This research focuses on the formulation and evaluation of a matrix drug delivery system for Aceclofenac using natural gum obtained from the Satpuda region. Aceclofenac, a non-steroidal anti-inflammatory drug (NSAID), is widely used to treat pain and inflammation but often causes gastrointestinal side effects when administered conventionally. To overcome this, a sustained-release drug delivery system was developed, aiming to release the drug over an extended period, thus improving patient compliance and minimizing adverse effects. The natural gum, chosen for its biodegradable and non-toxic properties, was used as the matrix-forming agent. Matrix tablets were prepared using different concentrations of the gum and other excipients via direct compression. The formulated tablets were subjected to evaluation for their physical properties, including hardness, friability, and drug content uniformity. In vitro drug release studies were conducted using dissolution tests over 12 to 24 hours, with the aim of achieving sustained drug release. The findings revealed that the natural gum effectively controlled the release of Aceclofenac, with drug release following a controlled pattern over time. The release mechanism was observed to be diffusion-controlled, supported by the swelling properties of the gum matrix. The formulation also demonstrated stability and compatibility between the drug and the gum, as confirmed by FTIR and DSC studies. In conclusion, the natural gum sourced from the Satpuda region proved to be an efficient and sustainable material for developing a matrix-based sustained-release drug delivery system for Aceclofenac. This approach offers the potential to enhance therapeutic outcomes while reducing dosing frequency and side effects, contributing to a more patient-friendly treatment for chronic pain and inflammation.
Aceclofenac, Matrix System, Sustained Release, Natural Gum, Satpuda Region, NSAID, Drug Delivery, Biodegradable Polymers
The quest for effective drug delivery systems has become increasingly important in the pharmaceutical field, particularly in improving the bioavailability and therapeutic efficacy of various medications. Aceclofenac, a non-steroidal anti-inflammatory drug (NSAID), is widely used for its analgesic and anti-inflammatory properties, especially in the management of osteoarthritis and rheumatoid arthritis. However, its therapeutic effectiveness is often limited by factors such as poor solubility, rapid metabolism, and short half-life. Matrix drug delivery systems have emerged as a promising approach to address these challenges. By controlling the release of the drug over an extended period, these systems can enhance bioavailability, minimize side effects, and improve patient compliance. Natural gums, derived from plant sources, offer several advantages in drug formulation, including biocompatibility, biodegradability, and the ability to form hydrophilic matrices that can modulate drug release profiles. The Satpuda region is known for its rich biodiversity, including various plants that produce natural gums with potential pharmaceutical applications. This study focuses on the formulation and evaluation of an aceclofenac matrix drug delivery system utilizing these natural gums. By leveraging the unique properties of these gums, the aim is to develop a sustained release formulation that enhances the therapeutic effects of aceclofenac while minimizing its side effects.
The present work will investigate the formulation parameters, physicochemical properties, in vitro release characteristics, and stability of the developed drug delivery system. Ultimately, this research seeks to contribute to the development of more effective and patient-friendly dosage forms for aceclofenac, utilizing locally sourced natural resources.
KHANDESH REGION
Khandesh is a significant geographic region in Central India, specifically situated in the northwestern part of Maharashtra state. This region encompasses the districts of Dhule, Jalgaon, and Nandurbar, and is bounded by the Baroda district of Gujarat to the north, Nimar district of Madhya Pradesh to the east, Jalgaon district to the south, and Nashik district to the west.
The Khandesh region is characterized by its diverse ecological landscape. To the east lies the Satpuda range, known for its rich vegetation and humid, semi-evergreen species. This biodiversity supports a variety of plant life, including those that produce natural gums with potential pharmaceutical applications. In contrast, the middle region of Khandesh features many deciduous species, contributing to the region's ecological variety. The western part of the region, however, primarily consists of dry scrub vegetation, reflecting the differences in climate and topography. The natural resources of Khandesh, particularly its flora, present opportunities for innovative research in pharmaceuticals. The unique properties of the natural gums found in this region can be harnessed for developing effective drug delivery systems, making Khandesh an important area for exploration in the field of pharmaceutical sciences.
DRY DECIDUOUS FOREST IN THE SATPUDA REGION
The dry deciduous forests of the Satpuda hills, located on the northern side of the Tapi River, play a vital role in the ecology of the Khandesh region. This area encompasses the Chopda, Yawal, and Raver ranges, characterized by a rainfall ranging from 70 to 101 cm annually. The dominant tree species in these forests include Tectona grandis (teak), Acacia catechu, Anogeissus latifolia, Terminalia tomentosa, Boswellia serrata, Terminalia arjuna, and Butea monosperma. These species contribute to the region's rich biodiversity and support various ecological functions.
In contrast, the scrub forests located on the southern side of the Tapi River exhibit a different ecological profile. With average rainfall varying from 38 to 63 cm and summer temperatures soaring up to 48°C, the vegetation here is less dense and more sparse. Prominent species in this area include Acacia catechu, Anogeissus latifolia, Boswellia serrata, Hardwickia binata, and Ziziphus jujuba. This region’s harsh climatic conditions limit the growth and diversity of plant life.
Khandesh, positioned in the northwestern corner of the Deccan Plateau, lies within the valley of the Tapi River. It is bounded by the Satpura Range to the north, the Berar (Vidarbha) region to the east, the Ajanta hills to the south, and the northern ranges of the Western Ghats to the west. The principal river, the Tapi, flows westward—a unique characteristic compared to most rivers in the Deccan, which typically flow eastward to the Bay of Bengal. The Tapi is fed by thirteen major tributaries, although none are navigable, and its deep riverbed historically hindered irrigation efforts.
Most of Khandesh is situated south of the Tapi River, drained by its tributaries such as the Girna, Bori, and Panjhra. The alluvial plain north of the river features some of the richest agricultural land in the region, while the landscape gradually ascends towards the Satpuda hills. Central and eastern Khandesh are predominantly flat, except for a few low ranges of barren hills. To the north and west, the terrain transitions into rugged hills, which are thickly forested and home to the tribal Bhil communities. This ecological diversity and the presence of unique flora provide significant opportunities for research and development in natural products and drug delivery systems.
KHANDESH REGION OVERVIEW
The Khandesh region, historically known as a former administrative division of British India, encompasses the modern-day districts of Jalgaon, Dhule, Nandurbar, and parts of Nashik in Maharashtra. This region is characterized by a rich tapestry of towns and villages, each contributing to its cultural and economic landscape.
KEY DISTRICTS AND TOWNS
- JALGAON DISTRICT: Prominent towns include Jalgaon, Amalner, Bhadgaon, Bhusawal, Bodwad, Chalisgaon, Chopda, Erandol, Dharangaon, Faizpur, Jamner, Pachora, Parola, Raver, Savda, and Yawal. This district is known for its agricultural output and various industries, including those related to bananas and onions.
- DHULE DISTRICT: Important towns include Dhule, Sakri, Sindkheda, and Shirpur. This district has a mix of urban and rural areas, with agriculture and textiles playing significant roles in its economy.
- NASHIK DISTRICT: This district features towns such as Deola, Kalwan, Malegaon, Nampur, Tarahbad, and Satana. Nashik is famous for its vineyards and is also an important pilgrimage site.
- NANDURBAR DISTRICT: The district headquarters is located in Nandurbar. It is bordered to the south and southeast by Dhule district, to the west and north by Gujarat, and to the north and northeast by Madhya Pradesh. Nandurbar was established on July 1, 1998, following the bifurcation of Dhule district. It consists of six talukas: Akkalkuwa, Akrani Mahal (Dhadgaon), Taloda, Shahada, Nandurbar, and Navapur. Covering an area of 5,034.23 square kilometers, Nandurbar has a population of approximately 1,311,709, with 65.5?longing to scheduled tribes. The Dhadgaon taluka has the highest tribal population at 94.95%.
HISTORICAL SIGNIFICANCE
Khandesh has historical significance, with Burhanpur (in Madhya Pradesh) serving as the capital of the old Khandesh province. Asirgarh, also in Madhya Pradesh, is part of this historical legacy.
GEOGRAPHY
Nandurbar District extends between 21°00’ to 22°30’ North latitude and 73°47’ to 74°47’ East longitude, and its northern boundary is defined by the Narmada River. The geography of Khandesh is diverse, featuring plains, hills, and river valleys, making it rich in natural resources and biodiversity.
This multifaceted region, with its blend of cultural heritage and ecological diversity, offers substantial potential for research and development, particularly in the areas of agriculture, natural products, and sustainable practices.
TRIBAL LIFE OF NANDURBAR DISTRICT
The tribal population of Nandurbar District primarily inhabits the valleys of the Satpura range, extending along the northern side of the Tapi River. This hilly region consists of six ranges: Taloda, Akkalkuwa (East and West), Kathi, Molgi, and Manibeli. These areas, known as tribal pockets, feature villages referred to as "Padav."
Major Tribes
- Bhil Pawara: The dominant tribe in the district, primarily located around Shahada, Toranmal, and Akrani. They communicate in Bhilori and Pawara dialects, and their cultural practices and social structures are significant in the region.
- Tadvi Gavit: Indigenous to the Satpura ranges, the Tadvi are a subgroup of Bhils who consider themselves superior to the Bhil community. Their identity reflects a blend of tradition and social hierarchy.
- Mavchi (Gavit): Concentrated in Nawapur tehsil, the Gavit are another group of Bhils, often referred to as Kokni Marathas due to their origins in Goa. They have unique customs and social structures.
- Vasave: Along with Bhils and Padvi, they are collectively referred to as 'Nayare' and share cultural and linguistic ties within the tribal community.
SOCIAL STRUCTURE AND PRACTICES
The social structure of these tribes often reflects patriarchal dominance, evidenced by practices such as polygamy, where families commonly consist of 2-3 wives and 10-12 children. Superstitious beliefs are prevalent, with witchcraft, locally referred to as "Dakin," being a common practice among these communities.
As part of modern interventions, government policies have facilitated the education of tribal children through Ashram schools, aiming to integrate them into the broader educational framework.
CULTURAL SIGNIFICANCE AND CONSERVATION
A notable aspect of tribal life is the reverence for sacred groves, such as the "Wagdeo gaga," which serve as critical elements of forest conservation. These sacred spaces reflect the tribes' deep-rooted connection to their environment and their role in maintaining ecological balance.
INDIGENOUS KNOWLEDGE AND MEDICINAL PRACTICES
The tribes possess a wealth of indigenous knowledge about the vegetation surrounding them, passed down through generations. This knowledge has led to the discovery of various plant-based remedies for ailments, showcasing the tribes' resourcefulness and understanding of their natural environment.
RESEARCH AND DOCUMENTATION
Given the rich tapestry of culture, tradition, and ecological knowledge within these tribes, the present investigation aims to document their indigenous practices and the native uses of different plants. This research not only honors their heritage but also contributes to the broader understanding of traditional medicine and conservation practices, providing valuable insights for both scientific study and cultural preservation.
NATURAL GUM FOUND IN THE KHANDESH REGION
The Khandesh region, with its diverse flora, is home to various natural gums that are integral to the local economy and the livelihoods of forest-dwelling communities. Gums are produced from woody plants through natural processes, such as exudation from bark cracks or damage caused by insects and animals. Additionally, gum production can be artificially induced through incisions in the bark, allowing for the collection of viscous nodules that are easily harvested.
CHARACTERISTICS OF GUMS
Gums are complex carbohydrate derivatives, primarily polysaccharides, and can either be soluble in water or form mucilages when absorbing large amounts of water. They exhibit hydrophilic properties and are generally composed of monosaccharide units linked by glucosidic bonds. These natural gums are typically insoluble in oils or organic solvents, making them valuable for various applications.
USES OF GUMS
- Culinary Applications: Gums are widely used in food products, enhancing texture, viscosity, and stability. They play crucial roles in confectionery, dairy products, and beverages, serving as emulsifiers and stabilizers.
- Pharmaceutical Applications: Gums function as demulcents and adhesives in pill manufacturing and are used as emulsifying agents in various formulations.
- Industrial Applications: In the industrial sector, gums find applications in adhesives, lithography, paints, and inks.
- Economic Importance: For many tribes in the Khandesh region, the collection and sale of gums provide a vital source of income. Unlike many non-timber forest products (NTFPs), which are typically available for only a few months each year, gums can be harvested for six to eight months, ensuring a more stable income stream.
PRODUCTION AND ECONOMIC IMPACT
India produces around 5,000 tons of plant-based gums annually, with major production states including Maharashtra, Madhya Pradesh, Chhattisgarh, and others. In 2006-07, India exported 1,730.24 tons of plant-based gums valued at approximately ?22.18 crore, while imports reached 19,464.08 tons valued at around ?58.79 crore.
COMMERCIALLY IMPORTANT GUMS
Some of the most significant gums produced in India include:
- Gum Arabic
- Gum Ghatti
- Gum Karaya
Gum tragacanth, derived from Astragalus species in Asia Minor, is particularly valuable as a natural emulsifier in food products like mayonnaise, though its high cost has led to a shift towards synthetic alternatives. Other gums of commercial interest come from plants such as carob (Ceratonia siliqua), gum mesquite (Prosopis latifolia), and Indian squill (Urginea indica).
GUMS FOUND IN THE KHANDESH REGION
The Khandesh region, particularly in the Nandurbar and Dhule districts, is rich in various natural gums sourced from diverse plant species. Below is a list of notable gum-producing plants, along with their local names:
- Sterculia urens
- Local Names: Kadai, Kadhay, Kadoni, Kewdi, Kandul, Kevda, Kudal
- Terminalia crenulata
- Local Names: Sadaba, Haijada, Sandadi
- Garuga pinnata
- Local Names: Kakad, Kakada, Kakod, Kakado
- Boswellia serrata
- Local Names: Dhupali, Salai, Goradu, Sal, Sayphal
- Azadirachta indica
- Local Names: Neem, Kadu-Neem, Neemada
- Acacia chundra
- Local Names: Khair, Esa, Esan, Kati
- Acacia nilotica spp. indica
- Local Names: Babhul, Telya-Babhul, Sadha Babhul
- Buchanania lanzan
- Pterocarpus
- (Specific species not mentioned, but typically includes various gum-producing trees)
- Butea monosperma
- (Commonly known as Flame of the Forest, it is also a source of gum)
- Dalbergia sissoo
- Local Names: Roxb (also known as Indian Rosewood)
- Lannea coromandelica
- (Commonly found in deciduous forests)
- Mangifera indica
IMPORTANCE OF GUMS
These gum-producing species not only contribute to the local economy but also play significant roles in traditional medicine, food, and various industrial applications. The collection and sale of these gums provide a vital source of income for many forest-dwelling communities in the Khandesh region.
ADVANTAGES OF NATURAL GUM
Natural gums offer a wide range of benefits across various industries, making them valuable resources. Here are some key advantages:
- Biodegradable: Natural gums decompose naturally, minimizing environmental impact compared to synthetic alternatives.
- Biocompatible and Non-toxic: They are safe for use in pharmaceuticals and food products, ensuring better compatibility with biological systems.
- Low Cost: Natural gums are often less expensive to produce and procure, providing cost-effective solutions for various applications.
- Environmentally-Friendly Processing: The extraction and processing of natural gums typically require less energy and fewer chemicals, promoting sustainable practices.
- Local Availability: Many natural gums are sourced from local flora, particularly in developing countries, supporting local economies and reducing transportation costs.
- Better Patient Tolerance and Public Acceptance: Due to their natural origins and safety profiles, natural gums are generally better tolerated by patients and more accepted by consumers.
- Versatile Uses in the Food Industry: Natural gums serve multiple roles, including:
Thickening Agents: Enhancing the texture of food products.
Gelling Agents: Providing structure to jellies, jams, and desserts.
Emulsifying Agents: Stabilizing mixtures of oil and water in dressings and sauces.
Stabilizers: Preventing separation in products like dairy and beverages.
- Diverse Applications in Other Industries: Beyond food, natural gums are used as:
- Adhesives: In woodworking and packaging.
- Binding Agents: In pharmaceuticals and cosmetics.
- Crystal Inhibitors: To prevent crystallization in products like syrups.
- Clarifying Agents: In beverages and other liquids to improve clarity.
- Encapsulating Agents: For controlled release in drug delivery systems.
- Flocculating Agents: In wastewater treatment.
- Swelling Agents: In hydrogels and controlled-release formulations.
- Foam Stabilizers: In food products and beverages to maintain texture.
DISADVANTAGES OF NATURAL GUMS
While natural gums offer numerous advantages, they also have some drawbacks that can affect their use across various applications. Here are key disadvantages:
- Microbial Contamination: Natural gums can be susceptible to contamination by bacteria, fungi, and other microorganisms, which can compromise their safety and efficacy, especially in food and pharmaceutical applications.
- Batch to Batch Variation: The composition and properties of natural gums can vary significantly between batches due to factors like plant source, harvesting methods, and environmental conditions. This variability can lead to inconsistent performance in formulations.
- Uncontrolled Rate of Hydration: Natural gums may absorb water at unpredictable rates, which can affect their functionality in formulations. This uncontrolled hydration can lead to issues in texture and stability, particularly in food and pharmaceutical products.
- Reduced Viscosity on Storage: Over time, natural gums can experience a reduction in viscosity during storage, impacting their effectiveness as thickening or stabilizing agents. This degradation can lead to changes in product consistency and quality.
CLASSIFICATION OF GUMS
Gums can be classified based on several criteria, including charge, source, shape, and chemical structure. Here’s a detailed overview:
1. According to Charge
-
- Non-ionic Seed Gums: Examples: Guar, Locust Bean, Tamarind, Xanthan, Amylose, Arabinans, Cellulose, Galactomannans.
- Anionic Gums: Examples: Gum Arabic, Gum Karaya, Gum Tragacanth, Gellan, Agar, Algin, Carrageenans, Pectic Acid.
2. According to Source
- Marine Origin/Algal (Seaweed) Gums: Examples: Agar, Carrageenans, Alginic Acid, Laminarin.
- Plant Origin:
- Shrubs/Tree Exudates: Gum Arabic, Gum Ghatti, Gum Karaya, Gum Tragacanth, Khaya, and Albizia gums.
- Seed Gums: Guar Gum, Locust Bean Gum, Starch, Amylose, Cellulose.
- Extracts: Pectin, Larch Gum.
- Tuber and Roots: Potato Starch.
- Animal Origin: Examples: Chitin, Chitosan, Chondroitin Sulfate, Hyaluronic Acid.
- Microbial Origin (Bacterial and Fungal): Examples: Xanthan, Dextran, Curidan, Pullulan, Zanflo, Emulsan, Baker’s Yeast Glycan, Schizophyllan, Lentinan, Krestin, Scleroglucan.
- Semi-synthetic:
- Starch Derivatives: Hetan Starch, Starch Acetate, Starch Phosphates.
- Cellulose Derivatives: Carboxy Methyl Cellulose (CMC), Hydroxy Ethyl Cellulose (HEC), Hydroxypropyl Methylcellulose (HPMC), Methylcellulose (MC), Microcrystalline Cellulose (MCC).
3. According to Shape
- Linear: Examples: Algins, Amylose, Cellulose, Pectins.
- Branched:
- Short Branches: Xanthan, Xylan, Galactomannan.
- Branch-on-Branch: Amyl Pectin, Gum Arabic, Tragacanth.
4. According to Monomeric Units in Chemical Structure
- Homoglycans: Examples: Amylose, Arabinans, Cellulose.
- Diheteroglycans: Examples: Algins, Carrageenans, Galactomannans.
- Tri-heteroglycans: Examples: Arabinoxylans, Gellan, Xanthan.
- Tetra-heteroglycans: Examples: Gum Arabic, Psyllium Seed Gum.
- Penta-heteroglycans: Examples: Ghatti Gum, Tragacanth.
APPLICATION IN SUSTAINED RELEASE DRUG DELIVERY SYSTEMS
Natural gums are particularly valuable in the formulation of sustained-release (SR) drug delivery systems. They offer several advantages:
- Simplified Production: The use of gum matrices eliminates the need for complex procedures like coating and pelletization.
- Controlled Drug Release: The rate of drug release can be controlled by adjusting the type and proportion of the polymer used.
- Improved Patient Compliance: SR formulations can be taken once or twice daily, compared to conventional forms that may require more frequent dosing.
- Therapeutic Concentration Maintenance: SR systems help maintain therapeutically effective drug concentrations in circulation over extended periods.
MATRIX SYSTEM CHARACTERISTICS
The matrix system is a widely utilized method for controlling drug release in pharmaceutical formulations. This system is essential for achieving a delayed and sustained release of drugs, where the active ingredient is either dissolved or dispersed within a matrix that resists disintegration. Here are the key characteristics that define matrix systems and differentiate them from other controlled release dosage forms:
1. Physical State of Drug
- Solid, Liquid, or Gaseous: The drug can exist in various physical states within the matrix. The solubility and distribution of the drug in the matrix significantly influence its release profile.
2. Chemical Nature of Support
- Polymer Characteristics: The type of polymer or excipient used as a support affects the matrix's physical properties and the drug's release rate. Different polymers can provide varied interactions with the drug, impacting solubility and permeability.
3. Route of Administration
- Oral, Transdermal, or Injectable: The route of administration plays a crucial role in determining the matrix design. Each route requires specific considerations regarding release kinetics, absorption, and bioavailability.
4. Matrix Shape and Alteration in Volume Over Time
- Shape and Size: The geometry of the matrix (e.g., tablets, beads, films) can influence the surface area available for drug release. Additionally, the matrix may swell, erode, or change shape over time, affecting the drug release dynamics.
5. Release Kinetic Model
- Release Mechanisms: Understanding the kinetics of drug release is vital. Common models include zero-order kinetics (constant release rate), first-order kinetics (dependent on drug concentration), and Higuchi kinetics (diffusion-controlled). The model helps predict how the drug will be released over time.
ADVANTAGES OF MATRIX SYSTEM
- Easy to manufacture.
- Patient compliance can be improved.
- Maintains therapeutic concentrations over prolonged periods.
- Reduction in toxicity by slowing drug absorption.
- Improvement in treatment efficacy.
- Safety margins of high potency drugs can be increased and the incidence of both local and systemic adverse side effects can be reduced in sensitive patients.
- Reduction in toxicity by slowing drug absorption.
DISADVANTAGES OF MATRIX DRUG DELIVERY SYSTEMS
- Remaining Matrix Removal: After the drug has been released, the leftover matrix may need to be removed, which can be inconvenient for patients and may require additional medical procedures.
- Increased Potential for First-Pass Metabolism: Some drugs may undergo extensive first-pass metabolism when administered via oral matrix systems, potentially reducing their effectiveness.
- Release Rates and Time Dependency: Drug release rates can be influenced by time, often following a square root of time relationship, which can complicate predictability and dosage adjustments.
- Poor In Vitro–In Vivo Correlation: There can be discrepancies between laboratory studies (in vitro) and actual biological performance (in vivo), making it difficult to accurately predict clinical outcomes based on lab results.
- Possible Reduction in Systemic Availability: The controlled release may lead to a decrease in the overall systemic availability of the drug, affecting its therapeutic effect.
- Stability Issues: Matrix systems can face stability problems, including degradation of the polymer or the drug itself, which may compromise the formulation's integrity over time.
- Need for Additional Patient Education: Patients may require more detailed instructions and counseling on how to use matrix systems effectively, which can increase the burden on healthcare providers.
- Compatibility Limitations: Not all drugs can be effectively blended with a given polymeric matrix, limiting the range of medications that can utilize this delivery method.
CLASSIFICATION OF MATRIX TABLET
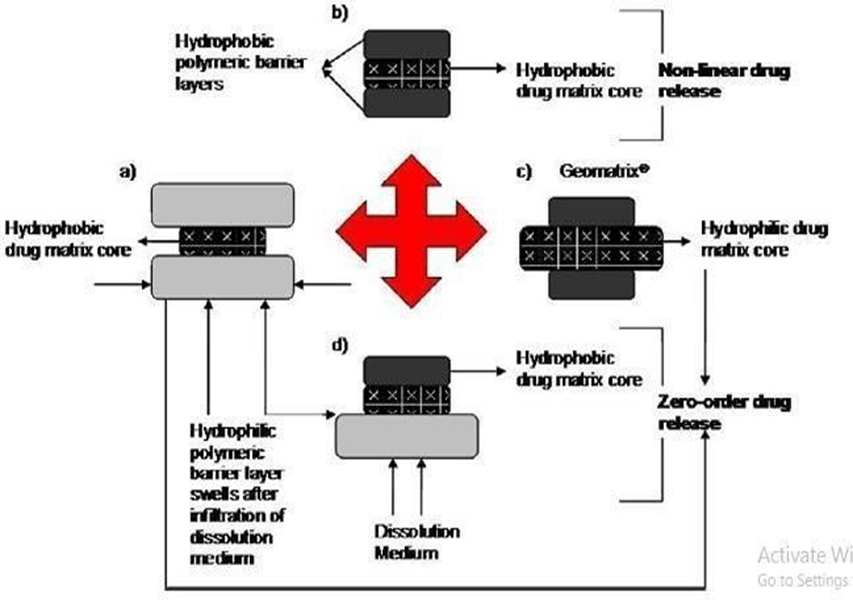
Fig. 1: Possible drug release mechanism from various matrix systems
A) LIPID MATRIX SYSTEM
Overview:
Lipid matrix systems are drug delivery formulations where the active pharmaceutical ingredient (API) is embedded within a hydrophobic lipid or waxy matrix. These systems are designed to provide controlled and sustained release of the drug.
Mechanism of Release:
- Matrix Composition: The matrix is primarily composed of lipid waxes and related materials, creating a stable and impermeable structure that encapsulates the drug.
- Porous Structure Formation: Upon exposure to an aqueous medium, a channeling agent within the matrix dissolves and leaches out. This process creates a porous network of capillaries throughout the matrix.
- Diffusion of the Active Ingredient: The drug, now dissolved in the aqueous medium, diffuses through the water-filled capillaries. The release rate is influenced by several factors, including:
- The nature of the lipid matrix.
- The solubility of the drug in the aqueous medium.
- The size and arrangement of the capillaries formed within the matrix.
ADVANTAGES OF LIPID MATRIX SYSTEMS
- Sustained Release: The hydrophobic nature of the matrix allows for prolonged drug release, reducing the frequency of dosing.
- Improved Stability: Lipid matrices can enhance the stability of certain drugs, protecting them from degradation.
- Reduced Variability: The controlled release mechanism can lead to more consistent plasma levels of the drug, improving therapeutic outcomes.
DISADVANTAGES OF LIPID MATRIX SYSTEMS
- Limited Drug Compatibility: Not all drugs are suitable for encapsulation in lipid matrices, particularly those with high solubility in water.
- Potential for Variable Release Rates: The release kinetics can vary based on the formulation, leading to challenges in achieving desired therapeutic levels.
- Challenges in Manufacturing: The preparation of lipid matrices can be more complex compared to other systems, requiring careful control of processing conditions.
B) INSOLUBLE POLYMER MATRIX SYSTEMS
Overview:
Insoluble polymer matrix systems are drug delivery formulations where the active pharmaceutical ingredient (API) is embedded within an inert, insoluble polymer matrix. This type of system is designed to provide controlled and sustained release of the drug over an extended period.
Mechanism of Release:
- Matrix Composition: The matrix is composed of insoluble polymers that do not dissolve in gastrointestinal fluids, providing a stable environment for the drug.
- Diffusion Through Capillaries: As the drug is released, it does not dissolve in the surrounding fluids. Instead, the drug molecules diffuse through a network of capillaries that form between compacted polymer particles. This network allows for the gradual release of the drug into the surrounding medium.
- Influence of Porosity and Tortuosity: The release rate of the drug can be modified by:
- Porosity: Increasing the porosity of the matrix enhances the release rate by allowing more space for the drug to diffuse through.
- Tortuosity: The complexity of the diffusion path can affect how quickly the drug moves through the matrix.
- Role of Pore-Forming Agents: The incorporation of hydrophilic salts or solutes can create additional pores within the matrix, significantly influencing the drug release rate by providing more pathways for diffusion.
ADVANTAGES OF INSOLUBLE POLYMER MATRIX SYSTEMS
- Sustained Release: These systems allow for prolonged release of the drug, leading to improved patient compliance and reduced dosing frequency.
- Stability: Insoluble polymers can protect sensitive drugs from degradation in the gastrointestinal tract, enhancing stability.
- Customizable Release Profiles: By altering the formulation (e.g., changing the type or amount of polymer, adding pore-forming agents), the release kinetics can be tailored to meet specific therapeutic needs.
DISADVANTAGES OF INSOLUBLE POLYMER MATRIX SYSTEMS
- Variable Release Rates: The release kinetics can be influenced by numerous factors, including the degree of polymer compaction and the presence of hydrophilic agents, which can complicate the design.
- Potential for Limited Solubility: Some drugs may have poor solubility in gastrointestinal fluids, potentially affecting the bioavailability of the drug.
- Manufacturing Complexity: Creating a consistent and reliable matrix can require precise manufacturing techniques, which may increase production costs.
C) HYDROPHILIC MATRICES
Overview:
Hydrophilic matrices, also known as swellable-soluble matrices, are a type of controlled drug delivery system that utilizes hydrophilic colloids to facilitate the sustained release of active pharmaceutical ingredients (APIs). These matrices are designed to swell upon contact with aqueous media, forming a gel-like structure that modulates drug release.
MECHANISM OF RELEASE:
- Swelling and Gel Formation: Upon contact with water, the hydrophilic components of the matrix absorb moisture and swell. This leads to the formation of a hydrated gel layer that surrounds the unhydrated core of the matrix.
- Diffusion Control: The drug release rate is primarily governed by the diffusion of the drug through this hydrated matrix layer. As water diffuses into the matrix, it dissolves the drug, which then diffuses out into the surrounding medium.
- Erosion and Dissolution: The outer hydrated layer gradually erodes and dissolves as it becomes more dilute, allowing for continuous release of the drug. The erosion rate is influenced by the specific nature of the hydrophilic colloids used in the formulation.
- Factors Influencing Release:
Nature of Colloid: Different hydrophilic polymers will have varying swelling capacities and erosion rates, which directly impact the drug release profile.
Viscosity and Molecular Weight: Higher viscosity or molecular weight polymers may result in slower drug release due to increased resistance to diffusion.
ADVANTAGES OF HYDROPHILIC MATRICES
- Controlled Release: The gel formation and subsequent erosion allow for a controlled and sustained release of the drug, improving therapeutic efficacy.
- Enhanced Patient Compliance: Reduced dosing frequency due to sustained release can enhance patient adherence to treatment regimens.
- Versatility: Hydrophilic matrices can accommodate a wide range of drugs, including those that are poorly soluble, providing flexibility in formulation design.
DISADVANTAGES OF HYDROPHILIC MATRICES
- Batch-to-Batch Variability: Variations in polymer properties can lead to inconsistent drug release profiles between batches.
- Sensitivity to Environmental Conditions: Factors such as pH, temperature, and the presence of other substances can affect the swelling and erosion behavior of the matrix, impacting drug release.
- Potential for Premature Release: If the matrix does not adequately control water ingress, there may be an uncontrolled release of the drug, reducing effectiveness.
D) BIODEGRADABLE MATRICES
Overview:
Biodegradable matrices are designed to release drugs in a controlled manner while gradually degrading within the body. These matrices are made from polymers that can be broken down by biological processes, making them suitable for various pharmaceutical applications.
MECHANISM OF RELEASE:
- Degradation Process: The polymer backbone of biodegradable matrices consists of monomers linked by functional groups that are susceptible to hydrolysis or enzymatic action. As the matrix degrades, it breaks down into smaller oligomers and monomers.
- Drug Release: As the matrix degrades, the encapsulated drug is gradually released into the surrounding environment. The rate of release can be influenced by the polymer's composition, molecular weight, and environmental conditions such as pH and temperature.
- Metabolism and Excretion: The resulting degradation products are typically non-toxic and can be metabolized by the body or excreted, reducing the risk of accumulation and associated side effects.
ADVANTAGES OF BIODEGRADABLE MATRICES
- Reduced Need for Surgical Removal:
Since these matrices are designed to break down naturally, they do not require surgical intervention for removal after drug release, minimizing patient discomfort and risk.
- Controlled Release Profiles:
The degradation rate can be tailored by selecting specific polymers and adjusting their chemical composition, allowing for precise control over the drug release kinetics.
- Compatibility with Biological Systems:
Biodegradable matrices are often made from biocompatible materials, reducing the likelihood of adverse reactions and improving patient tolerance.
- Potential for Localized Drug Delivery:
These systems can be used for targeted delivery of drugs to specific tissues or organs, enhancing therapeutic efficacy while minimizing systemic side effects.
DISADVANTAGES OF BIODEGRADABLE MATRICES
- Complexity of Formulation:
Developing biodegradable matrices with consistent and predictable release profiles can be challenging, requiring extensive characterization and optimization.
- Batch-to-Batch Variability:
Variability in polymer production can lead to differences in degradation rates and drug release characteristics, impacting overall efficacy.
Biodegradable materials may have limited shelf-life, especially if they are sensitive to moisture, temperature, or light.
- Potential for Incomplete Degradation:
In some cases, the matrix may not fully degrade, leading to concerns about residual materials causing inflammatory responses or other complications.
E) MINERAL MATRICES
Overview:
Mineral matrices are derived from natural polymers, primarily obtained from various species of seaweeds. These matrices utilize biopolymers like alginic acid, which is renowned for its unique properties that make it suitable for controlled drug delivery systems.
Key Features:
- Source: Mineral matrices are primarily derived from brown seaweeds (Phaeophyceae), with alginic acid being one of the most prominent examples. It is extracted through a process involving dilute alkali treatment.
- Composition: Alginic acid is a hydrophilic carbohydrate that consists of linear chains of ?-D-mannuronic acid and ?-L-guluronic acid. This unique structure contributes to its gel-forming abilities.
MECHANISM OF RELEASE:
- Hydration and Gel Formation: Upon contact with water, alginic acid swells and forms a gel-like matrix. This hydrated gel acts as a barrier, controlling the diffusion of the drug from within the matrix.
- Diffusion Controlled Release: The release of the drug occurs through diffusion mechanisms. The gel matrix allows for the gradual release of the encapsulated drug into the surrounding environment, leading to sustained therapeutic effects.
ADVANTAGES OF MINERAL MATRICES:
- Biocompatibility:
- Derived from natural sources, mineral matrices are generally biocompatible, reducing the risk of adverse reactions in patients.
- Controlled Drug Release:
- The gel-forming ability of alginic acid allows for tailored drug release profiles, enhancing the effectiveness of the treatment.
- Ease of Processing:
- Alginic acid and similar polymers are relatively easy to process, allowing for the development of various dosage forms.
- Thickening and Stabilizing Agent:
- In addition to drug delivery, mineral matrices like alginic acid can function as thickening agents in food and pharmaceutical formulations.
DISADVANTAGES OF MINERAL MATRICES:
- Variable Quality: The quality and properties of alginic acid can vary based on the source and extraction methods, potentially leading to batch-to-batch variability in drug release characteristics.
- Limited Release Control: The release profile can be influenced by factors such as pH and ionic strength, which may complicate the predictability of drug release.
- Potential for Gelation Issues: In some formulations, excessive gelation may hinder drug release, leading to slower-than-expected therapeutic effects.
COMPONENTS OF MATRIX TABLETS
Matrix tablets are designed for controlled release of active pharmaceutical ingredients (APIs) over an extended period. The formulation of matrix tablets involves various components, each playing a specific role in the drug delivery system. Here’s an overview of the key components:
- Active Drug: The therapeutic agent intended to provide the desired pharmacological effect. The selection of the active drug influences the overall formulation and release characteristics.
- Release Controlling Agent(s) (Matrix Formers): These are polymers or materials that form the matrix structure. They control the release rate of the active drug by affecting the diffusion and dissolution processes. Common examples include hydrophilic polymers (like hydroxypropyl methylcellulose) and hydrophobic materials (like ethyl cellulose).
- Matrix Modifiers:
Channeling Agents: These substances create pores or channels within the matrix, enhancing the rate of drug release. Examples include mannitol and certain surfactants.
Wicking Agents: These agents help draw water into the matrix, facilitating the drug’s dissolution and release. They can improve the hydration and swelling properties of the matrix.
- Solubilizers and pH Modifiers:
- Solubilizers: These enhance the solubility of the active drug, particularly for poorly soluble compounds. Examples include surfactants like polysorbate.
- pH Modifiers: These are used to adjust the pH of the formulation, which can influence the solubility and stability of the active drug.
- Lubricants and Flow Aids: These components improve the processing characteristics of the tablet formulation, ensuring smooth flow during tablet compression and preventing sticking to machinery. Common lubricants include magnesium stearate and stearic acid.
- Supplementary Coatings: Coatings can be applied to further control the drug release profile. They may extend lag time, provide taste masking, or protect the drug from environmental factors. Examples include enteric coatings that resist dissolution in the stomach but release in the intestines.
RATIONALE FOR DEVELOPING SUSTAINED RELEASE MATRIX DEVICES
The development of sustained release matrix devices is driven by several key objectives that enhance therapeutic efficacy and patient adherence. Here’s a closer look at the rationale:
- Extend the Duration of Action of the Drug:
Sustained release formulations are designed to release the drug over an extended period, maintaining therapeutic levels in the bloodstream for longer durations. This is particularly beneficial for drugs with short half-lives.
- Reduce the Frequency of Dosing:
By providing a slow and controlled release of the drug, these devices can decrease the number of doses a patient needs to take throughout the day. This is especially advantageous for chronic conditions, improving patient compliance.
- Reduce Inter and Intra-Subject Variability:
Sustained release formulations help achieve more consistent drug absorption and action among different individuals (inter-subject variability) and within the same individual over time (intra-subject variability). This results in more predictable therapeutic outcomes.
- Minimize Fluctuations in Plasma Levels:
These devices aim to maintain steady plasma drug concentrations, reducing peaks and troughs associated with conventional dosing regimens. This can lead to improved safety and efficacy, especially for narrow therapeutic index drugs.
- Improve Drug Utilization:Enhanced drug delivery efficiency can lead to better overall treatment outcomes, maximizing the therapeutic effects while minimizing side effects. This is particularly important in medications where maintaining a certain plasma concentration is crucial for effectiveness.
POLYMERS USED IN MATRIX TABLETS
There are number of polymers which may be used to formulate matrix tablets depending on the physicochemical properties of the drug substance to be incorporated into matrix system and drug release profile required. Polymers used for matrix tablets may be classified as:
- Hydrogels:
- Poly-hydroxyethyl methacrylate (PHEMA).
- Cross-linked polyvinyl alcohol (PVA).
- Cross-linked polyvinylpyrrolidone (PVP).
- Polyethylene oxide (PEO).
- Polyacrylamide (PA).
- Soluble polymers:
- Polyethylene glycol (PEG).
- Polyvinyl alcohol (PVA).
- Polyvinylpyrrolidone (PVP).
- Hydroxypropyl methylcellulose (HPMC).
- Biodegradable polymers:
- Polylactic acid (PLA).
- Polyglycolic acid (PGA).
- Polycaprolactone (PCL).
- Polyanhydrides.
- Polyorthoesters.
- Non-biodegradable polymers:
- Polyethylene vinyl acetate (PVA).
- Polydimethylsiloxane (PDS).
- Polyether urethane (PEU).
POLYMERS USED IN MATRIX TABLETS
The choice of polymer in matrix tablet formulation is critical as it influences the drug release profile, stability, and overall performance of the dosage form. Here’s a classification of commonly used polymers:
1. Hydrogels
- Poly-hydroxyethyl methacrylate (PHEMA): A versatile hydrogel used for its excellent swelling properties.
- Cross-linked polyvinyl alcohol (PVA): Provides a network structure for controlled drug release.
- Cross-linked polyvinylpyrrolidone (PVP): Known for its biocompatibility and ability to form gels.
- Polyethylene oxide (PEO): A hydrophilic polymer that enhances drug solubility and release.
- Polyacrylamide (PA): Used for its gel-forming ability, facilitating sustained release.
2. Soluble Polymers
- Polyethylene glycol (PEG): Commonly used for its solubility and flexibility in modifying drug release rates.
- Polyvinyl alcohol (PVA): Serves both as a soluble polymer and as a hydrophilic matrix-forming agent.
- Polyvinylpyrrolidone (PVP): Enhances solubility and stability, aiding in controlled release.
- Hydroxypropyl methylcellulose (HPMC): Widely used for its gel-forming ability and controlled release properties.
3. Biodegradable Polymers
- Polylactic acid (PLA): Biodegradable and used in long-acting formulations.
- Polyglycolic acid (PGA): Known for rapid degradation, suitable for short-term applications.
- Polycaprolactone (PCL): A slow-degrading polymer ideal for sustained release formulations.
- Polyanhydrides: Provide controlled drug release through hydrolytic degradation.
- Polyorthoesters: Used for their ability to degrade in biological environments.
4. Non-Biodegradable Polymers
- Polyethylene vinyl acetate (PVA): Offers stability and controlled release without degradation.
- Polydimethylsiloxane (PDS): Known for its flexibility and chemical stability.
- Polyether urethane (PEU): Provides mechanical strength and flexibility in formulations.
5. Mucoadhesive Polymers
- Polycarbophil: Enhances adhesion to mucosal surfaces, promoting localized drug delivery.
- Sodium Carboxymethylcellulose: Known for its gel-forming properties and mucosal adhesion.
- Polyacrylic acid: Offers high swelling capacity and mucoadhesive properties.
- Tragacanth: A natural gum that provides mucoadhesive properties.
- Methylcellulose: Used for its gel-forming ability and compatibility.
- Pectin: A natural polysaccharide that enhances mucoadhesion.
6. Natural Gums
- Xanthan gum: Provides thickening and stabilizing properties in formulations.
- Guar gum: Used for its gel-forming and water-retention abilities.
- Karaya gum: A natural gum with good mucoadhesive properties.
- Gum Arabic: Known for its emulsifying and stabilizing properties.
- Locust bean gum: Provides viscosity and stabilization in drug formulations.
1) MUCOADHESIVE POLYMERS
- Polycarbophil: Enhances adhesion to mucosal surfaces, promoting localized drug delivery and improving bioavailability.
- Sodium Carboxymethylcellulose: Known for its gel-forming properties and strong mucoadhesive characteristics, often used in sustained-release formulations.
- Polyacrylic Acid: Offers high swelling capacity and is effective in enhancing mucoadhesion, making it suitable for various drug delivery systems.
- Tragacanth: A natural gum that provides good mucoadhesive properties and helps in thickening formulations.
- Methylcellulose: Used for its gel-forming ability and compatibility, aiding in sustained release and mucoadhesion.
- Pectin: A natural polysaccharide that enhances mucoadhesion and is often used in oral drug delivery systems.
2) NATURAL GUMS
- Xanthan Gum: Provides thickening and stabilizing properties, commonly used in food and pharmaceutical formulations for its viscosity.
- Guar Gum: Known for its gel-forming abilities and water retention, making it useful in various formulations, including those for controlled release.
- Karaya Gum: A natural gum with good mucoadhesive properties, often used in formulations targeting the gastrointestinal tract.
- Gum Arabic: Recognized for its emulsifying and stabilizing properties, frequently employed in food and pharmaceutical applications.
- Locust Bean Gum: Adds viscosity and stability to drug formulations, often used in combination with other gelling agents.
TYPES OF MATRIX SYSTEMS
The matrix system can be categorized based on the types of retarding agents or polymeric materials used:
- Hydrophobic Matrix System: Utilizes hydrophobic materials to control drug release by creating a barrier that slows the diffusion of the drug from the matrix.
- Hydrophilic Matrix System: Involves hydrophilic polymers that swell upon contact with water, forming a gel layer that controls the rate of drug release through diffusion.
- Fat-Wax Matrix System: Combines fats and waxes to create a matrix that slowly dissolves or erodes, allowing for sustained drug release.
- Biodegradable Matrix: Composed of polymers that degrade in the body, releasing the drug as they break down into non-toxic byproducts.
- Mineral Matrix: Made from mineral-based polymers, such as alginates derived from seaweed, offering unique release characteristics.
CLINICAL CONTEXT: INFLAMMATION AND DRUG DELIVERY
Inflammation is a key feature in various conditions, including autoimmune diseases and is characterized by redness, heat, pain, swelling, and loss of function. Effective treatment requires maintaining consistent drug concentrations in the body. Sustained-release dosage forms are designed to deliver drugs at a slow release rate over an extended period, which is particularly beneficial in managing chronic inflammatory conditions.
Example: Aceclofenac
Aceclofenac is a non-steroidal anti-inflammatory drug (NSAID) used to treat rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. It has a short biological half-life of about 4 hours, necessitating frequent dosing. Formulating aceclofenac into sustained-release dosage forms can reduce the frequency of administration and improve patient compliance.
Example: Guggul
Guggul is the oleogum resin from Commiphora mukul, known for its hypolipidemic properties and various therapeutic uses, including treatment of nervous disorders, skin conditions, and hypertension. Guggul resin is utilized in pharmaceuticals as a binding agent and rate-retarding polymer, making it suitable for sustained-release formulations.

Fig: 2 - (a) Guggul tree (b) Guggul gum
Isolation of Guggul Gum
MATERIALS:
- Coarsely powdered oleo-gum resin
- Water
- Alcohol (e.g., ethanol)
- Muslin cloth
- Oven (set to 45°C)
- Sieve (80#)
PROCEDURE:
- Purification:
- Begin by adding water to the coarsely powdered oleo-gum resin.
- Stir the mixture to dissolve soluble impurities.
- Allow the mixture to settle, then decant the water to remove impurities.
- Extraction:
- Add alcohol to the purified oleo-gum resin.
- Stir the mixture thoroughly to facilitate the extraction of the gum.
- Allow the mixture to sit for a period, allowing the gum to precipitate out.
- Filtration:
- Filter the mixture through muslin cloth to separate the precipitated gum from the alcohol solution.
- Collect the filtered gum.
- Drying:
- Transfer the collected gum to an oven set at 45°C.
- Dry the gum until it is completely moisture-free.
- Powdering:
- Once dried, pass the gum through an 80# sieve to obtain a fine powder.
- Percentage Yield Calculation:
- Weigh the final dried gum powder.
NEED AND OBJECTIVE
Need for Study
Pain and inflammation pose significant challenges in modern medicine, particularly in the context of autoimmune diseases. Inflammation is a critical response of the immune system to various stimuli, including injury, infection, environmental agents, malignancy, and cellular alterations. This physiological process is characterized by redness, heat, pain, swelling, and loss of function, making effective management essential.
The success of anti-inflammatory treatments relies heavily on maintaining appropriate drug concentrations within the body. Sustained release dosage forms, which gradually release the drug over an extended period, are instrumental in achieving this goal. Among various routes of administration, the oral route has garnered the most attention due to its complexity, convenience, and safety profile. Matrix tablets, consisting of a drug combined with release-retarding materials, represent a straightforward approach to designing sustained release systems.
This study aims to develop and evaluate a safe and effective anti-inflammatory matrix tablet incorporating Aceclofenac with Boswellia serrata, utilizing a blend of hydrophobic and hydrophilic polymers. Aceclofenac, a non-steroidal anti-inflammatory drug (NSAID), is widely used for treating conditions such as rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. As a newer derivative of diclofenac, Aceclofenac has fewer gastrointestinal complications and a relatively short biological half-life of approximately 4 hours, necessitating multiple doses throughout the day. This characteristic makes it an ideal candidate for modified release formulations, aiming to enhance patient compliance by reducing dosing frequency.
Additionally, Guggul (from Commiphora mukul) is notable for its hypolipidemic properties, contributing further therapeutic potential when combined with Aceclofenac. The inclusion of Boswellia serrata, known for its anti-inflammatory effects, complements the formulation, enhancing its overall efficacy. Through this research, we seek to establish a sustained release matrix tablet that optimally delivers Aceclofenac, ultimately improving treatment outcomes for patients experiencing chronic pain and inflammation.n In the formulation of sustained release matrix tablets, inactive pharmaceutical ingredients play a crucial role as binding agents and rate-retarding polymers. These excipients not only enhance the mechanical properties of the tablets but also modulate the release profile of the active ingredient, ensuring a consistent therapeutic effect over time.
Guggul, derived from Commiphora mukul, serves as an effective binding agent and is rich in active compounds known for various therapeutic benefits. Its applications extend beyond its role in drug formulations; it is traditionally used to treat a range of conditions, including nervous diseases, leprosy, muscle spasms, skin disorders, ulcerative pharyngitis, hypertension, ischemia, and urinary disorders. The incorporation of guggul can potentially enhance the bioavailability and efficacy of the active drug.
Aceclofenac, classified as a Class II drug in the Biopharmaceutical Classification System (BCS), presents challenges related to solubility and gastrointestinal tolerance. Oral administration can lead to significant gastric irritation and side effects such as nausea and vomiting. To address these issues, a matrix tablet formulation was developed. By integrating Aceclofenac with natural polymers, the solubility and stability of the drug can be improved, leading to a more favorable release profile and enhanced patient compliance.
The combination of guggul and hydrophilic polymers in the matrix tablet aims to create an optimal environment for sustained drug release, mitigating the side effects associated with Aceclofenac while maximizing its therapeutic potential. This formulation strategy not only seeks to improve the patient experience but also contributes to more effective management of chronic pain and inflammation.
OBJECTIVES
The primary objective of this study is to develop and evaluate sustained release matrix tablets of Aceclofenac. The specific objectives are as follows:
- Preformulation Studies:
To determine the preformulation factors of Aceclofenac, including melting point and the establishment of a standard calibration curve in pH 7.4 phosphate buffer.
- Drug-Excipients Compatibility:
To conduct compatibility studies between Aceclofenac and various excipients to ensure stability and efficacy in the formulation.
- Pre-Compression Parameters:
To assess the pre-compression parameters of the powder blend, ensuring optimal flowability and compressibility for tablet formulation.
- Formulation Development:
To formulate sustained release matrix tablets of Aceclofenac using both natural (Guggul gum) and synthetic polymers (HPMC K 200M).
- Post-Compression Evaluation:
To evaluate the prepared sustained release matrix tablets by conducting various post-compression tests, including hardness, friability, and drug release studies.
- Investigation of Natural Polymers:
To study and investigate the effects of different concentrations of natural polymers on the sustained release profile of Aceclofenac and their impact on drug release kinetics.
PLAN OF WORK
- Literature survey
- Selection and Procurement of drug, excipients and polymers
- Preformulation study
- Characterization of Aceclofenac
- Drug excipients compatibility study
- FTIR studies
- DSC studies
- Flow Properties of drug, excipients, polymers & blend
- Standard Calibration Curve for drug
- Fabrication of Matrix tablet batches of Aceclofenac
- Evaluation of Matrix tablet batches of Aceclofenac
- Hardness
- Thickness
- Weight variation
- Friability
- Drug Content
- In vitro dissolution study
Flow chart of plan of work

DRUG AND EXCIPEINT PROFILE
DRUG, POLYMER AND EXCIPIENT PROFILE
ACECLOFENAC
Description:
Aceclofenac is a non-steroidal anti-inflammatory drug (NSAID) that exhibits site-specific action at the inflammation site, leading to enhanced efficacy in treating inflammatory conditions. It is generally well tolerated throughout the body.
Appearance: White or almost white crystalline powder.
Dosage: The maximum recommended dose is 200 mg, typically administered as two separate doses of 100 mg one tablet in the morning and one in the evening.
Solubility:
- Practically insoluble in water.
- Freely soluble in acetone.
- Soluble in alcohol and methanol.
CAS Registry Number: 89796-99-6
Molecular Weight: 354.19 g/ mol
Molecular Formula: C??H??Cl?NO?
BCS Classification: Class II (indicating low solubility and high permeability).
STRUCTURAL FORMULA OF ACECLOFENAC

Fig: 3 Structure of Aceclofenac
IUPAC Name: - 2-[(2, 6-Dichlorophenyl) amino] phenyl] acetyl] oxy] acetic acid.
Route - Oral Tablet
Half life - 4 - 4.3 hrs
MECHANISM OF ACTION OF ACECLOFENAC
Aceclofenac is a novel NSAID with a multifactorial mechanism of action, allowing it to effectively manage inflammation and pain. The key mechanisms include:
- Inhibition of Prostaglandin E2 (PGE2) Secretion: Aceclofenac directly blocks the secretion of PGE2 at the site of inflammation by inhibiting pro-inflammatory cytokines such as interleukin-1 beta (IL-1?) and tumor necrosis factor-alpha (TNF-?) in inflammatory cells. This reduces the inflammatory response.
- Synthesis of Extracellular Matrix: It stimulates the synthesis of the extracellular matrix in human articular cartilage, promoting cartilage health and repair, which is particularly beneficial in conditions like osteoarthritis.
- Inhibition of Neutrophil Activity: Aceclofenac inhibits the adhesion and accumulation of neutrophils at the inflammatory site during the early phase of inflammation. By doing so, it blocks the pro-inflammatory actions of neutrophils, further mitigating the inflammatory process.
- Selectivity for Cyclooxygenase-2 (COX-2): Compared to traditional NSAIDs like diclofenac sodium, Aceclofenac demonstrates greater selectivity for COX-2 over COX-1. This selective inhibition reduces the risk of gastrointestinal side effects while maintaining anti-inflammatory efficacy.
ABSORPTION OF ACECLOFENAC
- Rapid Absorption: Aceclofenac is rapidly absorbed after oral administration, leading to a quick onset of action.
- Bioavailability: The bioavailability of Aceclofenac is nearly 100%, indicating that nearly all of the administered dose reaches systemic circulation.
- Peak Plasma Concentration: Peak plasma concentrations are typically achieved within 1.25 to 3 hours following ingestion.
- Effect of Food: While the time to reach peak concentration (Tmax) may be delayed when taken with food, the overall degree of absorption remains unaffected.
DISTRIBUTION OF ACECLOFENAC
- Protein Binding: Aceclofenac is highly protein-bound, with over 99.7% of the drug binding to plasma proteins. This high level of binding can influence its distribution and therapeutic efficacy.
- Synovial Fluid Penetration: Aceclofenac effectively penetrates synovial fluid, where its concentration can reach approximately 60% of plasma levels. This characteristic is particularly beneficial for targeting inflammation in joint tissues.
- Volume of Distribution: The volume of distribution (Vd) of Aceclofenac is approximately 30 liters, indicating a moderate distribution throughout body tissues.
METABOLISM OF ACECLOFENAC
- Metabolic Pathway: Aceclofenac is primarily metabolized by the cytochrome P450 enzyme CYP2C9.
- Main Metabolite: The principal metabolite formed is 4-OH Aceclofenac, which contributes to its therapeutic effects.
- Additional Metabolites: Other metabolites include Diclofenac and 4-OH Diclofenac, which are also products of Aceclofenac metabolism and may play a role in its overall pharmacological profile.
ELIMINATION OF ACECLOFENAC
- Half-Life: The mean plasma elimination half-life of Aceclofenac is approximately 4 to 4.3 hours, indicating a relatively quick clearance from the body.
- Route of Elimination: About two-thirds of the drug is eliminated via urine, highlighting the importance of renal function in the drug's clearance.
- Clearance Rate: The clearance of Aceclofenac is estimated at around 5 liters per hour, reflecting its efficiency in being processed and eliminated from the system.
- Excretion of Unchanged Drug: Only about 1% of an oral single dose is excreted unchanged, indicating that the majority of the drug is metabolized before elimination.
CHARACTERISTICS IN PATIENTS
- Elderly Patients: No significant changes in the pharmacokinetics of Aceclofenac have been detected in elderly individuals, suggesting that age does not substantially affect the drug's absorption, distribution, metabolism, or elimination.
- Liver Function: In patients with decreased liver function, a slower rate of elimination of Aceclofenac has been observed. This indicates that liver function can influence the metabolism and clearance of the drug, potentially requiring dose adjustments.
- Renal Impairment: In patients with mild to moderate renal impairment, no clinically significant differences in the pharmacokinetics of Aceclofenac were observed after a single dose. This suggests that renal function may not significantly impact the drug's pharmacokinetics in these patients.
CONTRAINDICATIONS OF ACECLOFENAC
Aceclofenac is contraindicated in the following situations:
- Allergic Reactions:
- Patients with a history of hypersensitivity to Aceclofenac or any of its excipients.
- Asthma and Allergic Conditions:
- Patients who experience asthma attacks, bronchospasm, acute rhinitis, or urticaria triggered by substances with similar action (e.g., Aspirin).
- Gastrointestinal Issues:
- Patients with active or suspected peptic or duodenal ulcers.
- Individuals with a history of recurrent peptic or duodenal ulcers.
- Patients experiencing gastrointestinal bleeding or other active bleeding disorders.
- Severe Organ Impairment:
- Patients with severe heart failure or severely impaired hepatic (liver) or renal (kidney) function.
- Pregnancy:
- Use during the last three months of pregnancy is contraindicated.
INTERACTIONS WITH OTHER MEDICINAL PRODUCTS
Aceclofenac has potential interactions with various medications, and the following combinations should be avoided or monitored closely:
- Methotrexate:
- Aceclofenac can inhibit the tubular secretion of methotrexate, potentially leading to decreased clearance of methotrexate. This may increase the risk of methotrexate toxicity.
- Lithium:
- Aceclofenac may inhibit the renal clearance of lithium, resulting in elevated serum concentrations of lithium. This can increase the risk of lithium-related side effects.
- Anticoagulants and Antiplatelet Agents:
- Caution is advised when combining Aceclofenac with oral anticoagulants of the coumarin group, ticlopidine, thrombolytics, and heparin. These combinations should be avoided unless careful monitoring is in place due to the increased risk of bleeding.
COMBINATIONS REQUIRING DOSE ADJUSTMENTS AND PRECAUTIONS
- Methotrexate:
- When using low doses of methotrexate, especially in patients with decreased renal function, caution is advised due to the potential interaction with Aceclofenac that can decrease methotrexate clearance.
- Cyclosporine and Tacrolimus:
- Co-administration with Aceclofenac may increase the risk of nephrotoxicity due to reduced synthesis of prostacyclin in the kidneys.
- Aspirin and Other NSAIDs:
- Concomitant use may increase the frequency of side effects. Additionally, these combinations may counteract the antihypertensive effects of thiazide diuretics.
- Potassium-Sparing Medications:
- Aceclofenac can affect potassium levels, necessitating monitoring of serum potassium levels during treatment.
- ACE Inhibitors:
- The combination of Aceclofenac and ACE inhibitors carries a risk of acute renal failure in dehydrated patients, warranting careful monitoring.
- Antihypertensive Agents:
- While Aceclofenac was not found to affect blood pressure control when co-administered with bendrofluazide, the potential for interactions with other antihypertensive drugs, such as beta-blockers, should not be overlooked.
OTHER POSSIBLE INTERACTIONS
There have been isolated reports indicating that Aceclofenac may exhibit hypoglycemic effects. Therefore, when administering Aceclofenac, it is important to consider the following:
- Adjustment of Dosage: Healthcare providers should evaluate the need for dosage adjustments of antidiabetic agents that can produce hypoglycemia. This is particularly relevant for patients with diabetes who are being treated with medications such as insulin or sulfonylureas.
USE DURING PREGNANCY AND LACTATION
- Pregnancy:
Aceclofenac is contraindicated during pregnancy due to potential fetal effects. These effects may arise from the drug's inhibitory action on prostaglandin synthesis, which can lead to complications such as blocking uterine contractions and delaying delivery.
- Lactation:
It is not known whether Aceclofenac is excreted in human breast milk. Therefore, its use during lactation should be avoided unless deemed essential by a physician. In such cases, careful consideration of the benefits versus risks is necessary.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Patients taking Aceclofenac or other NSAIDs who experience dizziness or any central nervous system disturbances should refrain from driving or operating machinery. Such symptoms can impair coordination and reaction times, posing a safety risk.
Indications of Aceclofenac
Gonalgia (Knee Pain): Aceclofenac is indicated for the management of knee pain, known as gonalgia.
Clinical Study
A controlled double-blind study was conducted comparing Aceclofenac with Diclofenac in 40 patients suffering from acute or chronic gonalgia. The results showed that Aceclofenac demonstrated slightly superior activity compared to Diclofenac; however, the difference was not statistically significant.
ACECLOFENAC IN OSTEOARTHRITIS
Aceclofenac is indicated for the management of osteoarthritis, particularly in the knee.
Efficacy
In patients with knee osteoarthritis, Aceclofenac has been shown to:
- Decrease pain effectively.
- Reduce disease severity.
- Improve functional capacity of the knee.
The effectiveness of Aceclofenac in these areas is comparable to that of other NSAIDs such as Diclofenac, Piroxicam, and Naproxen.
MECHANISM OF ACTION OF ACECLOFENAC
Aceclofenac is a novel non-steroidal anti-inflammatory drug (NSAID) that exhibits a multifaceted mechanism of action:
- Inhibition of Prostaglandin E2 (PGE2) Secretion: Aceclofenac directly inhibits the secretion of PGE2 at the site of inflammation. This is achieved by blocking the activity of pro-inflammatory cytokines such as interleukin-beta (IL-?) and tumor necrosis factor-alpha (TNF-?) in inflammatory cells.
- Stimulation of Extracellular Matrix Synthesis: The drug promotes the synthesis of the extracellular matrix in human articular cartilage, which can aid in cartilage repair and maintenance.
- Inhibition of Neutrophil Activity: Aceclofenac reduces neutrophil adhesion and accumulation at inflammatory sites during the early phases of inflammation. This action helps to block the pro-inflammatory effects typically mediated by neutrophils.
- Selective Cyclooxygenase (COX) Inhibition: Aceclofenac exhibits greater specificity for the COX-2 enzyme compared to diclofenac sodium, which is significant in reducing gastrointestinal side effects while providing effective anti-inflammatory action.
ABSORPTION OF ACECLOFENAC
Aceclofenac is rapidly absorbed following oral administration, with nearly 100% bioavailability. The peak plasma concentration is typically reached between 1.25 to 3 hours after ingestion.
- Food Interaction: While the absorption degree is not significantly influenced by food, the time to peak concentration (Tmax) may be delayed when taken with meals. This means that while the overall absorption remains effective, the onset of action may be slower if the drug is consumed with food.
DISTRIBUTION OF ACECLOFENAC
Aceclofenac is characterized by its high protein binding, with over 99.7% of the drug bound to plasma proteins. This extensive binding influences its pharmacokinetics and therapeutic effects.
- Synovial Fluid Penetration: Aceclofenac effectively penetrates synovial fluid, achieving concentrations that are about 60% of those found in plasma. This property is particularly beneficial for treating inflammatory conditions affecting the joints, such as osteoarthritis and rheumatoid arthritis.
- Volume of Distribution: The volume of distribution (Vd) of aceclofenac is approximately 30 liters, indicating significant distribution throughout the body tissues.
METABOLISM OF ACECLOFENAC
Aceclofenac is primarily metabolized in the liver through the cytochrome P450 enzyme CYP2C9. The main metabolite produced is 4-OH Aceclofenac, which possesses anti-inflammatory properties.
- Other Metabolites: In addition to 4-OH Aceclofenac, the metabolism of aceclofenac also results in the formation of diclofenac and 4-OH Diclofenac. These metabolites may contribute to the overall therapeutic effects as well as the pharmacokinetic profile of the drug.
ELIMINATION OF ACECLOFENAC
- Half-Life: The mean plasma half-life of aceclofenac is approximately 4 to 4.3 hours, indicating a relatively quick elimination from the bloodstream.
- Route of Elimination: About two-thirds of the drug is eliminated through the urine, primarily as metabolites. Only about 1% of a single oral dose is excreted unchanged, highlighting the extensive metabolism that occurs before elimination.
- Clearance: The clearance of aceclofenac is estimated at 5 liters per hour, reflecting its efficiency in removal from the plasma.
CHARACTERISTICS IN PATIENTS
- Elderly Patients: No significant changes in the pharmacokinetics of aceclofenac have been observed in elderly individuals, suggesting that standard dosing can be maintained in this population.
- Liver Function: Patients with decreased liver function may experience a slower rate of elimination of aceclofenac. This could necessitate dose adjustments to avoid potential accumulation and associated side effects.
- Renal Impairment: In patients with mild to moderate renal impairment, no clinically significant differences in the pharmacokinetics of aceclofenac have been noted following a single dose. This indicates that the drug can generally be used without major concerns in this demographic.
CONTRAINDICATIONS OF ACECLOFENAC
Aceclofenac is contraindicated in the following situations:
- Allergic Reactions: Patients who have a history of hypersensitivity to aceclofenac or any of its excipients should not use this medication.
- Asthma and Allergic Conditions: Individuals for whom substances with similar actions (such as aspirin) trigger asthma attacks, bronchospasm, acute rhinitis, or urticaria should avoid aceclofenac due to potential cross-reactivity.
- Gastrointestinal Issues: Patients with active or suspected peptic or duodenal ulcers, a history of recurrent ulcers, gastrointestinal bleeding, or other active bleeding disorders should not take aceclofenac.
- Severe Organ Dysfunction: Contraindications also include patients with severe heart failure or significantly impaired hepatic or renal function.
- Pregnancy: The use of aceclofenac is contraindicated during the last three months of pregnancy due to potential risks to the fetus.
INTERACTIONS WITH OTHER MEDICINAL PRODUCTS
Aceclofenac may interact with several medications, and the following combinations should be avoided or used with caution:
- Methotrexate: Aceclofenac inhibits the tubular secretion of methotrexate, which can lead to decreased clearance of methotrexate and potentially increase its toxicity.
- Lithium: Aceclofenac can inhibit the renal clearance of lithium, resulting in elevated serum lithium concentrations. Monitoring of lithium levels is advised when these medications are used together.
- Anticoagulants: The combination of aceclofenac with oral anticoagulants (such as those in the coumarin group), ticlopidine, thrombolytic agents, and heparin should be avoided unless careful monitoring of coagulation parameters is implemented. This is due to the increased risk of bleeding.
INTERACTIONS REQUIRING DOSE ADJUSTMENTS AND PRECAUTIONS
Certain combinations of aceclofenac with other medications may necessitate careful monitoring, dose adjustments, or additional precautions:
- Methotrexate: When using low doses of methotrexate, particularly in patients with decreased renal function, caution is warranted due to the potential for interactions that can affect clearance.
- Cyclosporine and Tacrolimus: Co-administration with these immunosuppressants may increase the risk of nephrotoxicity, as aceclofenac can reduce prostacyclin synthesis in the kidneys.
- Aspirin and Other NSAIDs: Combining aceclofenac with aspirin or other NSAIDs can elevate the frequency and severity of side effects. Additionally, it may counteract the antihypertensive effects of thiazide diuretics.
- Potassium Levels: Concomitant use of aceclofenac with medications that affect potassium levels may require monitoring of serum potassium, as there is a risk of hyperkalemia.
- ACE Inhibitors: The combination of aceclofenac and ACE inhibitors carries a risk of acute renal failure, particularly in dehydrated patients, necessitating careful monitoring of renal function.
- Antihypertensive Medications: While co-administration with bendrofluazide does not seem to affect blood pressure control, potential interactions with other antihypertensive drugs, such as beta-blockers, should be considered.
OTHER POSSIBLE INTERACTIONS
There have been isolated reports indicating that aceclofenac may produce hypoglycemic effects. Consequently, when aceclofenac is administered alongside hypoglycemic agents (such as insulin or oral antidiabetic medications), it may be necessary to consider adjustments to their dosages to prevent excessive lowering of blood glucose levels.
Monitoring blood glucose levels in patients taking aceclofenac, especially those with diabetes or on antidiabetic therapy, is advisable to ensure safe and effective management.
USE DURING PREGNANCY AND LACTATION
- Pregnancy: Aceclofenac is contraindicated during the last three months of pregnancy due to the potential for fetal effects, likely stemming from its inhibitory effects on prostaglandin synthesis. These effects may include blocking uterine contractions and delaying delivery, which can pose risks to both the mother and the fetus.
- Lactation: It is not known whether aceclofenac is excreted in human milk. Therefore, the use of aceclofenac during lactation should be avoided unless deemed essential by a physician, who should weigh the benefits against potential risks to the nursing infant.
Effects on Ability to Drive and Use Machines
Patients who experience dizziness or other central nervous system disturbances while taking NSAIDs, including aceclofenac, should refrain from driving or operating machinery. It is important to ensure that they are fully aware of how the medication affects them before engaging in such activities.
INDICATIONS OF ACECLOFENAC
- Gonalgia (Knee Pain): A controlled double-blind study indicated that aceclofenac has slightly superior activity compared to diclofenac in managing acute or chronic knee pain, although the difference was not statistically significant.
- Osteoarthritis: In patients with osteoarthritis of the knee, aceclofenac effectively decreases pain, reduces disease severity, and improves functional capacity, demonstrating similar efficacy to diclofenac, piroxicam, and naproxen.
- Rheumatoid Arthritis: The anti-inflammatory and analgesic effects of aceclofenac are comparable to those of ketoprofen, indomethacin, tenoxicam, and diclofenac in treating rheumatoid arthritis.
- Ankylosing Spondylitis: Aceclofenac has been shown to reduce the duration of morning stiffness and pain intensity while improving spinal mobility, with effects similar to indomethacin, naproxen, and tenoxicam.
- Dental Pain: The analgesic efficacy of aceclofenac has been assessed in patients with moderate to severe tooth pain, including after the extraction of impacted third molars.
- Postoperative Pain: In women undergoing episiotomy, aceclofenac (100 mg) demonstrated superior analgesic efficacy compared to paracetamol (650 mg), particularly 3 to 5 hours after administration.
- Dysmenorrhea: In a non-comparative study involving 1,338 women, aceclofenac was effective in treating dysmenorrhea during the first three days of two consecutive cycles.
- Acute Lumbago: In a study of 100 patients, aceclofenac (150 mg intramuscularly for 2 days, followed by 100 mg orally twice daily) was superior to diclofenac in alleviating functional impairment due to acute lumbago.
- Musculoskeletal Trauma: Aceclofenac (100 mg twice daily) has been evaluated in non-comparative studies for its efficacy in managing musculoskeletal trauma.
Polymer Profile: Guggul Gum
Source and Description Guggul is the gum resin obtained from two plants: Commiphora mukul and Boswellia serrata.
- Synonyms: Guggul, Guggulu, Guggal, Guggala, Gulgulu, Guggalu, Maishakshi, Gukkal
- Family: Burseraceae
- Biological Source: Guggul gum is derived from the stem of Commiphora mukul and Commiphora wightii.
Isolation of Guggul Gum
- Preparation: Coarsely powdered oleo-gum resin is purified with water to remove impurities.
- Extraction: The purified oleo-gum resin is extracted with alcohol.
- Filtration: The precipitated gum is filtered through muslin cloth.
- Drying: The gum is dried in an oven at 45°C until completely dry.
- Final Processing: The dried gum powder is passed through an 80# sieve, and the percentage yield is calculated.
Properties
- Taste and Odor: The dried gum-resin has a bitter aromatic taste and a balsamic odor.
- Color: Guggul gum varies in color from transparent golden brown to dark brown.
- Solubility: It is soluble in most organic solvents.
- Combustion: It burns readily, releasing a pleasant aroma.
Uses
Modern therapeutic applications of guggul include:
- Nervous Diseases: Treatment for conditions like hemiplegia and neuralgia.
- Skin Disorders: Used for conditions such as leprosy, marasmus, scrofula, and ulcerative pharyngitis.
- Dental Issues: Effective in treating pyorrhea and spongy gums.
- Cardiovascular Health: Research shows guggul is beneficial in reducing cardiovascular disease risks, including lowering platelet stickiness and treating hyperlipoproteinemia effectively.
- Other Conditions: Includes management of hypertension, ischemia, urinary disorders, and respiratory issues like dyspnea and chest pain, especially in conjunction with Inula racemosa.
HYDROXYPROPYL METHYLCELLULOSE (HPMC)
Non-Proprietary Names:
- BP: Hypromellose
- JP: Hydroxypropylmethylcellulose
- PhEur: Hypromellosum
- USP: Hypromellose
Synonyms:
- Benecel MHPC
- E464
- Hydroxypropyl methylcellulose
- HPMC
- Methocel
- Methylcellulose propylene glycol ether
- Methyl hydroxypropylcellulose
- Metolose
- Tylopur
Overview
Hydroxypropyl methylcellulose (HPMC) is a semi-synthetic polymer derived from cellulose. It is widely used in pharmaceutical formulations, food products, and various industrial applications due to its unique properties.
KEY PROPERTIES
- Solubility: HPMC is soluble in cold and hot water, forming a gel-like consistency upon hydration.
- Viscosity: The viscosity can be modified by changing the degree of substitution, making it versatile for different applications.
- Film-forming Ability: HPMC forms a transparent, flexible film, which is beneficial in coatings and controlled-release formulations.
Applications
- Pharmaceuticals: Used as a binder, thickener, and film-forming agent in tablets and capsules. It's also employed in ophthalmic solutions and as a stabilizer in emulsions.
- Food Industry: Acts as a thickener, emulsifier, and stabilizer in various food products.
- Cosmetics and Personal Care: Utilized in lotions, creams, and gels for its thickening and emulsifying properties.
- Industrial Applications: HPMC is used in construction materials, paints, and coatings for its water retention and binding properties.
STRUCTURAL FORMULA:

Fig :4 Hydroxy Propyl Methyl Cellulose
HYDROXYPROPYL METHYLCELLULOSE (HPMC)
Chemical Information:
- Chemical Name: Cellulose hydroxypropyl methyl ether
- CAS Registry Number: 9004-65-3
Functional Category:
- Coating agent
- Film-former
- Rate-controlling polymer for sustained release
- Stabilizing agent
- Suspending agent
- Tablet binder
- Viscosity-increasing agent
APPLICATIONS IN PHARMACEUTICAL FORMULATION OR TECHNOLOGY
1. Oral Formulations:
- Tablet Binder: HPMC is primarily used as a binder in both wet and dry granulation processes. Typical concentrations range from 2% to 5% w/w.
- Extended-Release Tablets: High-viscosity grades can be incorporated at levels of 10-80% w/w to control drug release from matrix formulations.
- Film Coating: Concentrations of 2-20% w/w are utilized in film-forming solutions. Lower viscosity grades are preferred for aqueous solutions, while higher-viscosity grades are used with organic solvents.
COMMERCIAL EXAMPLES OF FILM-COATING MATERIALS:
- Spectracel
- Pharmacoat
- AcryCoat C
2. Ophthalmic Formulations:
- HPMC produces clear aqueous solutions with fewer undispersed fibers compared to methylcellulose, making it preferable for ophthalmic use.
- Used at concentrations of 0.45-1.0% w/w as a thickening agent in eye drops and artificial tear solutions.
3. Topical Formulations:
- Acts as an emulsifier, suspending agent, and stabilizing agent in gels and ointments.
- Functions as a protective colloid, preventing droplet and particle coalescence and sedimentation.
4. Additional Uses:
- Capsules: HPMC is used in the manufacture of capsules.
- Adhesive in Bandages: Acts as an adhesive in plastic bandages.
- Wetting Agent: Employed as a wetting agent for hard contact lenses.
- Cosmetics and Food: Also widely used in cosmetics and food products for its thickening and stabilizing properties.
Description
HPMC is an odourless and tasteless, white or creamy-white fibrous or granular powder.
Melting point
Browns at 190–200°C; chars at 225–230°C. Glass transition temperature is 170 – 180°C.
Solubility
Soluble in cold water, forming a viscous colloidal solution; practically insoluble in chloroform, ethanol (95%) and ether, but soluble in mixtures of ethanol and
dichloromethane, mixtures of methanol and dichloromethane, and mixtures of water and alcohol
Viscosity (dynamic)
A wide range of viscosity types are commercially available. Aqueous solutions are most commonly prepared; Dichloromethane and ethanol mixtures may also be used to prepare viscous Hypromellose solutions. Solutions prepared using organic solvents tend to be more viscous; increasing concentration also produces more viscous solutions.
Stability and storage
Hypromellose powder is a stable no. material, although it is hygroscopic after drying. Solutions are stable no. at pH 3–11. Hypromellose powder should be stored in a well- closed container, in a cool, dry place.
Incompatibilities
Hypromellose is incompatible with some oxidizing agents. Since it is non-ionic, Hypromellose will not complex with metallic salts or ionic organics to form insoluble precipitates.
Viscosity ranges of HPMC:
- HPMC K4 M-4000 (mPa s)
- HPMC K15 M-15000 (mPa s)
- HPMC K1OOM -1000 000 (mPa s)
- HPMC K200M -2000 000 (mPa s)
EXCIPIENT PROFILE MAGNESIUM STEARATE
Synonym: Magnesium octadecanoate, octadecanoic acid, magnesium salt; stearic acid, magnesium salt.
Chemical name: Octadecanoic acid magnesium salt.
Structural Formula: [CH3 (CH2)16COO] 2Mg

Fig :5 Magnesium Stearate
Empirical formula and molecular weight: C36H70MgO4; 591.34
Magnesium Stearate
Description:
Magnesium stearate is a very fine, light white powder that is precipitated or milled, characterized by a low bulk density. It has a faint odor of stearic acid, a greasy texture, and readily adheres to the skin.
- Molecular Weight: 591.27 g/mol
Functional Category:
- Tablet and capsule lubricant
Application in Pharmaceutical Formulation: Magnesium stearate is widely used in cosmetics, food products, and pharmaceutical formulations. It serves primarily as a lubricant in the manufacture of capsules and tablets, typically at concentrations between 0.25% and 5.0% w/w. It is also utilized in barrier creams.
Typical Properties:
- Density (Bulk): 0.159 g/cm?3;
- Density (Tapped): 0.286 g/cm?3;
- Solubility:
- Practically insoluble in ethanol, ether, and water
- Slightly soluble in warm benzene and warm ethanol (95%)
Stability and Storage Conditions: Magnesium stearate is stable and should be stored in a well-closed container in a cool, dry place.
Talc
Description:
Talc is a mineral composed of hydrated magnesium silicate. It is characterized by its light to dark green, brown, or white color and exhibits a foliated to fibrous crystal habit.
- Chemical Formula: H?Mg?(SiO?)? or Mg?Si?O??(OH)?
- Empirical Formula and Molecular Weight: Approximates to mg?(Si?O?)?(OH)?, with variable amounts of aluminum silicate and iron.
Synonyms:
- Altalc
- Hydrous magnesium calcium silicate
Functional Category:
- Anticaking agent
- Glidant
- Tablet and capsule diluent
- Tablet and capsule lubricant
Application of Talc:
Talc is used across various industries, including:
- Paper making
- Plastics
- Paint and coatings
- Rubber
- Food
- Electric cables
- Pharmaceuticals
- Cosmetics
- Ceramics
A coarse, greyish-green high-talc rock known as soapstone or steatite has applications in stoves, sinks, electrical switchboards, crayons, and soaps, owing to its resistance to heat, electricity, and acids.
APPLICATIONS IN PHARMACEUTICAL FORMULATION OR TECHNOLOGY:
Talc was traditionally used in oral solid dosage formulations as a lubricant and diluent, though its usage has decreased over time. It is still widely employed as a dissolution retardant in the development of controlled-release products.
Table no 1: Uses of Talc
|
USE
|
CONCENTRATION (%)
|
|
Dusting powder
|
90.0–99.0
|
|
Glidant and tablet lubricant
|
1.0–10.0
|
|
Tablet and capsule diluents
|
5.0–30.0
|
TALC
Description:
Talc is a very fine, white to grayish-white, odorless, impalpable, unctuous, crystalline powder. It adheres readily to the skin, is soft to the touch, and is free from grittiness.
Functional Uses:
- Lubricant: Used as a lubricant in tablet formulations and in a novel powder coating for extended-release pellets.
- Adsorbent: Effective in various applications as an adsorbent.
- Topical Preparations: Employed as a dusting powder, although it should not be used to dust surgical gloves due to potential contamination.
- Clarifying Agent: Used to clarify liquids.
- Cosmetics and Food Products: Utilized mainly for its lubricant properties.
Typical Properties:
- Moisture Content: Talc absorbs insignificant amounts of water at 25°C and relative humidity levels up to about 90%.
- Solubility: Practically insoluble in dilute acids, alkalis, organic solvents, and water.
Stability and Storage Conditions:
- Stability: Talc is a stable material.
- Sterilization: Can be sterilized by heating at 160°C for not less than 1 hour or by exposure to ethylene oxide or gamma irradiation.
- Storage: Should be stored in a well-closed container in a cool, dry place.
POLYVINYL PYRROLIDONE
Synonyms: Kollidon, Plasdone, Povidone
Chemical Name: 1-Ethyl, 2-pyrrolidone homopolymer
Molecular Weight: 2500 – 30, 00,000
Structure:

Fig: 6: Polyvinyl Pyrrolidone
Functional Category Disintegrant, dissolution aid, suspending agent, tablet binder.
Application.
- It is used as tablet binder, diluents, and coating agent at the concentration of 0.5- 5%.
- It is also used as suspending, stabilizing or viscosity increasing agent in topical oral and topical suspensions and solutions.
Description: It is a fine white to creamy white coloured, odourless, hygroscopic powder.
Solubility: Freely soluble in acids, chloroform, ethanol, ketones, methanol and water, practically insoluble in ether, hydrocarbons and mineral oil.
Viscosity: 1.3 -700 mPa s
Incompatibility: It is compatible with wide range of organic salts, natural andsynthetic resins.
LACTOSE
Non-Proprietary Names:
- BP: Anhydrous Lactose
- JP: Anhydrous Lactose
- PhEur: Lactose, Anhydrous
- USP-NF: Anhydrous Lactose
Synonyms:
- Anhydrous 60M
- Anhydrous Direct Tableting (DT)
- Anhydrous DT High Velocity
- Anhydrous Impalpable
- Lacto press Anhydrous
- Lacto press Anhydrous 250
- Lactosum anhydricum
- Lattosio
- Milk sugar
- SuperTab 21AN
- SuperTab 22AN
- Saccharum lactis
Chemical Name and CAS Registry Number: O-?-D-Galactopyranosyl-(1?4)-?-D-glucopyranose
CAS Number: 63-42-3
Empirical Formula and Molecular Weight:
- Formula: C??H??O??
- Molecular Weight: 342.30 g/mol
Description:
Anhydrous lactose appears as white to off-white crystalline particles or powder. It typically contains 70–80% anhydrous ?-lactose and 20–30% anhydrous ?-lactose.
Functional Category:
- Directly compressible tablet excipient
- Dry powder inhaler carrier
- Lyophilization aid
- Tablet and capsule diluent
- Tablet and capsule filler
Applications in Pharmaceutical Formulation or Technology: Anhydrous lactose is extensively used in:
- Direct Compression Tableting: Ideal for formulations requiring direct compression.
- Tablet and Capsule Filler and Binder: Serves as an effective filler and binder due to its compressibility and low moisture content.
- Moisture-Sensitive Drugs: Suitable for use with moisture-sensitive drugs because of its low moisture content.
- Intravenous Injection: Can also be utilized in formulations for intravenous injection.
METHODOLOGY
EQUIPMENTS:


 Utkarsh Mandage* 1
Utkarsh Mandage* 1
 Ketaki Gangavane 2
Ketaki Gangavane 2
 Priyanka Dhande 2
Priyanka Dhande 2
 Kiran Mapari 2
Kiran Mapari 2
 Pooja Gaikwad 3
Pooja Gaikwad 3
 Punam Badoge 4
Punam Badoge 4
 Pallavi Borse 1
Pallavi Borse 1



















 10.5281/zenodo.14267328
10.5281/zenodo.14267328