Abstract
The main aim of the present study is to develop a pharmaceutically equivalent, low-cost quality improved formulation and stable controlled release tablets of Paroxetine HCl comparable to innovator product. Paroxetine is a selective serotonin reuptake inhibitor, chemically unrelated to tricyclic, tetracyclic, or other antidepressants; presumably, the inhibition of serotonin reuptake from brain synapse stimulated serotonin activity in the brain. The conventional form of Paroxetine tablets have many side effects therefore a controlled release Paroxetine Hydrochloride is designed in order to improve its therapeutic profile and safety, and for reducing the dosing frequency.
Keywords
Paroxetine, antidepressants, Sustained – release, Dissolution, Povidone K30, Eudragit
Introduction
For many decades treatment of an acute disease or a chronic illness has been mostly accomplished by delivery of drugs to patients using various pharmaceutical dosage forms, including tablets, capsules, pills, suppositories, creams, ointments, liquids, aerosols and injectables as drug carriers. The design of oral sustained drug delivery system is subject to several inter-callated variables of considerable importance. Among these are the type of delivery system, the disease being treated, the patient, the length of therapy, a Pharmaceutical products designed for oral delivery and currently available in market are mostly the immediate-release type, which are designed for immediate release of drug for rapid absorption. 1,,2 All the pharmaceutical products formulated for systemic delivery via the oral route of administration, irrespective of mode of delivery (immediate, sustained or controlled release) and the design of dosage form (solid, dispersion or liquid) must be developed within intrinsic characteristic of gastrointestinal (GI) physiology.3,4
ADVANTAGES OF CONTROLLED RELEASE DOSAGE FORMS
- Patient compliance due to reduction in the frequency of dosing.
- Employ minimum drug.
- Minimize or eliminates local and systemic side effects.
- Obtain less protentiation or deduction in drug activity with chronic use.
- Minimize drug accumulation with chronic dosing.
- Improves efficacy in treatment.
DISADVANTAGES OF CONTROLLED RELEASE DOSAGE FORMS
- They are costly.
- Unpredictable and often poor in-vitro in-vivo correlations, dose dumping, reduced potential for dosage adjustment and increased potential first pass clearance.
- Poor systemic availability in general.
- Effective drug release period is influenced and limited by GI residence time.
RATIONALE OF CONTROLLED DRUG DELIVERY
The basic rationale for extended drug delivery is to alter the pharmacokinetic and pharmacodynamics of pharmacologically active moieties by using novel drug delivery systems or by modifying the molecular structure and/or physiological parameters inherent in a selected route of administration.5,6 It is desirable that the duration of drug action become more to design properly.7 Rate controlled dosage form, and less, or not at all, a property of the drug molecules inherent kinetic properties.8 As mentioned earlier, primary objectives of extended drug delivery are to ensure safety and to improve efficiency of drugs as well as patient compliance. This can be achieved by better control of plasma drug levels and frequent dosing. For conventional dosage forms, only the dose (D) and dosing interval (C) can vary and, for each drug, there exists a therapeutic window of plasma concentration, below which therapeutic effect is insufficient, and above which toxic side effects are elicited. This is often defined as the ratio of median lethal dose (LD 50) to median effective dose (ED50) 9, 10
MATERIALS AND EQUIPMENTS
CHEMICALS
EQUIPMENTS
EXPERIMENTAL WORK
PREFORMULATION STUDIES-
Organoleptic character
All the organoleptic character of paroxetine Hydrochloride was studied and it was found that all the character complies with IP standards.
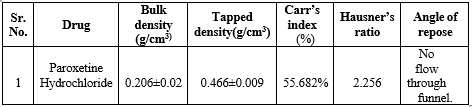
Bulk density and Tapped density
The density of Paroxetine Hydrochloride was found 0.206 g/ml .Tapped density was found 0.466g/ml. The results are shown in table 15.
Carr’s index
The measurement of free flowing powder can also be done by Carr’s index. The Carr’s index for all the formulations was found to be 55.682%, which reveals that the powder has poor flow character. The results are shown in table 15.
Angle of repose
The angle of repose for Paroxetine Hydrochloride was done as per the procedure. There was no proper flow through the funnel, indicates that powder has poor flow property. The results are shown in table 1.
PARTICLE SIZE
The Particle size was determined using mechanical sieve shaker as per the procedure. since 95% of the drug is retained on sieve # 50, the particle size of the drug lies between #50 and #18 i.e. 300 um an 1.00 mm. The results are shown in the table 2
Table 2:Particle size analysis

Solubility:
The solubility of Paroxetine Hcl was carried out in different buffers as per the procedure and the results are show in the table 3 and figure 1.
Table 3: Solubility of Paroxitine Hcl in different pH conditions
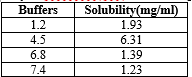

Figure 1 : Solubility of Paroxitine Hcl in different pH condition
PHYSICAL STABILITY OF THE ADMIXTURE
The drugs along with the excipients were kept under conditions specified and the results are given in table 4.
Table 4 : Drug – Excipient stability profile

There was no physical change observed in the admixture after one month at 60 ºC
Drug Excipient Compatibility Studies
FTIR analysis was conducted for the structure characterization and drug excipient Compatibility to which Paroxetine Hydrochloride showed the following character. All the FTIR characterization of drug excipient was analyzed and results showed that there was no shift of peak that correspond to pure drug as shown in fig 12.
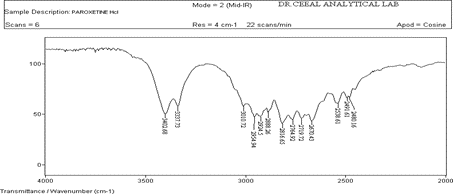
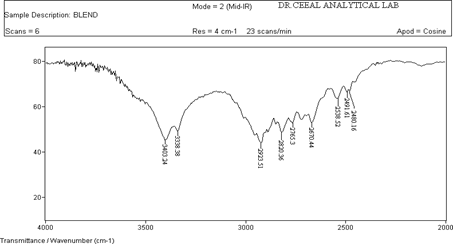
Figure 2 : IR of API(Paroxetine Hcl)
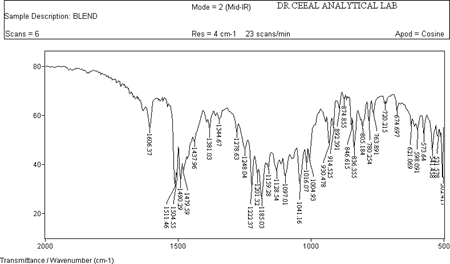

Figure 3: IR of API +Excipients
The FTIR of Paroxetine Hcl (drug) showed intense band at 3402.68 cm-1, 1606.26 cm-1 , 2954.94 cm-1 corresponding to the functional groups , NH, C=C and C-H bending as shown in Figure 11. The FTIR of drug and excipients shown intense bands at 3403.24 cm-1,1606.37 cm-1 , 2923.51 cm-1 indicates no change in the functional groups NH, C=C and C-H as shown in Fig 12. The FTIR of Placebo shown that there are no intense bands at groups NH, C=C and C-H this shows that drug peaks are missing in it as shown in Fig 10. From the above interpretation it is understood that there is no major shifting in the frequencies of above said functional groups. Hence these drug and polymers are compatible with each other.
Calibration curves
Table 5: Standard plot of Paroxetine Hydrochloride in 0.1 N HCl

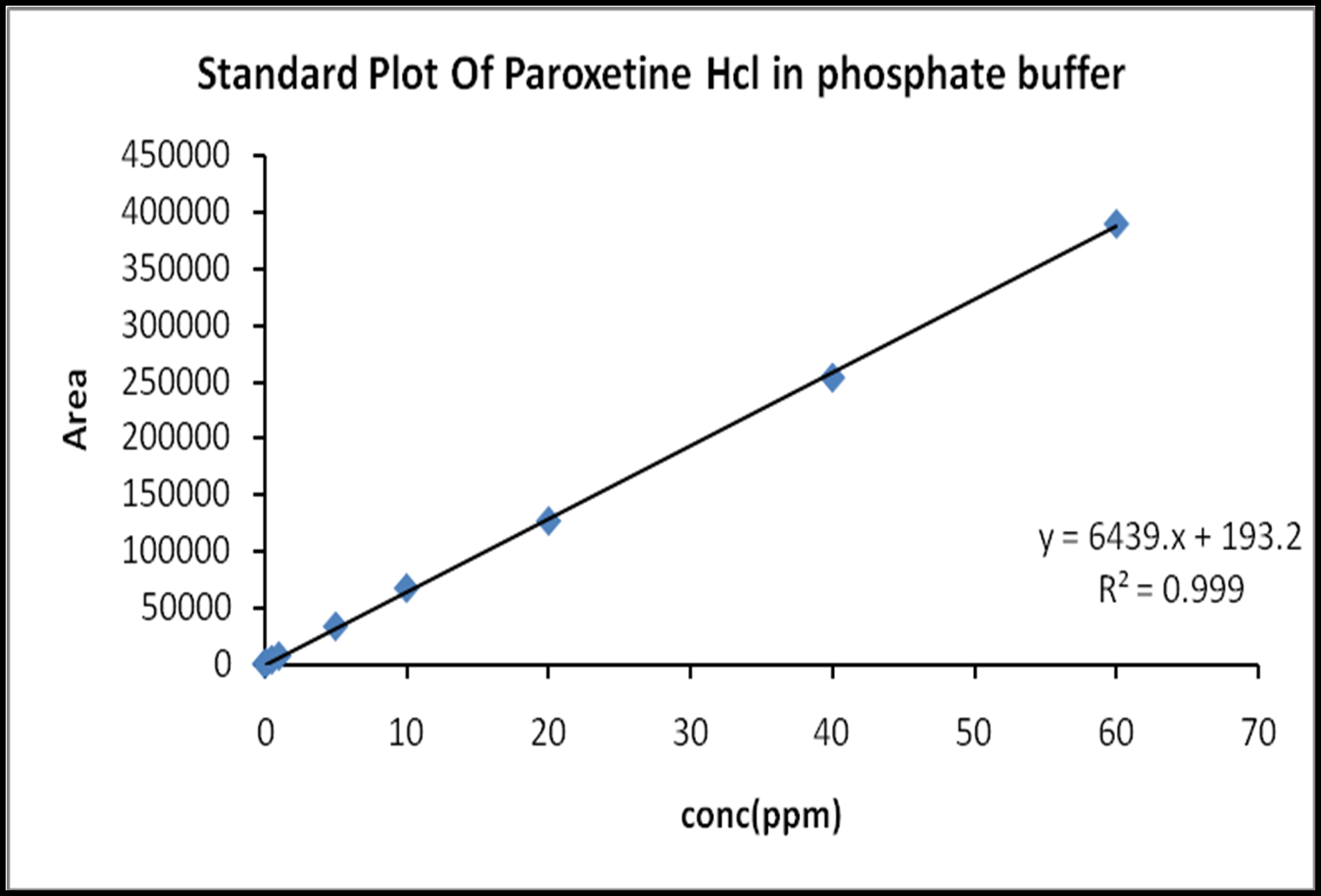
Figure 4: Standard plot of Paroxetine Hydrochloride in 0.1 N HCl

Table 6: Standard plot of Paroxetine Hydrochloride in phosphate buffer

Figure 5: Standard plot of Paroxetine Hydrochloride in phosphate buffer
The present analytical method obeyed Beer’s law in the concentration range of 0.1 to 60ppm and is suitable for paroxetine hydrochloride. The correlation coefficient (r) value for the linear regression equation was found to be 0.999, 0.999 in pH1.2, pH6.8 respectively, indicatingpositive correlation between the concentration of paroxetine hydrochloride and the corresponding area values. The summary of the calibrated curve is shown in the table 21,22.
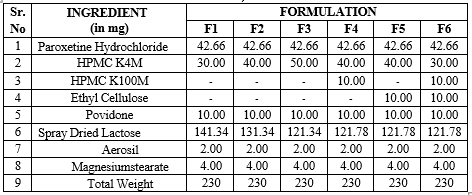
Table 7 : Formulation of Paroxetine Hydrochloride matrix tablets using different ratios of polymers (F1-F6)
ENTERIC COATING:
Coating solution preparation:
20% w/w of ACRYL-EZE.in water was prepared with continuous stirring for 1hr. It contains Methacrylic acid copolymer type C, Sodium carbonate, talc, silica, SLS and triethyl citrate. The final solution was passed through 100#. pH of the solution = 5.3
Table 8 : Enteric coating of formulation F7
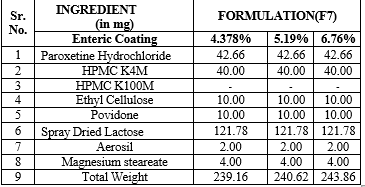
Table 9: Formulation of paroxetine hydrochloride matrix with varied concentrations of HPMC K4M and Ethyl cellulose
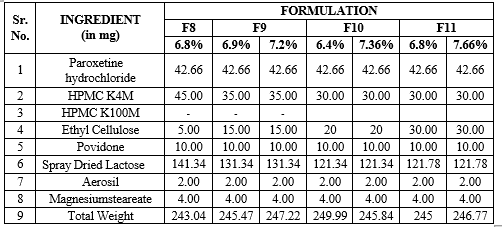
Enteric Coating for formulations F8, F9, F10, F11:
Coating solution was prepared using 400gm of Acryl white 20% w/w in water . Coating parameters:
Pan rpm = 35rpm
Pump rpm = 01
Pan size = 6”
Inlet temperature = 60 oC
Table 10: Development of different % of enteric coating
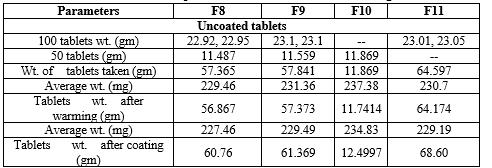
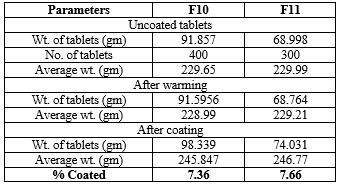
Table 11: Development of different % of enteric coating
EVALUATION OF TABLETS
CHARACTERIZATION OF PAROXETINE HYDROCHLORIDE MATRIX TABLETS (POST COMPRESSION PARAMETERS)
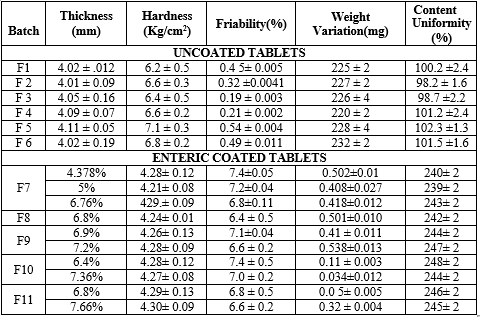
The tablets of different formulations of Paroxetine Hydrochloride were subjected to various evaluation tests, such as hardness, thickness weight variation, friability and drug content. All the result is shown in Table 20.
Thickness
The thickness of the tablets was found out using Vernier Caliper and the thickness found to be in the range of 4.02-4.12mm for the uncoated tablets (F1-F6). For the enteric coated tablets the thickness ranged from 4.21-4.30.Thus all formulations showed uniform thickness.
Hardness test
The hardness of tablet was measured by Monsanto hardness tester. Ten tablets from the batch were used for hardness studies and results showed they were in between 6.1-8.0 Kg/cm2. This is appropriate for matrix tablet.
Weight variation test
In a weight variation, the pharmacopoeial limit for the percentage deviation for tablets of more than 250 mg is ±5%. The average percentage deviation of all tablet formulations was found to be within the above limit, and hence all formulations passed the test for uniformity of weight as per official requirements.
Friability test
The Friability of all the formulation was below 1% as per IP specification. Drug content analysis/ Paroxetine Hydrochloride matrix tablet was tested for their drug content and all the formulation showed drug content more than 95%. All the tablet formulations showed acceptable pharmacotechnical properties and complied with the in-house specifications for weight variation, drug content, hardness, and friability.
Table 12: Characterization of Paroxetine Hydrochloride matrix tablets
In vitro dissolution studies
COMPARISION OF FORMULATIONS WITH MARKETED FORMULATION PAXIL CR USING SIMILARITY FACTOR (F2):
The similarity factor (f2) was calculated for all the formulations as per the procedure and the values are shown in the table 30.
Table 13:Similarity factor(f2)
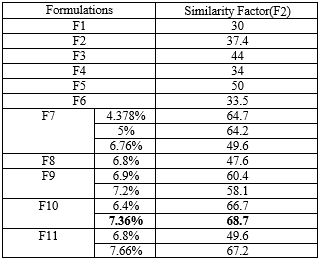
The similarity factor of all the formulation ranged from 30-70. The f2 value of formulation F10 was higher when compared to other formulations therefore F10 formulation was found to be similar to that of the marketed formulation (PAXIL CR).

Figure 7: Stability dissolution profile of formulation (F6) at 25ºc/ 60% RH
Table 14:cumulative drug release profile of stabilized formulation (F10)

Optimized formulation (F10) was kept for stability studies, and observed that assay after 1st, 2nd month was complies with optimized formulation. Dissolution profile of stability samples after 1st, 2nd months were compared with formulation (F10)(7.36%).There is no significant change in In vitro release profile in both conditions when compared with F10sssss. It shows that it is stable formulation.
CONCLUSION
In formulations (F7-F11) concentrations of HPMCK4M and ethyl cellulose in the formulations (F7-F11) were varied. The tablets were prepared by direct compression method after subjecting the blend to preformulation studies like Angle of repose, Bulk density, Tapped density, Carr’s Index. Post compression parameters like Hardness, Weight variation, Friability, Drug content analysis were carried out .The results obtained were satisfactory. Similarity factor (f2) was calculated for all formulations and found that f2 value was higher for F10 formulation. Among all formulations F10 was selected as optimized formulation as the in-vitro profile complied with the innovator Paxil CR.
Different kinetic models were applied to the formulation optimized and observed that formulation (F10) followed zero order kinetic model and it was complied with Paxil CR (Innovator sample). Stability studies were conducted for the optimized formulation and it was found that the product was stable. It may be concluded from the present study that slow, controlled release of Paroxetine over a period of 6 h was obtained from matrix tablets . It is evident from the results that Hydrophilic matrix of HPMC could not control the Paroxetine release effectively where as a combination of hydrophilic and hydrophobic matrix prepared by HPMCK4M and ethyl cellulose is a better system for controlled delivery of water-soluble drug like Paroxetine hydrochloride.
REFERENCES
- Martins Emeje olobayo o. Kunle1 sabinus Ofoefule Effect of the molecular size of carboxymethylcellulose and some polymers on the sustained release of theophylline from a hydrophilic matrix. Acta Pharm 2006;56:325-35.
- Chein YW, James Swarbrick, editors. Novel Drug Delivery System. 2nd ed. New York: Marcel Dekker; 1992.p.139-96.
- Robert S Langer, Donald L Wise, editors. Medical Application of Controlled release:classes of system. CRC Press; 1984.p.159.
- Brahmankar DM, Sunil B Jaiswal, editors. Biopharmaceutics and pharmacokinetics a treatise.1sted. Delhi: Vallabh Prakashan Publications;2001.p. 348-35.
- Steward PA. Review of Pharmaceutical Controlled Release Methods and Devices[online].[2000Nov11];Availablefrom:URL:www.initium.demon.co.uk.
- www.inintium.demon.co.uk Singh (1968) Nakagami(1991) Donbrow & Friedman (1975) [online].
- Xiaoling I, Bhaskar R.J. Design of Controlled Release Drug Delivery Systems, 2006.p. 155
- Peter G Welling, Michael R Dobrinska, Robinson JR, Lee VHL, editors. Controlled Drug Delivery: Fundamentals and Applications. 2nd ed. New York: Marcel Dekker; 1987.p. 255-259.
- Lippincott Williams, Wilkins, Thomas Wai- Yip Lee, Joseph R Robinson, editors. Remington’s The Science and Practice of Pharmacy. 20th ed. Maryland; 2000.p.903-914.
- Robinson JR, Lee VH. Controlled drug delivery: fundamentals & application. 2nd ed. New York(NY): Marcel Dekker; 1987.p. 36.
- Welling PG, Dobrinska MR, Robinson JR, Lee VH. Controlled Drug Delivery: Fundamentals and Applications, 2nd ed. New York: Marcel Dekker; 1987.p. 255-259.
- Howard C. Ansel, Loyd V. Allen, Nicholas G. Popovich. Ansel’s Pharmaceutical Dosage forms and Drug Delivery Systems: 268
- Sandip BT, Krishna Murthy T, Raveendra Pai M, Pavak RM, Chowdary P. Controlled Release Formulation of Tramadol Hydrochloride Using Hydrophilic and Hydrophobic Matrix System. AAPS PharmSciTech. 2003;4(3):31.
- Gruenhagen HH. Polymer/drug-melt extrusion: therapeutic and technological appeal. Pharmaceutical Technology Europe1996;11:22-27.
- Aulton ME editor. Pharmaceutics- The Sciences of Dosages form design. International student Edition: Churchill Livingston; p.129-191.
- 16. Leon Lachmann, editor. Theory and Practice of Industrial Pharmacy. 3rd ed. Philadelphia: Lea & Febiger; 1986.p .430-456.
- Remingtons, Pharmaceutical Sciences. 16th ed. Mack Publishing Company: 1980.p.1677-1683.
- Sunil Kamboj, Gupta GD, Jagmohan Oberoy. Matrix Tablets:An Important Tool for Oral Controlled-Release Dosage Forms. 2009;7(6).


 Tammanna Donadkar* 3
Tammanna Donadkar* 3
 Reetesh Yadav 1
Reetesh Yadav 1



















 10.5281/zenodo.12602803
10.5281/zenodo.12602803