Abstract
Indapamide, a thiazide-like diuretic, is widely used in the management of hypertension and edema. The accurate quantification of indapamide in pharmaceutical formulations and biological samples is crucial for ensuring its efficacy and safety. High-performance liquid chromatography (HPLC) is the preferred analytical technique for the quantification of indapamide due to its high sensitivity, selectivity, and efficiency. This review provides an overview of the development and validation of reversed-phase HPLC (RP-HPLC) methods for the estimation of indapamide in various matrices. The review discusses different aspects of method development, optimization, validation parameters, and applications in pharmaceutical analysis and pharmacokinetic studies.
Keywords
Indapamide, RP-HPLC, Method Development, Validation, Pharmaceutical Analysis.
Introduction
INTRODUCTION
Indapamide (IND), chemically known as 4-chloro-N-(2-methyl-2,3-dihydroindol-1-yl)-3-sulfamoylbenzamide (fig.1), is a thiazide-like diuretic, has emerged as a cornerstone in the treatment regimen for hypertension and edema associated with various cardiovascular conditions [1]. It acts by inhibiting sodium reabsorption in the distal convoluted tubules of the kidneys, leading to increased urine production and reduced blood pressure. Its efficacy in reducing blood pressure and managing fluid retention has positioned it as a pivotal therapeutic agent in clinical practice. However, the effective utilization of indapamide necessitates accurate quantification methods to ensure appropriate dosing and monitor patient response [2].
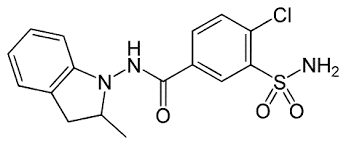
Figure 1: Structure of Indapamide
In pharmaceutical formulations, precise quantification of indapamide is indispensable to guarantee product quality and adherence to regulatory standards. Moreover, in pharmacokinetic studies and clinical settings, the ability to accurately measure indapamide levels in biological samples such as plasma and urine is crucial for understanding its pharmacological behavior, optimizing dosing regimens, and ensuring patient safety [3].
Among the various analytical techniques available for quantifying pharmaceutical compounds, high-performance liquid chromatography (HPLC) stands out as a preeminent method due to its versatility, sensitivity, and reliability [4]. Within the realm of HPLC, reversed-phase HPLC (RP-HPLC) has garnered significant attention for its effectiveness in separating a wide range of compounds, including indapamide, based on their hydrophobicity [5].
The development and validation of RP-HPLC methods for indapamide quantification represent a critical aspect of pharmaceutical analysis. These methods not only provide accurate and precise measurements but also offer the advantage of being robust, reproducible, and amenable to high-throughput analysis. Moreover, the versatility of RP-HPLC allows for the analysis of indapamide in diverse matrices, ranging from pharmaceutical formulations to biological fluids, facilitating comprehensive pharmacological investigations [6].
This review aims to provide a comprehensive overview of the development and validation of RP-HPLC methods for the estimation of indapamide. By synthesizing existing literature and highlighting key methodologies, optimization strategies, validation protocols, and applications, this review seeks to elucidate the significance of RP-HPLC in indapamide analysis within the context of pharmaceutical sciences and clinical practice. Furthermore, it aims to identify current challenges, emerging trends, and future directions in the field, thereby contributing to the advancement of analytical methodologies for indapamide quantification.

Table 1: Drug Profile of Indapamide [7]
Mechanism of Action of Indapamide:
The mechanism of action of indapamide, like other thiazide diuretics, primarily involves its effects on renal tubular function. Here's a breakdown of its mechanism [8-13]:
Inhibition of Sodium Reabsorption:
Indapamide acts predominantly on the distal convoluted tubule (DCT) of the nephron in the kidneys. It inhibits the sodium-chloride symporter (NCC) located on the luminal membrane of the DCT cells. By blocking NCC, indapamide prevents the reabsorption of sodium and chloride ions from the tubular fluid into the DCT cells. This inhibition disrupts the normal electrochemical gradient for sodium, leading to decreased reabsorption of water and other ions downstream in the nephron (fig.2).
Increased Excretion of Sodium and Water:
By inhibiting sodium reabsorption, indapamide promotes the excretion of sodium, chloride, and consequently water, into the urine. This diuretic effect reduces extracellular fluid volume and decreases blood pressure, making it effective in the management of hypertension and edema.
Potassium Conservation:
Unlike some other diuretics, such as loop diuretics, indapamide has a milder effect on potassium excretion. While it enhances sodium and water excretion, indapamide retains potassium to a significant extent. This is achieved through mechanisms such as increased delivery of sodium to the aldosterone-sensitive distal nephron segments, where potassium secretion occurs, and through direct effects on potassium channels.
Vasodilatory Effects:
In addition to its diuretic action, indapamide exhibits mild vasodilatory properties. This effect is thought to be mediated through several mechanisms, including the inhibition of vascular smooth muscle cell proliferation, improvement in endothelial function, and modulation of vascular tone. The vasodilatory effect contributes to the antihypertensive action of indapamide, helping to reduce peripheral vascular resistance.
Antihypertensive Action:
By promoting sodium and water excretion and inducing vasodilation, indapamide effectively reduces blood volume and peripheral vascular resistance. This combined action results in a decrease in systemic blood pressure, making it a valuable therapeutic agent in the management of hypertension.
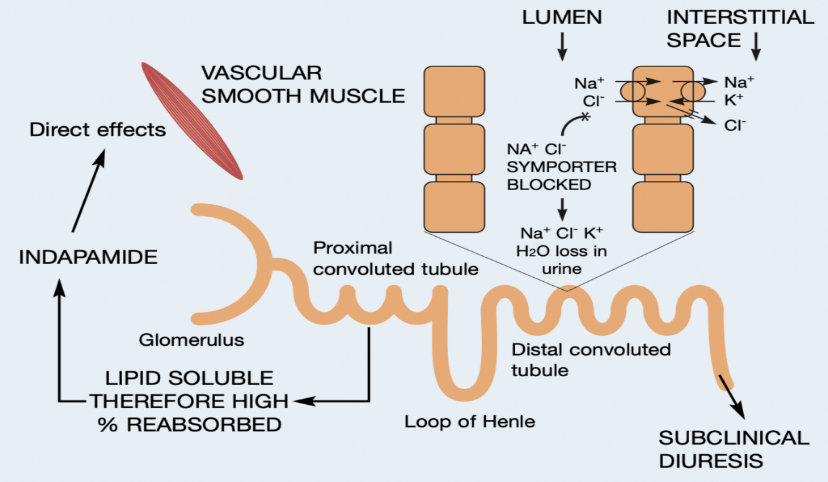
Figure 2: Mechanism of Action of Indapamide
Pharmacokinetics:
Here's a general overview of the pharmacokinetics of indapamide [14-18]:
Absorption:
Indapamide is well absorbed after oral administration, with peak plasma concentrations reached within 1 to 2 hours after ingestion. Food does not significantly affect its absorption, so it can be taken with or without food.
Distribution:
Indapamide is extensively distributed throughout the body, with a volume of distribution ranging from 16 to 20 liters. It binds strongly to plasma proteins, primarily to albumin.
Metabolism:
Indapamide undergoes minimal metabolism in the liver. The primary metabolic pathway involves hydroxylation to form an inactive metabolite. The cytochrome P450 enzyme system does not play a significant role in the metabolism of indapamide.
Excretion:
The drug and its metabolites are primarily excreted through the kidneys. Approximately 70% of the administered dose is excreted in the urine, with only a small amount eliminated in the feces. The elimination half-life of indapamide ranges from 14 to 24 hours, allowing for once-daily dosing.
Renal Impairment:
In patients with renal impairment, the elimination half-life of indapamide may be prolonged, necessitating dose adjustment based on renal function.
Hepatic Impairment:
Indapamide is not extensively metabolized by the liver, so hepatic impairment is not expected to significantly alter its pharmacokinetics. However, caution may be warranted in severe hepatic impairment due to potential alterations in drug metabolism and clearance.
Drug Interactions:
Indapamide has a low potential for drug interactions. However, concurrent use of other drugs that affect renal function or potassium levels (such as NSAIDs or potassium-sparing diuretics) may require dosage adjustment or monitoring.
Conditions for chromatography:
A 25 cm x 4.6 mm i.d. HiQ Sil-C18 HS column (Kya Tech, Japan) was used for the separation. There was a 1.0 mL min?1 flow rate. There was a 20µl injection volume. At 242 nm, the detecting wavelength was chosen. The ratio of acetonitrile, methanol, and water in the mobile phase was 40:50:10 (v/v/v). The column temperature was kept at room temperature and the run duration was fixed at ten minutes. The column was equilibrated for 30 minutes with mobile phase before the analyte was injected. After mixing, the mobile phase was filtered using a 0.45 µm membrane filter and then sonicated to remove any remaining gas [19,20].
Developing an RP-HPLC Method for Indapamide:
Reversed-phase HPLC (RP-HPLC) methods have gained prominence for the quantification of indapamide due to their ability to separate polar and non-polar compounds effectively [21]. The development of RP-HPLC methods involves optimization of various parameters such as mobile phase composition, pH, column type, column temperature, and detection wavelength to achieve optimal separation and quantification of indapamide [22,23].
In order to create a method, the RP-HPLC process was optimized (Table 2). Acetonitrile: Methanol: Water in the ratio of 40:50:10 (v/v/v) was found to provide a strong, well-defined peak with extremely good symmetry and low t R (3.233 min) out of numerous solvents and solvent mixtures examined (fig.3). Several other mobile phases that were attempted in the past were either ignored or failed to produce a clearly defined peak quickly. Based on peak shape (peak area, peak asymmetry, and tailing factor), baseline drift, analysis time, and solvent cost, the final decision regarding the composition and flow rate of the mobile phase was made. The ideal mobile phase was determined to be acetonitrile: methanol: water in a ratio of 40:50:10 (v/v/v). The retention time under these circumstances was 3.233 ± 0.01 minutes [24-26].

Table 2: Optimized Chromatographic Conditions
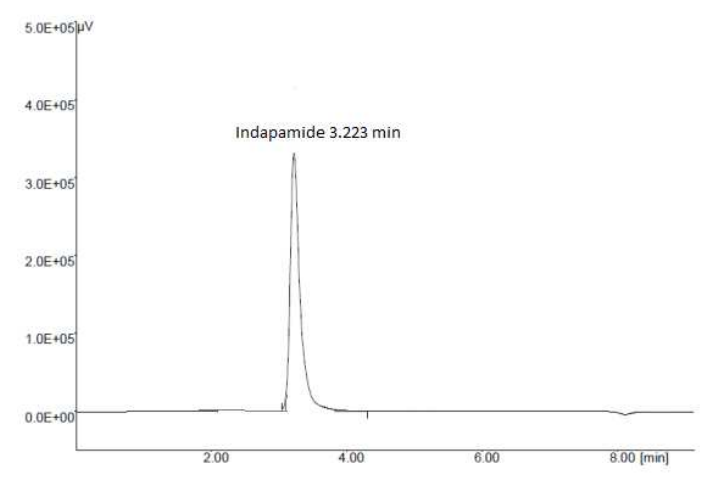
Figure 3: Typical chromatogram of Indapamide
Method Validation:
Linearity:
After dissolving 100 mg of indapamide in 100 ml of mobile phase, a stock solution containing 1000 µg mL?1 was created. From this stock solution, solutions with varying concentrations (10–60 µg mL?1) were created for the purpose of creating calibration plots. During column equilibration, the mobile phase was passed through a 0.45 µm membrane filter and supplied at a rate of 1.0 mL min?1. The baseline was continually observed throughout this procedure. 242 nm was the detecting wavelength. Following a series of injections of the created dilutions, each dilution's peak area was determined, and the concentration was plotted against the peak area (fig.4) [27].
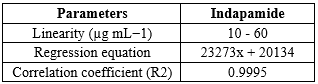
Table 3: Statistical data of calibration curves of Indapamide
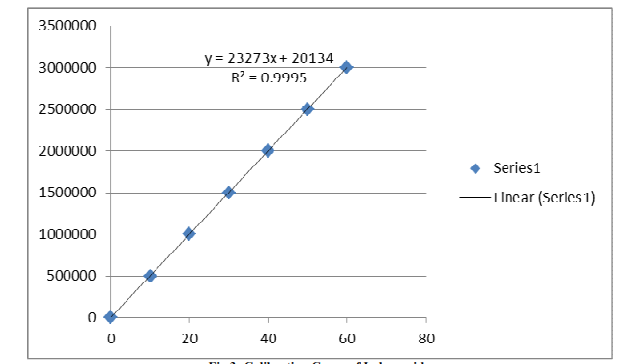
Figure 4: Calibration Curve of Indapamide
Accuracy: In various literature, researchers used the conventional addition approach to determine accuracy. The suggested procedure was applied to previously examined IND (10 µg mL?1) samples that had been spiked with 80, 100, and 120?ditional IND standard. Three separate people carried out the experiment. For every concentration, recovery (%), RSD (%), and standard error (SE) were computed (Table 4) [28-31].
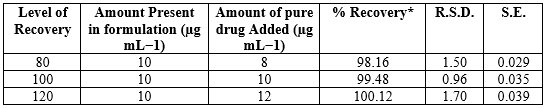
Table 4: Result of Recovery Studies of Indapamide
Precision: In compliance with ICH guidelines, precision was defined as both repeatability and intermediate precision. The assessment of inter-day variation was used to establish intermediate precision and intra-day variation was used to determine repeatability of sample injection. Single concentration solutions of indapamide were found for both intra-day and inter-day fluctuation (Table 5) [32].

Table 5: Result of Precision Studies of Indapamide
Reproducibility: By measuring precision on an alternative column while doing analysis with a different analyst, the reproducibility of the approach was verified. Six determinations of IND solutions at single concentrations (10µg mL?1) were made for both intra-day and inter-day variance [33].
Limit of Detection (LOD) and Limit of Quantification (LOQ):
By using the standard deviation (S y/x) technique, LOD and LOQ were ascertained. LOD and LOQ were calculated using the formulas LOD = 3.3 × S y/x /S and LOQ = 10 × S y/x /S based on the slope (S), of the calibration plot, S y/x [33,34].
The literature reported that the method's LOD and LOQ, ascertained by the standard deviation approach, were 0.52 and 0.78 µg mL?1, respectively [34]. These results suggest that the method is suitable for the detection and quantification of IND throughout a broad concentration range.
Robustness: The method's robustness was evaluated in order to evaluate the impact of a minor but intentional alteration to the chromatographic conditions on the identification of IND. By adjusting the mobile phase flow rate to 0.9 and 1.1 mL min?1 and the methanol content to 48 and 52%, robustness was assessed (Table 6) [35-38].

Table 6: Robustness of the method
Stability:
Repeated analyses of samples taken during the experiments on the same day as well as after the drug solution was stored for 48 hours under laboratory bench settings (33 ± 1°C) and under refrigeration (8 ± 0.5°C) were used to determine the stability of the medication in solution during analysis [39].
Applications of RP-HPLC Methods:
RP-HPLC methods have been successfully applied for the analysis of indapamide in various matrices, including tablets, capsules, injections, and biological fluids such as plasma and urine. These methods have been utilized in quality control laboratories for routine analysis of pharmaceutical formulations and in pharmacokinetic studies to determine the drug's concentration-time profile in vivo [40].
Future Perspectives:
Future research directions in the field of RP-HPLC for indapamide analysis may include the development of green analytical methods, automation of sample preparation and analysis, and exploration of hyphenated techniques such as HPLC-MS/MS for enhanced sensitivity and selectivity. Moreover, the application of quality-by-design (QbD) principles in method development and validation could further improve method robustness and reliability.
This review provides valuable insights into the development and validation of RP-HPLC methods for the estimation of indapamide, highlighting their significance in pharmaceutical analysis and clinical research.
CONCLUSION
In conclusion, the development and validation of a reliable RP-HPLC method for the estimation of indapamide represent a critical aspect in pharmaceutical analysis. Through a comprehensive review, it is evident that the establishment of such a method requires meticulous attention to various parameters including chromatographic conditions, validation parameters, and specificity. The significance of this method lies in its ability to accurately quantify indapamide, ensuring the quality, safety, and efficacy of pharmaceutical formulations containing this active ingredient. Furthermore, the review highlights the importance of thorough validation to ensure the robustness, precision, and accuracy of the analytical procedure, thereby facilitating its application in routine quality control laboratories. Overall, the synthesis of existing literature underscores the necessity for continued research and standardization in the development and validation of RP-HPLC methods for indapamide analysis to meet the evolving demands of pharmaceutical analysis.
REFERENCES
- Anthony, C. M., & Osselton, M. D. (2004). Clarke’s analysis of drugs and poisons in body fluids and post-mortem material (2nd ed.). Pharmaceutical Press, 1132-1137.
- O’Neil, M. J. (2006). The Merck Index (14th ed.). Merck & Co, Inc., Whitehouse Station.
- Indian Pharmacopoeia. (2022). Govt. of India, The Indian Pharmacopoeia Commission. Ghaziabad, Ministry of Health & Family Welfare.
- United States Pharmacopeia and National Formulary (USP 30-NF 25) (2007). Vol. 2. Rockville, MD: United States Pharmacopoeial Convention. Page 2340.
- Pai, J., Shetty, S. K., Chenna, G., Gopinath, B., & Ahmed, M. (2011). Development of new spectrophotometric methods for the determination of indapamide in bulk and pharmaceutical formulations. International Journal of ChemTech Research, 3(2), 755-760.
- Pai, J., Shetty, S., Gopinath, B., & Chenna, G. (2011). Development and validation of RP-HPLC method for quantitative estimation of indapamide in bulk and pharmaceutical dosage forms. International Journal of ChemTech Research, 3(3), 1482-1487.
- Legorburu, M. J., Alonso, R. M., Jiménez, R. M., & Ortiz, E. (YEAR). Quantitative determination of indapamide in pharmaceuticals and urine by high-performance liquid chromatography with amperometric detection. Journal of Chromatographic Science, 37(8), 283-287.
- Hang, T. J., Zhao, W., Liu, J., Song, M., Xie, Y., Zhang, Z., Shen, J., & Zhang, Y. (2006). A selective HPLC method for the determination of indapamide in human whole blood: Application to a bioequivalence study in Chinese volunteers. Journal of Pharmaceutical and Biomedical Analysis, 40(1), 202-205.
- Sharma, B. K. (2005). Instrumental methods of chemical analysis (20th ed.). GOEL Publishing House.
- International Conference on Harmonization (ICH). (2005). Q2 (R1), Text on validation of analytical procedures. Geneva, Switzerland.
- Giri, C., Kondawar, M., & Chougule, D. (2010). Simultaneous estimation of nebivolol hydrochloride and amlodipine besylate in combined tablet dosage form by q-analysis method. International Journal Pharma Development, 2, 13-14.
- Thomas, A., Jagdale, S., Dighe, S., & Nanda, R. (2010). Simultaneous spectrophotometric estimation of amlodipine besylate and telmisartan in tablet dosage form. International Journal of Pharma Tech Research, 2, 1334–1341.
- Prajapati, J., Patel, M., Prajapati, R., & Prajapati, N. (2011). Simultaneous determination of perindopril erbumine and amlodipine besylate by absorption factor method. International Journal of Applied Bio Pharma Technology, 2, 230–233.
- Rathee, P., Rathee, S., Thakur, S., & Kumar, V. (2010). Simultaneous estimation of amlodipine besylate and lisinopril dihydrate as A.P.I. and in tablet dosage forms by modified form of simultaneous equation method using derivative UV Spectrophotometry. International Journal of Pharma Tech Research, 2, 556–562.
- O’Neil, M. J. (2001). The Merck Index: An encyclopedia of chemicals, drugs, and biologicals (13th ed.). Merck & Co.
- United States Pharmacopeia 23 NF 18. (1995). United States Pharmacopeial Convention, Inc.
- British Pharmacopoeia. (2005). Her Majesty’s Stationery Office.
- Liang, Y., Li, H., Chen, W. D., Liu, X. D., Wang, G. J., & Xie, L. (2006). Anal. Letters, 39, 1365-1379.
- Zendelovska, D., Stafilov, T., & Stefova, M. (2003). J. Chrom. B, 788, 199-206.
- Dangi, M., Chaudhari, D., Sinker, M., Racha, V., & Damle, M. (2010). Stability indicating HPTLC method for estimation of nebivolol hydrochloride and amlodipine besylate in combination. Eurasian Journal of Analytical Chemistry, 5, 161–169.
- Chabukswar A, Jagdale S, Kumbhar S, Kadam V, Patil V, Lokhande P. Simultaneous HPTLC estimation of telmisartan and amlodipine besylate in tablet dosage form. Archives of Applied Science Research, 2010;2: 94–100.
- Chaudhari B, Patel N, Shah P. Stability indicating RP-HPLC method for simultaneous determination of atorvastatin and amlodipine from their combination drug products Chemical and Pharmaceutical Bulletin, 2007;55: 241–6.
- Chauhan V, Prajapati S, Patel C. A validated RP-HPLC method for simultaneous estimation of amlodipine And lisinopril in pharmaceutical dosage form. International Journal of Pharmaceutical Sciences and Research, 2011;2: 1712–15.
- Naidu K, Kale U. Shingare M. Stability indicating RP-HPLC method for simultaneous determination of amlodipine and benazepril hydrochloride from their combination drug productJournal of Pharmaceutical and Biomedical Analysis, 2005;39: 147–55.
- Basavaiah K, Chandrashekar U, Nagegowda P. Spectrophotometric and high performance liquid chromatographic determination of Amlodipine besylate in Pharmaceuticals. Science Asia, 2005; 31: 13–21.
- Pawar P, Gaikwad P, Bankar V, Pawar. Development and validation of uvspectrophotometric method for simultaneous estimation of atenolol and indapamide in bulk and tablet dosage form. International Journal of PharmTech Research, 2010;2: 876–885.
- Barot T.G., Prajapati V., Patel P.K., Shah N., Patel L.D., Int. J. Pharm. Tech. Research 2009, 1(4), 287-296.
- Sweetmann S.C., Martindale-The Complete Drug Reference, 33rd ed, London, Pharmaceutical press, 2002, 913.
- Block J.H., Beale J.M., Wilson and Gisvold’s Textbook of Organic Medicinal & Pharmaceutical Chemistry, 11th ed., Lippincott Williams & Wilkins, Wolters Kluner Company: Philadelphia, 2004, 607-610.
- Hang T.J., Zhao W., Liu J., Song M., Xie Y., Zhang Z., et.al., J. Pharm. & Biomed. Analysis, 2006, 40, 202-205.
- Singhvi I., Goyal A., Indian J. Pharm. Sci., 2010 Jul 9, 69(1), 164-165.
- Agrawal Y.K., Majumdar F.D., Anal. Letters, 1995 Jul, 28(9), 1619-1627.
- Rodriguez J.C., Barciela J., Garcia S., Herrero C., Pena R. M., J. AOAC Int., 2005 Jul, 88(4), 1148- 1154.
- X. Gao, J. Chen, N. Mei, W. Tao, W. Jiang, Xinguo Jiang. HPLC Determination and Pharmacokinetic Study of Indapamide in Human Whole Blood, Chemistry and Materials Science Chromatographia, Vol 61, Numbers 11- 12(2005), 581-585.
- Beckett, A. H.; Stenlake, J.B. Practical Pharmaceutical Chemistry. Fourth edition, Part 2, CBS Publishers and Distributors, New Delhi, 2002, 275-278.
- ICH, Q2 (R1), Harmonized tripartite guideline, Validation of analytical procedures: text and Methodology International Conference on Harmonization.
- ICH, Harmonised Tripartite Guideline, Text on Validation of Analytical Procedures, International Conference on Harmonization: Geneva, October1994, 1-5.
- Sethi P.D., High Performance Liquid Chromatography-Quantitative Analysis of Pharmaceutical Formulations, CBS Publishers and Distributors: New Delhi, 2001, 215-263.
- Skoog D.A., West D.M., Holler F.J., Crouch S.R., Fundamentals of Analytical Chemistry, 8th ed., Thomson Brooks/Cole: Singapore, 160-167.
- George L., Schmuff N., HPLC Methods for Pharmaceutical Analysis, John -Wiley and Sons Inc: New York, 1996, 682-69.


 Shubham G. Padol*
Shubham G. Padol*
 Sonali A. Waghmare
Sonali A. Waghmare










 10.5281/zenodo.11355337
10.5281/zenodo.11355337