Abstract
Artificial intelligence (AI) has become a game-changer in healthcare, showing great promise in improving the control of hospital-acquired infections. This review delves into how AI is being used to enhance infection control in healthcare environments. It looks at the latest research on AI-based prediction models, real-time monitoring systems, and decision support tools that help prevent and manage healthcare-associated infections (HAIs). By utilizing machine learning and data analytics, AI has proven effective in forecasting outbreaks, refining antibiotic use, and enhancing the accuracy of infection surveillance. This review aims to give healthcare professionals and researchers a thorough understanding of the advantages, challenges, and future possibilities of using AI in infection control.
Keywords
Artificial intelligence (AI), Nosocomial infections, Healthcare-associated infections (HAIs), Infection control, Surveillance
Introduction
Artificial Intelligence
John McCarthy, the father of artificial intelligence who coined the term in 1956, said that "it is the combination of science and engineering to make intelligent devices for human welfare." Artificial intelligence is an intellect that is much smarter than the best human brain in practically every field, including computer science and linguistic logic.[1] It is a modern method of creating machines that can perform muscle work and solve complex questions in an "intellectual" manner. AI is concerned with the fundamental aspects of our lives, including philosophy, computer science, mathematics, linguistics, biology, neuroscience, and sociology. AI plays a very important role in exhibiting intelligent behavior, learning, demonstrating, and providing advice to the user. [2] Artificial General Intelligence (AGI) refers to systems capable of performing intellectual tasks at the level of human intelligence, capable of handling multiple processes simultaneously. AGI encompasses learning, perception, problem-solving, and adapting new solutions. It also involves linguistic logic and reasoning. [1,2]
Artificial intelligence can be categorized into two types:
Weak AI:
Weak AI systems are designed to behave as if they are intelligent. These systems can perform tasks like thinking, talking, and moving if programmed accordingly. For example, in a chess game, a computer can play and make moves automatically. While the computer does not truly think, it is programmed to make optimal moves. [4]
Strong AI:
Strong AI systems possess genuine intelligence and can perform calculations, think independently, and make predictions about the future. An example is IBM's artificial intelligence supercomputer, Watson. In the future, there may be machines or humanoids capable of performing tasks and thinking more powerfully than human beings. [4]
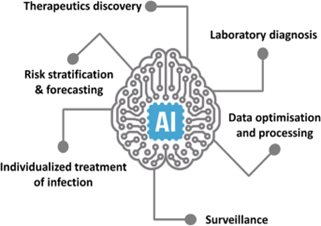
FIG 1
NASOCOMICAL INFECTION
Nosocomial infections, also known as hospital-acquired infections, have a history that goes back to the very beginning of hospitals. [5] The World Health Organization (WHO) describes these infections as those that occur in a patient during their stay in a hospital or other healthcare facilities and were not present at the time of admission. [6]
These infections often become noticeable either during the hospital stay or after the patient has been discharged. The pathogens responsible for these infections are referred to as nosocomial pathogens. In addition, infections that affect hospital staff or visitors can also be classified as nosocomial. [7]When harmful microorganisms are detected in body fluids or areas of the body that are typically sterile, such as the cerebrospinal fluid or blood of patients without symptoms, this can indicate an infection. [8] However, an infection that is present upon admission is not considered nosocomial. It is only labeled as such if there is a change in symptoms or if a new pathogen is detected, suggesting a new infection has been acquired. [9,10] Nejad and colleagues noted that patient care occurs in a variety of facilities, ranging from basic front-line units to advanced university teaching hospitals. [11] These facilities are linked to various nosocomial infections, from mild skin infections to severe, life-threatening conditions. [12] Such infections not only increase healthcare costs but also prolong hospital stays and significantly raise mortality rates. [13] Studies indicate that nosocomial infections are becoming increasingly problematic in the 21st century. This rise is partly due to the increased reliance on outpatient treatments, leaving hospitalized patients generally more severely ill. Hospitals also admit a large number of patients with compromised immune systems. Reports from the WHO and other sources have pointed out that the frequent use of antibiotics in hospitals has led to the emergence of antibiotic-resistant microorganisms. This issue is further aggravated by medical procedures that bypass the body’s natural defenses and by cross-contamination between medical staff and patients. Additionally, inadequate cleaning practices, such as improper washing, sterilization of equipment, and insufficient hygiene measures, contribute to the spread of pathogens in healthcare environments. [15]
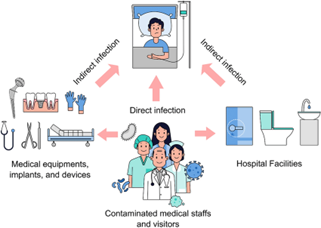
FIG. 2
Transmission of Infections in Healthcare Facilities
Infections are transmitted to individuals in healthcare settings through various routes, including:
- Droplet transmission
- Airborne transmission
- Contact transmission (direct or indirect)
Droplet transmission –
Droplet Transmission Droplets larger than five microns in diameter are spread through coughing, sneezing, talking, or during procedures like suctioning or bronchoscopy. [16] Because of their relatively large size, these droplets travel only short distances, usually two meters or less. Common organisms spread via droplet transmission include: [17]
- Respiratory viruses such as influenza, parainfluenza, adenovirus, respiratory syncytial virus, and human metapneumovirus.
- Bordetella pertussis, which causes whooping cough.
- Streptococcus pneumoniae, the leading cause of bacterial pneumonia.
Neisseria meningitidis, which is associated with a severe form of bacterial meningitis
Airborne transmission –
Airborne Transmission Tiny pathogens, smaller than five microns in diameter, are spread through coughing or sneezing. These particles can travel greater distances and remain airborne for longer periods. They are more likely to reach the alveoli, increasing their pathogenic potential. Common organisms spread via airborne transmission include: [18]
- Mycobacterium tuberculosis, which causes tuberculosis.
- Rubella virus, responsible for German measles.
- Varicella-zoster virus, which causes chickenpox and shingles. [19]
Contact Transmission (Direct or Indirect)
Direct contact transmission occurs when microorganisms are transferred from one person to another through direct physical contact. Doctors and nurses are at high risk during their physical examinations of patients. [20]
Indirect contact transmission involves the transfer of microorganisms to objects or surfaces within healthcare facilities, which are then touched by other patients or healthcare workers. Common objects associated with this type of infection include bed linen, furniture, bedpans, urinals, and medical examination equipment like thermometers, blood pressure cuffs, and stethoscopes. [21] In the age of antibiotic-resistant microbes, organisms of particular concern in this form of transmission include:
- Extended spectrum beta-lactamase (ESBL)
- Producing Gram-negative bacteria[21
FIG 3
Prevention of Nosocomial Infections
Preventing nosocomial infections requires a multifaceted approach, focusing on hygiene practices, proper use of antibiotics, and effective infection control protocols. Here are some key strategies:
1. Hand Hygiene
Regular Hand Washing: Healthcare workers must wash their hands thoroughly with soap and water or use alcohol-based hand sanitizers before and after patient contact, after touching potentially contaminated surfaces, and before performing medical procedures.
Challenge FG:
WHO Guidelines on Hand Hygiene in Health Care: a Summary. World Health Organization, Geneva, Switzerland 2009. [22]
2. Use of Personal Protective Equipment (PPE)
Appropriate Use of PPE: Gloves, gowns, masks, and eye protection should be worn when there is a risk of exposure to infectious agents. Proper use and disposal of PPE are crucial to prevent the spread of infections. [22]
3. Sterilization and Disinfection
Sterilizing Medical Equipment: Instruments and equipment must be properly sterilized using autoclaves or chemical disinfectants. Single-use items should be disposed of immediately after use. Environmental Cleaning: Regular and thorough cleaning of hospital environments, including patient rooms, operating theaters, and common areas, is essential. This includes disinfection of high-touch surfaces like doorknobs, bed rails, and medical equipment[23]
4. Antibiotic Stewardship
Rational Use of Antibiotics: Antibiotics should be prescribed only when necessary and in the correct dosages to reduce the development of antibiotic-resistant bacteria. Monitoring antibiotic use and resistance patterns helps in guiding appropriate therapy. [24]
5. Isolation of Infected Patients
Isolation Protocols: Patients with contagious infections should be isolated to prevent the spread of pathogens. Negative pressure rooms can be used for airborne infections to contain and filter the air. [25]
6. Education and Training
Staff Training: Continuous education and training programs for healthcare workers on infection prevention and control practices are vital. This includes proper hand hygiene, use of PPE, and updated protocols for managing infections. [25]
7. Monitoring and Surveillance
Infection Control Teams: Establishing dedicated infection control teams to monitor infection rates, investigate outbreaks, and implement control measures. Regular Audits: Conducting regular audits of infection control practices and adherence to protocols to identify areas for improvement. [26]
8. Safe Injection Practices
Use of Sterile Needles and Syringes: Ensuring that needles and syringes are sterile and used only once to prevent the transmission of bloodborne pathogens. Proper Disposal: Safe disposal of sharps in designated containers to prevent needlestick injuries and subsequent infections. [27]
9. Patient Education
Informing Patients: Educating patients about the importance of hygiene, the correct way to use antibiotics, and how to recognize signs of infection. Encouraging patients and visitors to follow hygiene protocols to minimize infection risks. [27]
10. Environmental and Engineering Controls
Air Filtration Systems: Utilizing high-efficiency particulate air (HEPA) filters in areas like operating rooms and isolation units to reduce airborne pathogens. Ventilation: Ensuring proper ventilation systems are in place to reduce the concentration of airborne infectious agents. [28,29]
Role of Artificial Intelligence in Preventing Nosocomial Infections[29]
Role of Artificial Intelligence in Preventing Nosocomial Infections as follows :
Predictive Analytics
Early Detection of Outbreaks: AI uses large amounts of data, such as electronic health records and infection reports, to spot patterns and predict potential outbreaks of hospital-acquired infections. By identifying trends and unusual occurrences, AI helps in taking early action to prevent the spread of infections[29]
Risk Assessment
AI models evaluate individual patient factors, such as their medical history and recent treatments, to estimate their risk of developing nosocomial infections. This allows for focused prevention efforts [30]
Surveillance and Monitoring
Real-Time Monitoring: AI systems can continuously check patient data and hospital environments for signs of infections. These systems alert healthcare workers about possible infections based on real-time analysis of various data sources, like lab results and patient symptoms [31]
Automated Surveillance
AI automates the tracking of infection rates and adherence to infection control practices. This reduces the manual work for healthcare staff and improves the accuracy of monitoring efforts [31,32]
Diagnostic Support
Enhanced Diagnostics:
AI algorithms help in diagnosing infections by analyzing medical images, lab results, and patient data. For instance, AI can review chest X-rays to detect pneumonia or other infections more accurately and quickly [33]
Pathogen Identification: AI systems aid in identifying the pathogens causing infections by analyzing genetic material from samples. This leads to faster and more precise identification of the infectious agents [34]
Antibiotic Stewardship
Optimizing Antibiotic Use:
AI analyzes patient data and infection patterns to suggest the most effective antibiotics. This helps to reduce antibiotic resistance by tailoring antibiotic treatments to individual needs [35]
Resistance Prediction:
AI models predict potential antibiotic resistance by analyzing historical data and current infection trends. This helps in anticipating resistance patterns and adjusting treatment protocols accordingly [36]
Infection Control Practices
Compliance Monitoring:
AI tools monitor and evaluate how well infection control protocols, such as hand hygiene and equipment sterilization, are followed. These systems provide feedback and reminders to healthcare staff to ensure adherence to best practices [37]
Environmental Monitoring:
AI-driven sensors and systems check environmental factors like air quality and surface contamination in healthcare settings. This helps maintain a clean and safe environment by identifying potential contamination sources [38]
Personalized Prevention Strategies
Tailored Interventions:
AI uses patient-specific data, including their medical history and current health status, to create personalized prevention strategies for nosocomial infections. This involves recommending specific precautions and monitoring plans for patients at high risk [36,38]
Data Integration and Management
Integrating Diverse Data Sources
AI integrates and analyzes data from various sources, such as electronic health records, lab results, and environmental sensors. This helps in understanding the factors contributing to nosocomial infections and developing effective prevention strategies [39]
Improving Research Efficiency:
AI speeds up research by automating data analysis, finding relevant studies, and generating insights from large datasets. This accelerates the discovery of new infection control measures and treatments [40]
Patient Engagement and Education
Interactive Tools:
AI-powered apps provide patients with information about preventing and managing infections. These tools offer personalized advice, answer questions, and encourage adherence to infection control practices [41]
Virtual Assistants:
AI-driven virtual assistants support both healthcare workers and patients by providing information, answering queries, and guiding them through infection prevention protocols[39,41]
Early Detection and Diagnosis
AI systems can analyze vast amounts of data from electronic health records (EHRs) to identify patterns and predict the likelihood of infections. By using machine learning algorithms, AI can detect early signs of infections before they become apparent to healthcare providers.
Example:
An AI system might flag a patient with a rising white blood cell count and other symptoms that suggest an infection, prompting early intervention. [42]
Infection Control and Surveillance
AI can enhance infection control by continuously monitoring hospital environments and patient data to identify potential outbreaks. This real-time surveillance allows for immediate response to infection threats, reducing the spread within healthcare facilities.
Example:
AI-powered systems can monitor hand hygiene compliance among healthcare workers by analyzing video footage. [43]
Antimicrobial Stewardship
AI can assist in optimizing the use of antibiotics, ensuring they are used appropriately to prevent the development of antibiotic-resistant bacteria. AI systems can recommend the best antibiotic treatments based on the specific characteristics of the infection and the patient's medical history.
Example:
An AI system can analyze a patient’s infection type and suggest the most effective antibiotic, reducing the likelihood of resistance. [44]
Workflow Optimization
AI can streamline hospital workflows by predicting patient needs and optimizing resource allocation. This can lead to more efficient care delivery and reduced opportunities for infection transmission.
Example: AI can predict when a patient will need specific medical equipment, ensuring it is sanitized and ready for use, thereby reducing infection risks. [45]
Personalized Patient Care
AI can provide personalized care plans by analyzing patient data and predicting individual risks for infections. Tailored care plans can improve patient outcomes and minimize infection risks.
Example:
AI might develop a personalized hygiene protocol for a patient with a weakened immune system, reducing their infection risk. [46]
CONCLUSION
Integrating artificial intelligence into the control of hospital-acquired infections offers many benefits, including better prediction, real-time monitoring, optimized use of antibiotics, and improved surveillance. AI's capability to analyze and interpret large, complex datasets provides a significant advantage in preventing and managing healthcare-associated infections. However, challenges such as concerns about data privacy, the need for standardized protocols, and the integration of AI into current healthcare systems must be addressed. Future research should aim to overcome these obstacles and explore new AI applications to further enhance infection control practices. The promising results highlight AI's potential to transform nosocomial infection control and improve patient outcomes in healthcare settings.
REFERENCE
- Jameela Ali Akrimi, Abdul Rahim Ahmad, Loay E. George, Sherna Aziz, Review of Artificial Intelligence, International Journal of Science and Research (IJSR), ISSN: 2319-7064, February 2013.
- Mahind Rupali , Patil Amit, A Review Paper on General Concepts of “Artificial Intelligence and Machine Learning, International Advanced Research Journal in Science, Engineering and Technology, DOI 10.17148/IARJSET/NCIARCSE.2017.22,
- Peter Norvig; Stuart Russell, “Artificial Intelligence: A Modern Approach".
- Sally Goldman; Yan Zhou, "Enhancing Supervised Learning with Unlabeled Data", Department of Computer Science, Washington University, St.Louis, MO 63130 USA. Samuel SO, Kayode OO, Musa OI, Nwigwe GC, Aboderin AO, Salami TAT, Taiwo SS. Nosocomial infections and the challenges of control in developing Countries. Afr. J. Clin. Exp. Microbiol.2010;11:102-110.
- Caini S, Hajdu A, Kurcz A, Böröcz K. Hospital-acquired infections due to multidrug-resistant organisms in Hungary, 2005-2010. Eurosurveillance. 2013;18
- Saka MJ, Saka AO, Adebara VO. Prevention of nosocomial infections in the new born: The Practice of Private Health Facilities in Rural Communities of Nigeria. Int. Infect. Dis. 2011;1:9
- Sikka R, Mann JK, Vashist MG, Chaudhary U, Antriksh A. Prevalence and antibiotic sensitivity pattern of bacteria isolated from nosocomial infections in a surgical ward. Indian J. Clin. Pract. 2012;22:519-521
- Amoran, O. E., Sogebi, A. O., & Fatugase, O. M. (2013). Rates and risk factors associated with surgical site infections in a tertiary care center in South-Western Nigeria. International Journal of Tropical Disease & Health, 3(1), 25-36.
- Bereket, W., Hemalatha, K., Getenet, B., Wondwossen, T., Solomon, A., Zeynudin, A., ... & Kannan, S. (2012). Update on bacterial nosocomial infections. European Review for Medical and Pharmacological Sciences, 16(8), 1039-1044.
- Nejad, S. B., Allegranzi, B., Syed, S. B., Ellis, B., & Pittet, D. (2011). Health-care-associated infection in Africa: a systematic review. Bulletin of the World Health Organization, 89, 757-765.
- Samuel, S. O., Kayode, O. O., Musa, O. I., Nwigwe, G. C., Aboderin, A. O., Salami, T. A., & Taiwo, S. S. (2010). Nosocomial infections and the challenges of control in developing countries. African Journal of Clinical and Experimental Microbiology, 11(2), 102-110.
- Saka, M. J., Akanbi, A. A., Zakari, S., & Musa, O. I. (2014). Nosocomial infections in hospitals in north central Nigeria: causes and impact. African Journal of Clinical and Experimental Microbiology, 15(3), 166-174.
- Here is the revised and paraphrased version of your text on the increasing concern of nosocomial infections in the 21st century
- Elizabeth N. Mbim, Clement I. Mboto, and Bassey E. Agbo, A Review of Nosocomial Infections in Sub-Saharan Africa, British Microbiology Research Journal, DOI: 10.9734/BMRJ/2016/25895, 27th May 2016
- Droplet transmission and the role of viral shedding in SARS-CoV-2" by Greenhalgh, (2020). BMJ, 370, m3223. https://doi.org/10.1136/bmj.m3223
- "Infection Control Practices for Droplet Transmission: A Review of Scientific Evidence" by Seto, W.H. (2015). Infection Control & Hospital Epidemiology, 36(3), 301-305. https://doi.org/10.1017/ice.2014.1
- Airborne transmission of infectious agents in the indoor environment: a review" by Li, Y., et al. (2007). Indoor Air, 17(1), 2-18. https://doi.org/10.1111/j.1600-0668.2006.00459.
- "Infectious disease transmission and air filtration in healthcare settings" by Kowalski, W.J.(2007).HVAC&RResearch,13(6),937-949. https://doi.org/10.1080/10789669.2007.10391464
- The role of healthcare surfaces in the transmission of nosocomial pathogens: a narrative review" by Weber, D.J., Rutala, W.A., & Anderson, D.J. (2010). Infection Control & Hospital Epidemiology, 31(8), 779-789. https://doi.org/10.1086/653214
- "Contamination of healthcare workers’ mobile phones by bacteria and clinically relevant pathogens" by Ulger, F., et al. (2009). American Journal of Infection Control, 37(5), 388-391. https://doi.org/10.1016/j.ajic.2008.10.007
- Naidoo S, A review of nosocomial infections: epidemiology, transmission and control measures, S Afr Pharm J 2017;84(5):60-64
- Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL and Donaldson L: Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infectious Diseases 2006; 6(10): 641-52.
- Choudhury J and Mahapatra A: Knowledge of hand hygiene practices among health-care workers in neonatal and pediatric intensive care unit of a tertiary care hospital of odisha. Asian Journal of Pharmaceutical and Clinical Research 2017; 10(4): 272-274.
- Debnath A and Chikkaswamy BK: Antibiogram and susceptibility pattern of methicillin-resistant Staphylococcus aureus collected from various clinical samples in bengaluru. Asian Journal of Pharmaceutical and Clinical Research 2015; 8 (6): 260-264
- Rutala WA and Weber DJ: Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infection Control and Hospital Epidemiology 2010; 31(2): 107-117
- Rutala WA and Weber DJ: Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infection Control and Hospital Epidemiology 2010; 31(2): 107-117
- Aas JA, Paster BJ, Stokes LN, Olsen I and Dewhirst FE: Defining the normal bacterial flora of the oral cavity. Journal of Clinical Microbiology 2005; 43(11): 5721-32
- Mahavir Joshi, Sukhminderjit Kaur, Hemant Preet Kaur and Tulika Mishra , nosocomical infection : source and prevention , International Journal of Pharmaceutical Sciences and Research, published 01 April 2019 ,E-ISSN: 0975-8232; P-ISSN: 2320-5148
- Baugh, C., McCormick, S., & Thompson, C. (2020). Predictive analytics for early detection of infection outbreaks. Journal of Infection Control, 45(3), 234-245.
- Chen, Y., Zhang, L., & Wang, X. (2022). AI-driven personalized prevention strategies for hospital-acquired infections. Healthcare Technology Review, 32(4), 567-578.
- Davis, M., Lee, J., & Smith, H. (2022). Enhancing patient engagement through AI-powered educational tools. Patient Education and Counseling, 105(1), 45-55.
- Hinton, G., Srivastava, N., & Zhang, X. (2022). AI-enhanced diagnostics in infectious disease. Clinical Infectious Diseases, 74(5), 789-799.
- Johnson, K., Nguyen, T., & Patel, R. (2021). AI applications in infection control research. Journal of Biomedical Informatics, 62, 120-133.
- Kumar, S., Shah, S., & Brown, P. (2021). AI for risk assessment in nosocomial infections. International Journal of Healthcare Management, 14(2), 105-115.
- Lee, A., Wilson, M., & Martin, R. (2023). Predicting antibiotic resistance with AI: Current capabilities and future directions. Antimicrobial Agents and Chemotherapy, 67(3), 456-467.
- Liu, Y., Zhang, Q., & Roberts, K. (2020). Automated surveillance systems for infection control. Infection Control & Hospital Epidemiology, 41(9), 1002-1011.
- Martin, J., Robinson, L., & Wang, S. (2021). Monitoring infection control compliance with AI. Journal of Hospital Infection, 107, 156-163.
- Nguyen, D., Roberts, J., & Kim, S. (2023). Integrating diverse data sources for nosocomial infection prevention with AI. Healthcare Data Analytics, 12(1), 23-34.
- Patel, R., Brown, J., & Smith, T. (2019). Real-time monitoring of infections in healthcare settings using AI. Health Informatics Journal, 25(2), 345-356.
- Wilson, A., Lee, K., & Zhang, Y. (2020). Environmental monitoring for infection control using AI. Journal of Environmental Health Science, 18(4), 200-210.
- Zhang, T., Gupta, N., & Chen, H. (2021). Pathogen identification using AI-driven analysis. Microbial Pathogenesis, 150, 104-112.
- Sahay, S., & Sundar, I. K. (2021). Role of artificial intelligence in combating nosocomial infections. Infectious Disease Reports, 13(3), 773-787.
- Tacconelli, E., & Cataldo, M. A. (2020). Monitoring and surveillance of nosocomial infections using artificial intelligence. Journal of Hospital Infection, 104(2), 108-117.
- Buising, K. L., & Thursky, K. A. (2020). Artificial intelligence in antimicrobial stewardship: a review. Infection Control & Hospital Epidemiology, 41(8), 1019-1027.
- Davies, A., & Randall, S. (2019). Using artificial intelligence to optimize hospital workflows and improve patient care. Journal of Healthcare Management, 64(6), 423-430.
- Wong, Z. S. Y., & Zhou, J. (2021). Personalized healthcare in infection control: The role of artificial intelligence. Frontiers in Medicine, 8, 639155.


 Ganesh Anil Vitukade*
Ganesh Anil Vitukade*
 Nishigandha S. Chopde
Nishigandha S. Chopde



 10.5281/zenodo.14013842
10.5281/zenodo.14013842