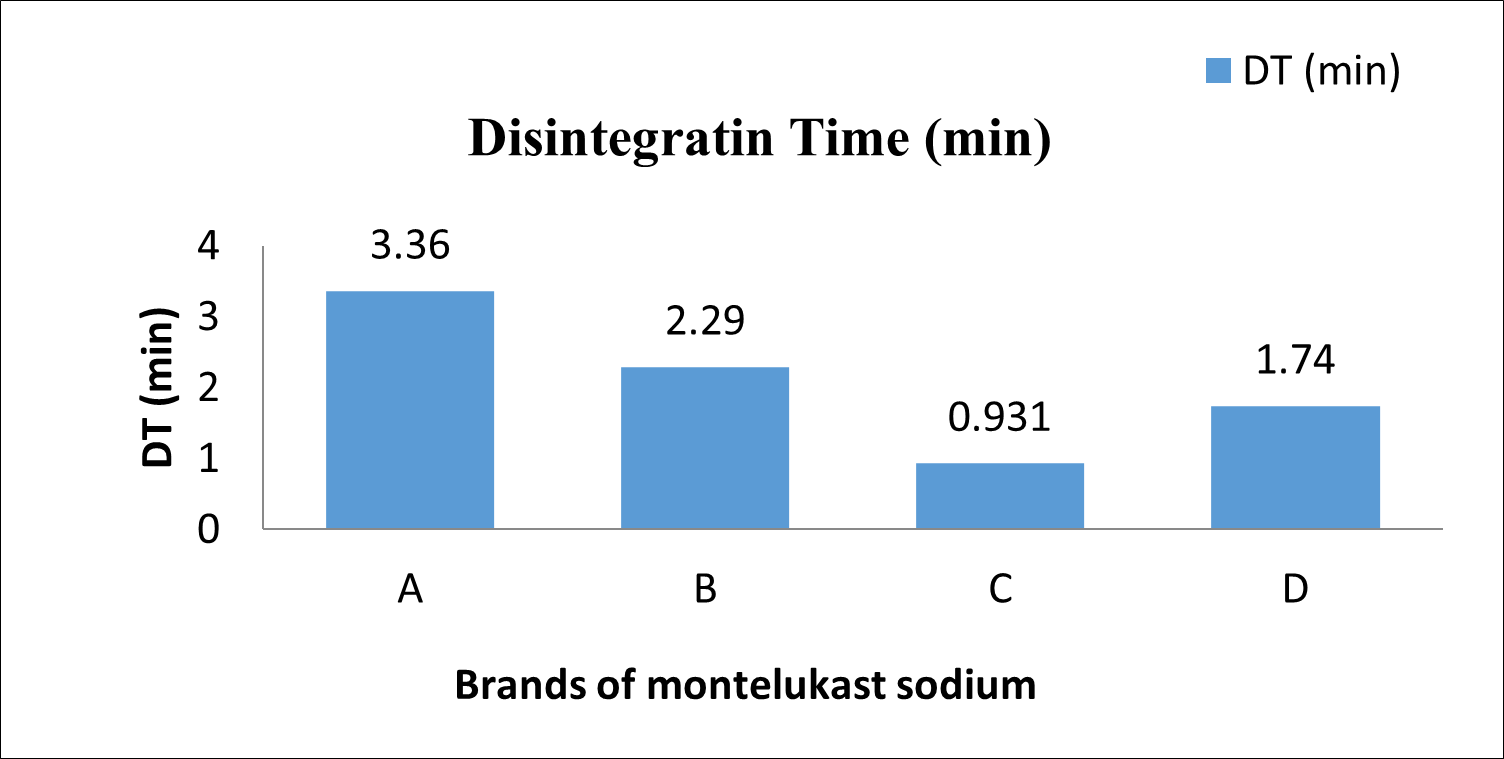
Fig. 6: Disintegration of diff. brands
- Standard Calibration Curve of Montelukast sodium
Calibration Curve of Standard was constructed by plotting Absorbance versus Concentration. Figure 7 represents the standard calibration curve. A correlation coefficient higher than 0.9986 indicated the presence of uniformity in the concentration range of 0 – 25µg/ml. The absorbance of the Montelukast tablet was determined by UV spectrophotometer mentioned in Table 10.

Table 10: Absorbance determined by UV Spectrophotometer
The calibration curve of Montelukast sodium in 0.5% SLS at 240 nm is shown in Fig7.
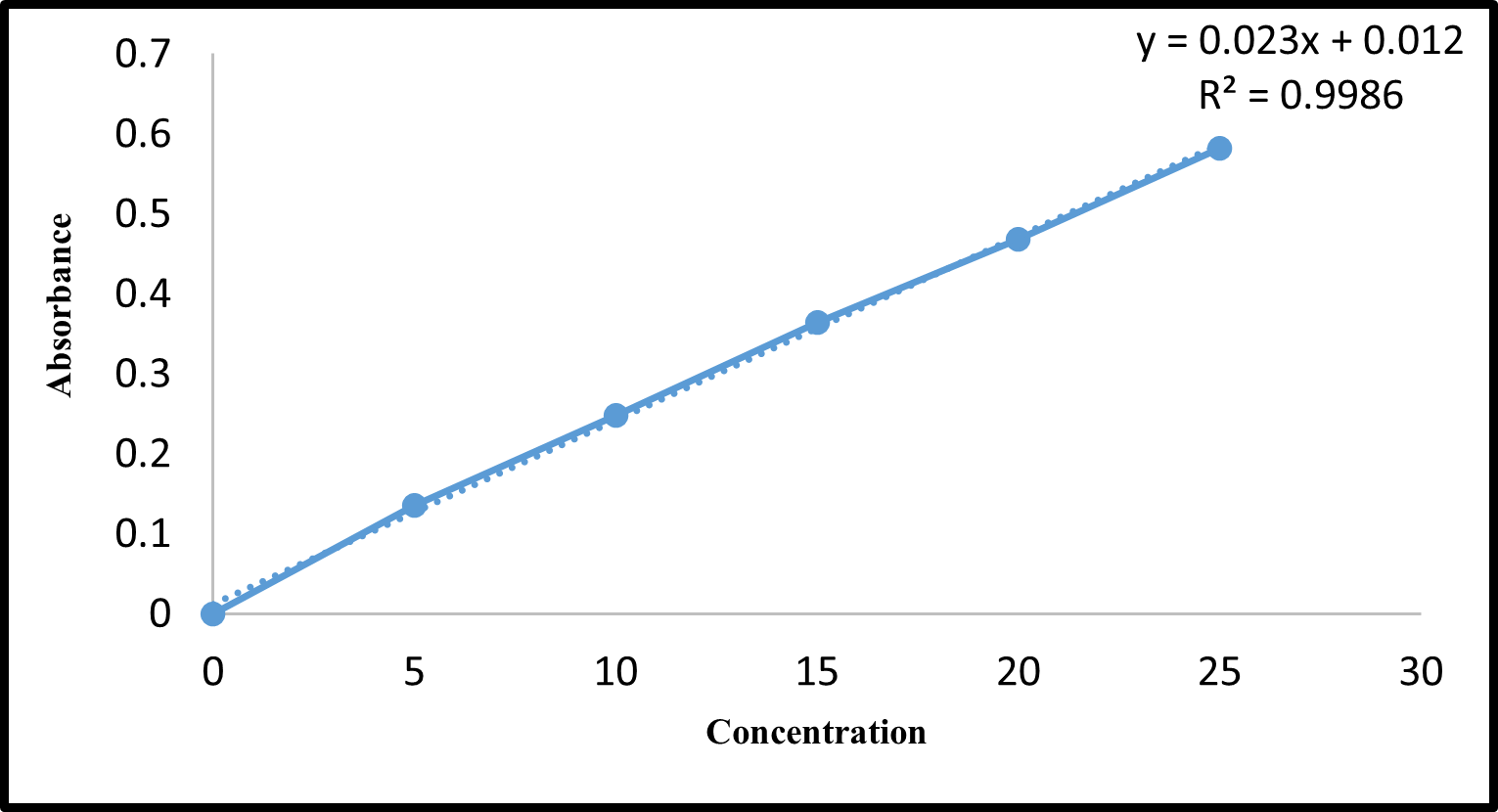
Fig. 7: UV estimation of Montelukast sodium in 0.5% SLS at 240 nm.10
- In Vitro Dissolution Study
The In vitro dissolution study is shown in Table 11.

Table 11: Dissolution time and % drug release
Dissolution study of Montelukast tablets in 0.5% SLS was carried out as shown below Fig 8.

Fig. 8: Cumulative % drug release
The percentage drug release is shown in Fig 9.
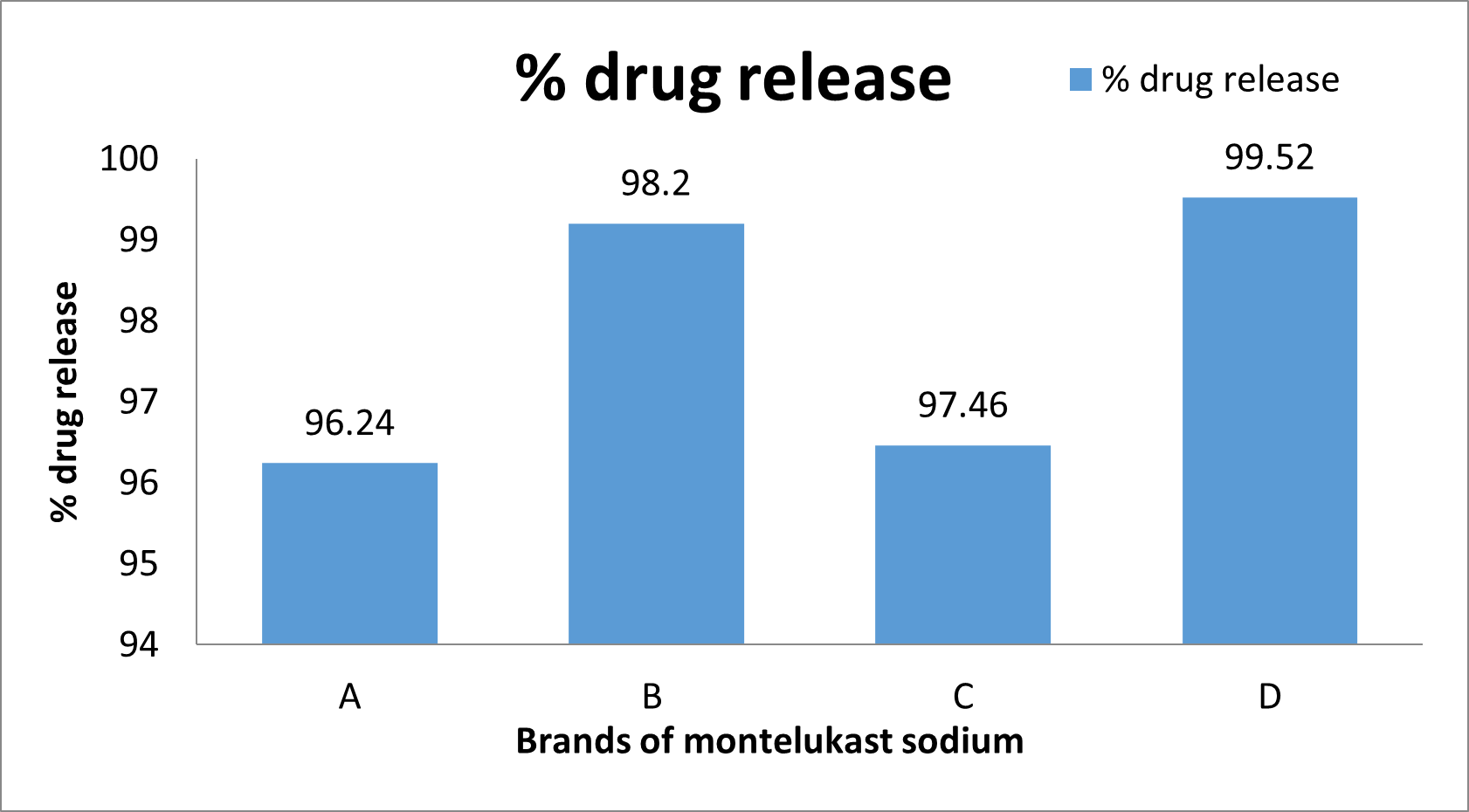
Fig. 9: Drug release of diff. brands
DISCUSSION
By comparing the quality control parameters of various marketed Montelukast Sodium tablet with the standards of Indian pharmacopeia. According to IP standards, all four brands' Montelukast tablets were found to be within ±10% of the average tablet weight. All four brands performed within the specified ranges. As per results it is found that brand A having more weight variation than the brand B but Brands C and D have the same weight variation (Table 5). It could be due to less flow ability of powder during compression. Hardness range is up to 4 to 10 kg/cm2. According to hardness tests, the average hardness values for Brands A and B are 5.8 kg/cm2 and 4.28 kg/cm2, respectively, while the average hardness values for Brands C and D are 4.49 kg/cm2 and 4.14 kg/cm2, respectively (Table 7). This indicates that both brands pass the disintegration test since all four Hardness values are below the pharmacopoeia limit. The pharmacopoeia states that 0.5 to 1% tablet loss during transportation is acceptable, and friability equipment used this. Friability tests reveal that Brands A and B average mass losses of 0.04% and 0.017%, respectively. The average mass losses for brands C and D are 0.00% and 0.07%, respectively. (Table 8)This indicates that the friability test was passed by all four tablet brands. The results indicate that Brands A and B disintegrate within a 15-minute interval, with timings of 3.36 min and 2.29 min, respectively. The disintegration time limit for uncoated tablets is 15 minutes, while for film-coated tablets it is 30 minutes. Brands C and D also meet this criterion, with disintegration times of 0.931 min and 1.74 min, respectively (Table 9). This means that all four disintegration times are under the pharmacopeia limit so both brands conform to the disintegration test. A Comparison of dissolution profiles reveals that Brand B (98.2%) exhibits better drug release compared to Brand A (96.24%) among film-coated tablets. Similarly, among uncoated tablets, Brand D (99.52%) demonstrates superior drug release compared to Brand C (97.46%) (Table 11). However, all brands comply with the drug release standards set by the Indian Pharmacopeia. All of the brands A, B, C, and D's dissolving tests confirmed the rate of drug release; in 30 minutes, nearly 95–99% of the drug was released, which was good from a dissolving approach. An examination of the dissolution was done multiple times to confirm the pharmacopoeia monograph.
CONCLUSION
To determine the quality of the tablets, the quality control requirements of four distinct brands of Montelukast tablets were analysed and compared. Tests for quality control, such as disintegration, friability, hardness, and weight variation, have been carried out. A UV Spectrophotometer was used to assess an in vitro dissolution research and calculate the proportion of the medication released after 30 minutes, which could be a predictor of how well the medicine performs in vivo. The Montelukast sodium tablets manufactured by various pharmaceutical companies correspond to Indian Pharmacopoeia criteria and are of uniform quality with minimal to no variation between them.
REFERENCES
- Krishnamurthy M, Abdullah B. Efficacy of Montelukast in Allergic Rhinitis Treatment: A Systematic Review and Meta-Analysis. Drugs. 2020; 80(17):1831-1851. doi: 10.1007/s40265-020-01406-9.
- Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007; 67(6):887-901. doi: 10.2165/00003495-200767060-00005.
- Paggiaro P, Bacci E. Montelukast in asthma: a review of its efficacy and place in therapy. There Adv Chronic Dis. 2011 Jan; 2(1):47-58. doi: 10.1177/2040622310383343.
- Barbosa JS, Almeida Paz FA, Braga SS. Montelukast medicines of today and tomorrow: from molecular pharmaceutics to technological formulations. Drug Deliv. 2016; 23(9):3257-3265.doi: 10.3109/10717544.2016.1170247.
- Ali AE, Elasala GS, Ibrahim RS, Kolkaila AS. Synthesis, characterization, spectral, thermal analysis and biological activity studies of some Montelukast sodium complexes. J. Chem. Res. Adv.2021; 02 (01):26-41.
- Singh RM, Saini PK, Mathur SC, Singh GN, Lal B. Development and Validation of a RP-HPLC Method for Estimation of Montelukast Sodium in Bulk and in Tablet Dosage Form. Indian J Pharm Sci. 2010; 72(2):235-237. doi:10.4103/0250-474X.65023
- Jain A, Chaudhary J, Saini A, Mehan N. Quality Assessment and Comparative Study of different Marketed Brands of Metformin. Research J. Pharm. and Tech. 2019; 12(3): 1357-1360. doi: 10.5958/0974-360X.2019.00228.2
- Gupta MM, Khoorban A, Ali A, Ramlogan O, Talukdar D. Comparative Quality Control Study of Different Brands of Diclofenac Sodium Tablet Available in Local and Government Pharmacies by In-Vitro Testing. Cureus. 2020; 12(11):11348. doi: 10.7759/cureus.11348.
- Pujari AS, Gaikwad NA, Mane IV, Vambhurkar GB. In-Vitro Evaluation of Different Marketed Brands of Paracetamol Tablets Using Quality Control Tests. Asian J. Pharm. Tech. 2018; 8 (3):119-122. doi: 10.5958/2231-5713.2018.00019.3
- Othman GQ, Battah MM, Halboup AM, Hassan HM. Comparative study of seven brands of Levofloxacin 500 mg film coated tablet marketed in Yaman. International Journal of Applied Pharmaceutics.2021; 13(2):264–268.https://doi.org/10.22159/ijap.2021v13i2.40217.
- Chaturvedi H, Garg A, Rathore US. Post-compression evaluation parameters for tablets-an overview. European journal of pharmaceutical and medical research.2017; 4(11): 526-530.
- Salve GS, Rokade AS, Ade AV. IPQC & FPQC Quality control tests for solid oral dosage forms. International Research Journal of Modernization in Engineering Technology and Science.2023; 5(4):6530-6541.
- Srilakshmi N, Pedireddi S, Das A, Rani T. Formulation, development and evaluation of press coated tablets of Montelukast sodium. Int. J. Adv. Pharm. Biotech., 2015; 1(1): 45-53 doi.org/10.38111/ijapb.20150101005.
- Indian pharmacopoeia. Ghaziabad: Controller of Publications; 2018;2(8):2630–2631.


 Trupti Yogesh Pawar* 1
Trupti Yogesh Pawar* 1
 Tanishka Anil Pawar 1
Tanishka Anil Pawar 1
 Rahul Keshav Godge 2
Rahul Keshav Godge 2



















 10.5281/zenodo.10853399
10.5281/zenodo.10853399