Abstract
Right now, a larger part of cancer treatment methodologies is based on the evacuation of tumor mass mainly by surgery. Chemical and physical medications such as chemo- and radio therapies have moreover made a major contribution in hindering rapid development of harmful cells. Moreover, these approaches are frequently combined to improve therapeutic indications. It is popularly known that surgery, chemo- and radiotherapy moreover hinder ordinary cells' development. In inclusion, these treatment modalities are associated with severe side effects and high toxicity which in turn lead to low quality of life. This survey envelops novel techniques for more viable chemotherapeutic conveyance sighting to generate superior prognosis. As of now, cancer treatment is a tremendously dynamic field and remarkable advances are being made in the improvement of novel cancer treatment techniques. In contrast to conventional cancer therapeutics, novel approaches such as ligand or receptor-based targeting, triggered discharge, intracellular drug targeting, quality conveyance, cancer stem cell treatment, magnetic drug targeting and ultrasound-mediated drug conveyance, have included modern modalities for cancer treatment. These approaches have driven to specific discovery of malignant cells leading to their eradication with negligible side effects. Bringing down, multi-drug resistance and involving intrusion transportation in targeted drug conveyance to cancer cells can to contribute essentially in the therapeutic conciliation in cancer.
Keywords
Chemotherapeutics; Cancer; Immunotherapy; Targeted Delivery; Efflux Pump; Gene Therapy; Cancer stem cell; Nanoparticles; Resistance.
Introduction
Cancer is a group of disorders abnormal cell growth and can spread to other body part worldwide cancer-death rates come second after the death related to cardiac system disease and are examined the most serious universal health issues. Due to this commonness cancer therapy has become a joint conversation. Among medical management providers researchers. [1] Cancer Involve a scale of diseases, with unpredictable of new cases emerges every year and this range are anticipated to increase yearly [2] for that reason regardless of all the attempt, it remains a leading health problem globally and a notable cause of death. Current cancer treatment option includes Surgical intervention, radiotherapy and Systemic therapies, such as chemotherapy targeted therapy and Immunotherapy. [3] Cancer comprises a scale of diseases that emerge as a result of the unregulated growth of destructive cells, which have the ability to occupy or increase to other body parts. With more than 10 million new cases annually, cancer linked deaths are estimate to increase in the virtually future with an approximation by the Global Health Organization of 13-1 million cancer-related death by the year 2030. [4] But, the death rate has decreased in the past 5 years due to a good apprehension of tumor biology and better diagnostic devices and medicant attention. Current cancer treatment Options work primarily by DNA Synthesis and mitosis, leading to the death of Speedily growing and splitting cancer cells. The Representative agents are non-selective and can also cause fine and common tissues, be the cause of severe unintended and undesirable side effect- e.g. loss of appetite and nausea. In point of fact very bad adverse effect induced by chemotherapeutic drugs on fine tissues and on organs are a main reason behind the more death rate of the cancer Sufferer. As well as the bio-accessibility of these drugs to tumor issues is Proportionally poor, higher doses are needed, most significant to elevated toxicity in normal cells and a growth occurrence of various drug resistance. For that reasons it is advantageous to enlarge chemotherapeutic that can either passively or actively kill the cancerous cells. Consequently, making less adverse side effect While upgrade therapeutic efficacy. In the last few years., a good apprehension of tumor biology and growing availability of versatile material including polymer. [5][6][7][8]
2.Intracellular Targeted Drug Delivery
The most compelling procedures to target tumor cells is to coordinate DNA hindering drug
molecules to nuclei of cancer cells. Nuclear focusing on not solitary causes essentially tumor cell
death but at the same time minimizes vandalism to adjoining typical cells. The major problem of such targeted drug conveyance is to refrain from translocation of dynamic specialists into
endosomal or lysosomal vesicles. Drug conveyance mechanism requires dynamic particles to
escape from subcellular cytoplasmic vesicles and translocate into cores [9]. The cancer
cells advance intracellular resistance mechanisms such as overexpression of medicate efflux
pumps, catabolism and sequestration into acidic compartments and deactivation [10]. Sui et
al. portrayed two nuclear drug conveyance procedures. One technique necessitates indirect nuclear
targeting in which drug particles are carried into cytosol in large amounts along these lines
allowing absolute sequestration of nuclear DNA. The other technique gives direct nuclear
targeting in which nanocarriers carry particles to cancer cells over the cell membrane
into cytosol and at last localize in the nuclei where the active particles may be discharged [11].
2.1. Barriers for Nuclear Drug Delivery
(a)Plasma Membrane: - The preeminent barrier to nuclear drug conveyance to mammalian cells is the plasma layer that confine the section of charged and huge hydrophilic particles [12]. Huge nanocarriers are taken by distinctive endocytotic mechanisms into the cell [13]. Physical properties of nanocarriers such as geometry may have a reaction on phagosomes formation. Pinocytosis may be segmented into clathrin mediated endocytosis (CME), caveolin-mediated endocytosis, clathrin and caveolin- autonomous endocytosis, and macro pinocytosis. Trafficking of nanocarriers through diverse sorts of pinocytotic mechanisms is a vital factor to progress targeted treatments. For illustration, polarized MDCK epithelial cells that shortfall caveolae on apical surface cationic as well as anionic nanoparticles are trafficked by clathrin mediated endocytosis (CME) [14]. In any case, anionic particles are trafficked through CME and caveolae interceded endocytosis considering that cationic particles are limited to CME and micropinocytosis in non-polarized HeLa cells [15]. In MDCK cells anionic particles’ ingress lysosomal compartments through cationic particles are directed to transcytosis [16]. These probes highlight the importance of shape and charge of the particulate delivery system which must be considered to overcome the foremost obstruction of nuclear drug conveyance.
(b) Nuclear Membrane & Nuclear Transport: - Multicellular nature of eukaryotes permits for compartmentalized engineering to direct cell separation. The nuclear envelope adjoins the nucleus isolating the nucleoplasm and hereditary substance from cytoplasm. The nuclear envelope comprises nuclear pore complex (NPC) through which swapping of particles takes place [17]. NPC comprises a central channel, a nuclear and cytoplasmic ring which are made up of 50 diverse proteins called nucleoporins [18]. Each independent NPC trans locates roughly 1000 proteins per second in a bidirectional way [19]. Atoms ?45KDa or 8.5nm in diameter are transported though targeted signals to enter or exit the nucleus. On the other hand, small atoms pass through the NPC by passive propagation [20]. Interestingly, a few probes describe the ingress of particles over 99 nm [21]. Therefore, there is no consensus about exact mechanisms of transport beyond the NPC [22].
2.2. Approaches for Targeted Nuclear Delivery
Four major nuclear targeted delivery systems have been investigated broadly, i.e.,
1)NLS mediated conveyance system 2) TAT conjugated nuclear targeting 3) cationic polymer-based delivery system and 4) pH triggered charge inversion approach. In vitro/In vivo probes demonstrated efficacious nuclear localization of NLS functionalized nanocarriers which are presented in Table 1. Positive charge residues in NLS and CPP arrangements render them non-specific for in vivo applications. Indeed, despite brain penetration of CPP was reported, CPP and NLS manifest quick clearance mechanisms [23, 24]. Block copolymer micelles (BCM) surface-grafted with Trastuzumab-Fab-NLS spawned 5-fold higher tumor uptake in HER2 overexpressing mice demonstrate. Besides, the clearance of NLS conjugated Tm-Fab-NLS conjugates is ascribed to mononuclear phagocyte system (MPS) without bringing down tumor aggregation [25]. TAT functionalized liposomes conjugated to DOX escalated cancer cell apoptosis by 37.1%. PEG altered liposomes are activated extracellularly by cysteine to achieve higher in vivo tumor assimilation [26]. Additionally, DOX conjugated dextran and phenylboronic acid cholesterol (chol-PBA) nano-micelles illustrated noteworthy lysosomal-acidity dependent nuclear assimilation of DOX nano-micelles. DOX-loaded DEX/ CholPBA nano assembly exemplified 100% survival rate and diminished tumor volume in mice model [27]. Cytotoxicity of DOX is largely reliable on its intercalation between two base pairs of DNA’s shaping DNA adduct subsequently inducing division of DNA strands and DNA helicase [28]. Surged DNA cleavage action and nuclear aggregation are dual functions that may improve the efficacy of an anticancer drug. On the other hand, Wang et al. developed Graphene Quantum Dots conjugated DOX (DOX/GQD) and detailed significant nuclear DOX aggregation and improved DNA cleavage. Assist, at cellular level DOX/GQD conjugates sidestepped efflux transporters overexpressed in MCF-7/ADR cell lines [29].
TABLE 1: - Drug delivery vehicles and their effect on nuclear translocation
|
Drugs
|
Formulation
|
In vivo
|
In vitro
|
Reference
|
|
Doxorubicin
|
TAT modified micelles consisting 2 block co polymers
|
Suppressed tumor growth in Xenograft mice models
|
|
[30]
|
|
Camptothecin
|
PLL & PAMAM dendrimers
|
N/A
|
Facilitates nuclear accumulation by endosomal and lysosomal uptake
In SKOV3 ovarian cancer cells
|
[31]
|
|
Doxorubicin
|
Dox-NLS loaded PLGA NP
|
N/A
|
Six times higher uptake than native DOX in MCF -7 cell line
|
[32]
|
|
Carboplatin
|
PEGylated NLS-carboplatin conjugate
|
N/A
|
Quick internationalization and assimilation in nucleus in M109FR cell lines that have overexpressed folic acid receptors
|
[33]
|
|
Doxorubicin
|
Targeted charge reversal nanoparticle [TCRN]
|
N/A
|
Increased antitumor activity in SKOV3 ovarian cancer cell
|
[34]
|
2.3. GENE Therapy: - Clearly, more than 2100 DNA Segment therapy clinical trials have been conducted as well as approved early till to date. A few DNA Segment therapy approaches have been instigated which include suicide gene therapy, immunomodulatory DNA Segment treatment, hereditary rectification, pro-apoptotic and restorative gene treatment, antiangiogenic gene treatment, and siRNA therapy [35, 36]. In comparison to customary chemotherapy which can cause elevated perniciousness due to the lack of specificity, gene treatment supplies an interesting and effective approach to combat cancer. Gene treatment works at infinitesimal level, in which hereditary materials or operative genes are inserted into patients’ cells to either repair or take over the flawed genes. The cancer cells carry transformed genes such as p53, bax and other oncogenes. Hence, gene treatment can play crucial part in treating cancer.
2.3.1. Mechanism of gene therapy in cancer treatment: -Gene therapy implies the delivery of hereditary materials into cancer cells. A vector is utilized as a carrier to confirm the delivery of hereditary materials. After therapeutic genes are transported into cells, these sequences apply their reaction through different mechanisms such as silencing, up or down regulation, repair, or alteration of the specific target genes. Suicide genes may cause cell death and/or tumor corruption. Gene silencing hampers cell development and tumor regression. While gene alteration may lead to higher response from other combination treatments (e.g. chemotherapy, immunotherapy, or radiation).
2.3.2. Gene delivery —Several conveyance strategies have been created for gene therapy, which for the most part can be isolated into two categories, viral and non-viral delivery system.
2.3.2.1. Viral vector: - Viruses are minuscule irresistible agents that can imitate in living cells [37]. Analysts have utilized viruses to convey therapeutic genes into cell nuclei because of high transfection proficiency, capacity to penetrate, express and duplicate in host cells [38]. In order to utilize virus as vector, it is vital to expel the pathogenic portion of viral genes and displace with the restorative genes [38]. The remaining non-pathogenic segments in virus carries restorative gene constituting the viral vector.
i. Adeno-Associated viruses (AAV) are minute single-stranded (SS) DNA viruses which offer a number of preferences such as wide host range, low resistant response (due to deficiency of pathogenic) and long-term expression [39]. Latterly a variety of AAV-mediated genes have been evolved to treat cancers such as prostate cancer, glioblastoma, cervical and breast cancer, nasopharyngeal carcinoma, and lung carcinoma [40-43]. The harmlessness of cytotoxic T lymphocytes (CTLs) with recombinant AAV has been assessed in cancer patients [44]. AAV-mediated bevacizumab has been described to hinder ovarian tumor development [45]. In another investigation, down-regulations of p16 (INK4), p27 (KIP1), p21 (WAF1), and p53 tumor suppressors utilizing AAV type 2 was noted [46]. Another breakthrough of AAV related cancer treatment was the consent of Glybera (alipogene tiparvovec) by European Union in 2013. It is an adeno-associated viral vector to treat lipoprotein lipase insufficiency [47].
ii. Adenovirus (Ads) are non-enveloped viruses that have linear DS (double-stranded) DNA [48]. Ads can cause transduction securely along with high transgene expression, which makes it an exceptionally capable vector to treat glioblastoma multiforme (GBM) [48]. In any case, the application of Ads in gene treatment is constrained by the low restorative adequacy after systemic administration and serious toxicity [49]. Numerous trials have been made to minimize virulence and improve restorative adequacy. Yao et al. illustrated higher tumor-selective transgene expression with PEGylated adenovirus vector relative to Ads [50, 51]. The breakthrough in Ads gene treatment was the consent of Gendicine (Ad-p53) in 2003 in US and Oncorine/ONYX-015 (E1B-defective Ad) in 2005 in China. Both drugs are affirmed for treating head and neck squamous cell carcinoma [52].
iii. Lentiviruses are ssRNA genome retroviruses. They have come to light as a promising vectorin cancer gene treatment. Lentiviruses dominates many preferences over other sorts of viral systems i.e. in low immunogenicity and the capability of transducing a diversification of cells [53]. Various trials have been made with lentivirus as vector in cancer gene treatment. Lentivirus interceded RNA intervention was employed to target protein phosphatase magnesium/manganese-dependent 1D (PPM1D) [54] or high mobility group box1(HMGB1) [55]. These builds repress development of bladder cancer. Additionally, other research varieties have petitioned lentivirus-mediated short hairpin RNA to knockdown PPM1Din lung [56] and colorectal carcinoma [57].
iv. Herpes viruses are huge wrapped dsDNA viruses that can carry huge transgene. A combination of oncologic herpes simplex virus and vinblastine to convey interleukin (IL)-12 to improve antitumor repercussion in prostate cancer demonstrate has been evaluated [58]. Zeng et al. demonstrated synergistic impacts of a combination treatment with paclitaxel and oncologic herpes simplex virus G47? [59] in breast cancer. Additionally, Goshima et al. moreover appeared promising repercussion of a combination of HF-10, herpes simplex virus (HSV) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in Murine ovarian cancer [60].
2.3.2.2. Non-viral: Numerous non-viral systems have been examined for gene conveyance, including the infusion of naked DNA or physical strategies such as electroporation, gene gun, hydrodynamic conveyance, sonoporation, and nanocarriers (nanoparticles and neutral or cationic liposomes) [61]. Non-viral strategies are predominant for large-scale generation, low immunogenicity, and they furnish high level of transfection efficiencies compared to viral methods [62].
a. Hydrodynamic is another effortless but successful non-viral quality conveyance strategy. It works by utilizing a physical constrain that increments intravascular pressure [63]. This process is widely petitioned for gene conveyance in animals. Hydrodynamic gene conveyance is potential of delivering transgenes into mammalian cells in proficient and secure manner [64]. Yazawa et al. have inspected hydrodynamic infusion of plasmid encoding a dissolvable form of fetal liver kinase-1 (Flk-1) gene for angiogenesis hindrance in mouse demonstrate [65].
b. Naked DNA: This is the least complex way to convey restorative genes with undeviating infusion of free DNA into specific tissues arising in quality expression. In cancer gene treatment, DNA can be specifically infused either interior the tumor or into tumor-surrounding tissues to express tumor antigens that might work as cancer antibody. This strategy is less immunogenic and comparatively conservative in terms of generation cost. In any case, the low overall expression level limits its utilization. In spite of restrictions, a few victories have been reported regarding clinical trials of naked DNA plasmid conveyance [66], [67], [68].
c. Electroporation (or electron-permeabilization) is a method in which electrical field is applied to improve the penetrability of DNA into cells [69] [70]. Electroporation offers several preferences such as precise conveyance of restorative genes, localized gene expression and less unfavorable impacts. Ponders have been conducted to guarantee the security and tolerability of this strategy in cancer and irresistible infections. The first clinical trial begun in 2004 [71], [72]. Daud et al. have illustrated the viability of interlieukin-12 plasmid electroporation in metastatic melanoma patients in a stage I trial [73], [74]. Another ponders reported an intravaginal restorative DNA immunization by electroporation. This includes coding calreticulin connected to E7 (CRT/E7) against cervicovaginal cancer [75].
d. Nanocarriers is another extensively examined zone in cancer gene treatment including nanoparticle-based conveyance of hereditary material. These artificially synthesized non-bioactive nonviral vectors contribute an effective way to convey hereditary material to cells. Low immunogenic, less toxic and adaptable for chemical alterations are interesting preferences associated with this approach. However, moderately low transfection proficiency is the fundamental drawback with this strategy [76]. The nano vectors i.e. nanoparticles or nano capsules are usually prepared with biodegradable materials. These 10 to 100 nm particles frame a nano complex by embodiment or adsorption of hereditary materials. The adaptability to chemical modification of nanomaterials yields fabulous capability to adsorb, concentrate and protect hereditary materials. Such Nano vectors can be isolated into two categories: polymeric Nano vectors made of dendrimers, lipids, PLGA, and chitosan; and inorganic Nano vectors made of silica, iron oxide, and gold nanoparticles [77] [78] [79] [80]. Endocytosis is considered as the fundamental conveyance mechanism. In addition, coupling of particular atoms (monoclonal antibodies, and peptides) to the aspect of nanoparticles may be utilized to target tumor complex through authoritative to specific receptors on cell surface. The targeted genes can thus be securely and successfully transfected. The nanocarriers utilized for gene therapy in cancer are summarized in Table 2.
TABLE 2: - Nanocarriers for gene therapy in cancer
|
Nanoparticles types
|
Genes/Drugs
|
Target Cancer
|
Reference
|
|
Neutral/Zwitterionic Liposomes
|
Chlorotoxin-coupled nanoparticle for antisense oligonucleotides or siRNAs
|
glioblastoma
|
[81,82]
|
|
Gold
nanoparticles
|
MicroRNA
|
Prostate and breast cancer
|
[83]
|
|
siRNA delivery with poly ion complexes and gold nanoparticles
|
Cervical cancer
|
[84]
|
|
siRNA by novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold NPs
|
retinoblastoma
|
[85]
|
|
Cationic
Liposomes
|
ZEBI siRNA and doxorubicin
|
Lung cancer
|
[86]
|
|
Anti Prostate-specific membrane antigen (PSMA) liposome complex
|
Prostate cancer
|
[87]
|
|
Hyaluronic acid based
|
siRNA
|
Lung cancer
|
[88]
|
|
Magnetic
nanoparticles
|
Vascular endothelial growth factor (VEGF) siRNA
|
Ovarian cancer
|
[89]
|
|
siRNA with multimodal mesoporous silica-based nanocarriers.
|
Lung cancer
|
[90]
|
|
Notch- 1 shRNA
|
Breast cancer
|
[91]
|
|
Chitosan based
|
Systemic delivery of survivin (SVN) siRNA
|
Prostate cancer
|
[92]
|
|
siRNA
|
ovarian cancer
|
[93]
|
2.4. Cancer Stem Cell Therapy
Cancer stem cell therapy Self-renewal and multiplication of cancer stem cells (CSC) cause tumor start, development, metastasis and habituation. So far, CSC has been uncovered in a wide diversity of solid tumors, comprising lung, colon, prostate, ovarian, and brain cancer, as well as melanoma. The essential reasons for treatment disappointment in numerous malignancies incorporate resistance and need of selectivity for chemotherapeutic. Moreover, CSC populations are safer to tradition chemotherapy relative to non-CSC population. Subsequently, elimination of CSC is significant in cancer treatment. Latterly, numerous novel restorative systems have been surveyed for eradication of CSC and modification of microenvironment which supports the development of such cells. Chen et al. has proclaimed miscellaneous targets of current cancer treatment methodologies. (Fig. 1). Surface markers and signaling routeways are the two potential targets. Numerous potential CSC restorative targets, comprising the ABC superfamily, anti-apoptotic variables, detoxifying enzymes, DNA repair proteins and particular oncogenic cascades have been recognized. Right now, a few restorative techniques have been instigated that can annihilate CSC effectively whereas others are still beneath preclinical and clinical stages [94].

Fig. 1: - Cancer Stem Cell Therapy
2.4.1. Key signaling cascades-based therapy- Understanding the anti-apoptotic pathway and inert pro apoptotic pathway/ mechanisms are rising ranges for the development of cancer chemotherapeutic. Notch1 signaling plays a major character in the development of tumors. Xanthohumol (XN) that hinders Notch1 signaling pathway leading to quickened apoptosis of tumor cells [95]. Merkelbach and co-workers portrayed apoptosis modulation of Kit. Various mRNAs can initiate apoptosis such as the PI3K/AKT signaling pathways in gastrointestinal stromal tumors [96]. Various other signaling cascades encompassing c-Met [97], MAPK [98], TGF-?, Wnt [99], NF-?B [100], Hedgehog [101], APC/ ?catenin, Ras/ Raf/ Mek/ Erk and others play a vital role in the Habituation and maintenance of CSC.
2.4.2. Tumor microenvironment-based therapy- Tumor microenvironment too plays an important part in making an aperture for nursing and ensuring CSC from drugs-initiated apoptosis. Caro et al. outlined a dominant role of tertiary lymphoid tissue in T cell recruitment and local initiation of immune cells in the tumor microenvironment. Specific approaches including targeting myeloid cells [102], bone microenvironment [103], Versican, (an ‘bridge’ connecting swelling with tumor development) [104], spliceosome [105] and NPDH oxidase-derived responsive oxygen species [105] have been surveyed.
3. Chemotherapy and Drug Resistance
Chemotherapy is one of the first therapeutic intercessions in cancer. In spite of progresses in drug discovery and treatment conventions, patients gain multidrug resistance (MDR). Consequently, reaction to chemotherapy remains far below desires. MDR is a phenomenon where tumor cells advance resistance to functionally and fundamentally irrelevant anticancer drugs [106]. Whereas cancer cells at first respond to chemotherapy but retrogress is common. Clinically drug resistance follows earlier to or in response to chemotherapy. It is either procured or inherent and can be caused by means of numerous pathways. The mechanisms of drug resistance accommodated by cancer cells incorporate alterations in drug consumption and transport, gene transformation, intensification of drug targets and hereditary rewiring leading to gene repair and impaired apoptosis. Tumor heterogeneity (heterogeneous population of cells with distinct hereditary fingerprints) is another perspective of drug resistance where a small subpopulation remains dormant and inert to chemotherapy. But these cells afterward emerge as destructive type which gotten to be troublesome to treat [107]. Onset of drug resistance is a complex process. It necessitates pharmacokinetic and pharmacodynamic mechanisms. Extensive surveys are accessible on the mechanisms of drug resistance [108–111]. Pharmacokinetic mechanisms comprising sub-therapeutic concentration, raised efflux, due to overexpression of MDR genes and upgraded biotransformation by cytochrome P450 (CYP) metabolizing enzymes are involved. All these mechanisms demonstrate up-regulation of efflux transporters and metabolizing enzymes that constitute a major resistance phenotype. A concerted impact of efflux transporters and metabolizing enzymes leading to MDR may happen due to overlapping substrate specificity and facilitated regulation of their expression. The expressions of MDR genes and CYP are basically represented by atomic receptors, especially pregnane X receptor (PXR). These mechanisms may work independently or synergistically, leading to treatment disappointment. Subsequently, ways to overcome such integrated role of efflux transporters [P-glycoprotein (P-gp), multidrug resistant proteins (MRP) and breast cancer resistance protein (BCRP)] along with CYP action have been examined in the following segments:
3.1 Role of Transporter in Drug Delivery
Transporters are fundamentally portion of cell layer proteins. These proteins are complicatedly associated with selective absorption of endogenous substances (substances such as anions and cations, vitamins, sugars, nucleosides, amino acids, and peptides) and prohibition of harmful elements. In fact, invasion transporters permit absorption of fundamental supplements and ions whereas efflux transporter remove cellular metabolites and xenobiotics. These proteins play a crucial role in drug absorption, conveyance, elimination, as well as drug-drug interactions. In spite of the fact that the phenomenon of multidrug resistance in bacteria was recognized more than fifty years prior, role of P-gp as a major characteristic for efflux of xenobiotics has been established latterly. It is clear now that, these efflux proteins are exceedingly expressed on different tumor cells which leads to drug resistance in chemotherapy. In fact, these transporters play a critical role in the process of drug absorption, distribution, metabolism and excretion (ADME). Nearly 2000 genes have been distinguished for expression of transporters or transporter-related proteins [112]. Roughly 400 membrane transporters, in two super families, have been distinguished and characterized for specific tissue localization and a number of these transporters have been cloned [112]. From pharmacological point of view two major super families, ABC (ATP binding cassette) and SLC (solute carrier) transporters, are most vital. Significance of drug transporters in the process of ADME and drug-drug association is presently well reported. Lately US Food and Drug Administration (FDA) and International Transporter Consortium (ITC) together have considered a few transporters i.e., organic anion transporter (OAT), organic anion transporting polypeptide (OATP), organic cation transporter (OCT), peptide transporter (PEPT), P-gp, multidrug resistance protein (MRP) and breast cancer resistance protein (BCRP) which are pivotal for xenobiotics absorption, and drug-drug interactions. From pharmacokinetic view point, localization and function of these transporters in the digestive system, liver and kidney are getting preeminent consideration.
(a) ABC Transporters: The ABC transporter genes stand for the biggest family of transmembrane proteins. So distant 49 human genes have been recognized. Based on arrangement homology and domain structure, these proteins are categorized into seven diverse
classes (ABCA-ABCG). So distant, thirteen distinctive transporters have been recognized from classes A, B, C and G and these transporters play a remarkable part in the advancement of multidrug resistance (MDR) [111, 113]. Biedler and Riehm [114] reported that Chinese hamster ovary (CHO) cells recognized for resistance to actinomycin D, moreover transmit cross-resistance to numerous other drugs (duramycin, democoline, mithramycin, mytomycin C, puromycin, vinblastin and vincristine). These efflux pumps moreover transmit resistance to HIV-protease inhibitors. Mammalian cells can procure cross resistance to an assortment of restorative agents with different chemical structures and capacities. This marvel is referred to as multidrug resistance. Juliano and Ling uncovered that drug resistance in CHO cells is due to the presence of P-glycoprotein. These ABC transporters work in a unidirectional way as contradicted to several others transporters which are bidirectional [115]. All ABC proteins appear in common a negligible prerequisite of two transmembrane domains (TMDs) and two nucleotide binding domains (NBDs) except BCRP. The hydrolysis of ATP is required as a standard control for translocation by these transporters [116]. The auxiliary necessities of the ABC transporter for drug binding have been examined already [117, 118].
(b) Efflux transporters: Three vital ABC transporters: P-gp, MRP and BCRP are expressed at physiological boundaries (e.g. intestinal absorption barrier, BBB, placental barrier, corneal and retinal barriers), in the liver, cancer cells, and other tissues and are capable for clearance and excretion of xenobiotics. These transporters altogether impact ADME of a number of drugs and their metabolites and cause drug resistance for numerous restorative agents. Such proteins along with essential metabolizing protein cytochrome P450 (CYP) together constitute a profoundly proficient barrier for oral drug absorption. P-gp is the most extensively considered efflux transporter which capacities as a biological barrier by extruding toxins and xenobiotics into extracellular liquid. BCRP and MRP moreover have a place to the same ABC super family. Substrate specificity and tissue localization of P-gp and BCRP vary from MRP. As a result of efflux, drug absorption is diminished and bioavailability of xenobiotics decreases at the target tumor tissue. In later surveys we have elaborately discussed the role of these efflux transporters in drug resistance [119]. A list of anticancer agents that are substrate for different efflux transporters is displayed in Table 3
Table 3: - A list of chemotherapeutic agents which are substrates for various efflux transporters
|
Efflux Transporter
|
Chemotherapeutic Substrates
|
|
BCRP
|
Bisantrene, daunorubicin, DOX, epirubicin, etoposide, gefitinib, imatinib, irinotecan,methotrexate, mitoxantrone, SN-38, teniposide, topotecan
|
|
MDR1
|
Bisantrene, daunorubicin, docetaxel, DOX, epirubicin, etoposide, idarubicin, methotrexate, mitoxantrone, paclitaxel, teniposide, vinblastine, vincristine
|
|
MRP1
|
Daunorubicin, DOX, epirubicin, etoposide, imatinib, melphalan, methotrexate,mitoxantrone, paclitaxel, vinblastine, vincristine
|
|
MRP2
|
Cisplatin, DOX, etoposide, irinotecan, methotrexate, SN-38, vinblastine, vincristine
|
|
MRP3
|
Carboplatin, cisplatin, etoposide, methotrexate, teniposide
|
|
MRP4
|
Methotrexate, topotecan
|
|
MRP5
|
5-Fluorouracil, methotrexate
|
|
MRP6
|
Cisplatin, etoposide, teniposide
|
|
MRP7
|
Docetaxel, paclitaxel, vinblastine, vincristine
|
|
MRP8
|
5-Fluorouracil
|
3.2. Strategies to overcome efflux
A major obstacle in the effective conveyance of anticancer agents is efflux. Consequently, developing methodologies to dodge efflux proteins are indispensable.
3.2.1. Pharmacological hindrance of efflux proteins—Co-administration of appropriate chemical agents that can invert the activity of efflux proteins either by competitive or non-competitive binding may be a smart technique. The efflux modulators can efficiently avoid the action of efflux proteins and subsequently increase the penetrability of desired drug atoms in the targeted tumor. A schematic of this mechanism is portrayed in Fig. 2.
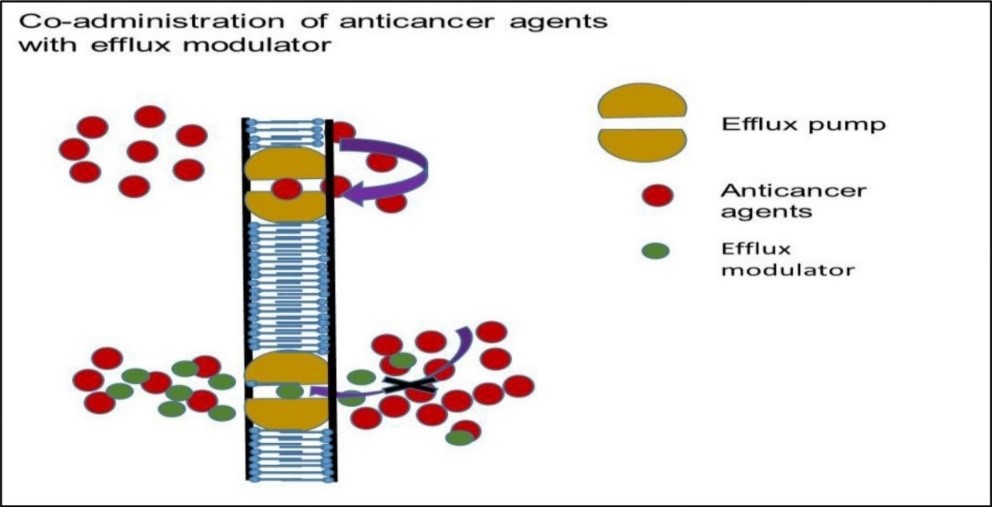
Fig. 2. Mechanism of action of combination therapy: efflux pump modulator upon co-administration with anticancer agents- substrates of MDR efflux transporter
(a) First generation MDR modulators: Clinically affirmed, Ca2+ channel blocker verapamil can improve cellular aggregation of vincristine in P-gp overexpressing cell lines [120, 121]. however, these first-generation inhibitors are strong for MDR reversal in vitro, these compounds require exceptionally high dosages to hinder drug efflux pumps in human causing severe toxicity [122].
(b) Second generation MDR modulators: In order to dodge the complications and toxicities correlated with the first-generation modulators, MDR modulators with lower toxicities were augmented. Auxiliary analogs of verapamil and cyclosporine A, such as dexverapamil and PSC-833 (valspodar) individually are produced as second-generation of MDR modulators [123, 124]. These compounds modify the pharmacokinetic properties of co-administered anticancer drugs, such as paclitaxel, and DOX [125, 126] along with nonspecific interactions with CYP proteins and other resistance proteins such as P-gp [127]. Often a result of these changes is related with dosage alteration of co-administered anticancer drugs, which may otherwise unfavorably affect the outcome of treatment.
(c) Third generation MDR modulators: These agents are outlined to overcome the limitations of the prior modulators. Zosuquidar [128], tariquidar [129] and elacridar [130] are the MDR inhibitors that have appeared potential in pre-clinical mouse models and in the clinic. These agents do not cause pharmacokinetic interactions with other restorative agents and drug metabolizing (CYP-450) proteins. Preclinical ponders demonstrate MDR-bearing human tumor diminution in mice and delayed survival rate [131]. No alterations in pharmacokinetic parameters of anticancer drugs such as DOX, etoposide, daunorubicin, vincristine and paclitaxel, have been revealed upon co-administration. Distinct Phase I and phase II ponders were conducted with co-administration of zosuquidar with docetaxel and daunorubicin and DOX for the treatment of breast cancer, leukemia and non-Hodgkin’s lymphoma [132–135]. The results indicate clinical enhancements. Various tyrosine kinase inhibitors (TKI) and other kinase inhibitors are being developed as MDR modulators. Quantitative structure-activity relationship to overcome the issues related with first- and second-generation modulators are progressing. TKI modulators (imatinib, nilotinib, ponatinib, icotinib, laptinib, erlotinib, canertinib, telatib, sunitinib, and vandetanib) are exceptionally effective at nanomole concentrations and clinically important for cancer treatment [136].
3.3. Ritonavir and reversal of Chemo-resistance
Our research facility has inspected the strategy of combining ritonavir-a retroviral protease inhibitors with anticancer drugs to control medicate efflux, and metabolism subsequently permitting sufficient drug entry into tumor cells [137]. Vinblastine intervened induction of efflux transporters (MDR1, MRP2), metabolizing enzyme (CYP3A4) and nuclear receptor (PXR) is repealed in the presence of ritonavir in human colon adenocarcinoma cells (LS-180). Vinblastine initiated overexpression of these genes totally deactivated co-treated cells with ritonavir. Uptake of (3H) Lopinavir and VIVID™ test further confirmed the functional activity of interpreted genes upon co-treatment. When any one of the anticancer agents (DOX, paclitaxel, tamoxifen and intestine) was combined with ritonavir, a significantly decreased cell multiplication, cell relocation and increased caspase activity are observed. This process leads to improved apoptosis of human breast adenocarcinoma cells (T47D) and prostate cancer cells (PC-3) (Tables 4 and 5). These results demonstrate enhanced activity of chemotherapeutic in the nearness of ritonavir. In summary, combination treatment of anticancer drug with ritonavir may overcome drug resistance by deactivating the overexpression of efflux transporters and metabolizing enzymes and reprogramming cell death. In this manner, drug regimens containing ritonavir may improve therapeutic presentation of cancer cells to anticancer agents, possibly progressing chemotherapeutic viability with lower resistance development.
Table 4: - Summary of activity changes of chemotherapeutics in presence of ritonavir in T47D cells
|
Drugs
|
Cell Proliferation (IC50)
|
Migration
|
Apoptosis
|
|
Doxorubicin
|
2.86
|
2.08
|
1.49
|
|
Paclitaxel
|
3.66
|
2.08
|
1.53
|
|
Tamoxifen
|
3.18
|
1.41
|
1.41
|
|
Vinblastine
|
7.71
|
1.52
|
1.78
|
Table 5: - Summary of activity changes of chemotherapeutics in presence of ritonavir in PC-3 cells
|
Drugs
|
Cell Proliferation (IC50)
|
Migration
|
Apoptosis
|
|
Doxorubicin
|
3.91
|
2.96
|
2.79
|
|
Paclitaxel
|
4.32
|
3.63
|
2.01
|
|
Tamoxifen
|
2.98
|
2.18
|
2.47
|
|
Vinblastine
|
3.17
|
2.51
|
3.17
|
3.4. Metabolizing Enzymes
Apart from MDR, metabolizing enzymes such as cytochrome P450 (CYP450) are moreover induced by anticancer drugs causing quick biotransformation arising in treatment failure. Several chemotherapeutic agents require enzymatic actuation to apply cytotoxic impacts. For example, a nucleoside drug cytarabine (demonstrated for leukemia) requires phosphorylation by deoxycytidine kinase to active cytarabine triphosphate. To diminish the drug effect, cancer cells procure resistance by lowering drug activation. Such resistance advances through down regulation/mutation of included metabolic enzyme [138, 139]. Two fundamental approaches have been investigated so far to overcome efflux pumps and metabolizing enzymes. These incorporate either co-administering an anticancer agent with an efflux modulator or utilizing anticancer agents which are not substrates of these efflux transporters. Tragically there are no modulators, so far distinguished, which can act at molecular level to avoid activation of MDR gene and metabolizing enzymes simultaneously. A novel common modulator which can repress the resistance mechanisms (activation of efflux transporters and metabolizing enzymes) needs to be surveyed for successful chemotherapy. Table 6 portrays representative chemotherapeutic agents which are metabolized by CYP450.
Table 6: - Chemotherapeutic agents metabolized by different isoforms of CYP450
|
CYP450 isoforms
|
Chemotherapeutic substrates
|
|
IA1
|
Dtx, erlotinib, tamoxifen
|
|
IA2
|
Erlotinib, etoposide, flutamide, imatinib, tamoxifen
|
|
2B6
|
Cyclophosphamide, ifosfamide, tamoxifen
|
|
2C9
|
Cyclophosphamide, ifosfamide, imatinib, tamoxifen
|
|
2C19
|
Cyclophosphamide, ifosfamide, imatinib, tamoxifen, teniposide
|
|
2D6
|
Imatinib, tamoxifen, vinorelbine
|
|
2E1
|
Cisplatin, etoposide, tamoxifen, vinorelbine
|
|
3A4/5
|
Cisplatin, cyclophosphamide, cytarabine, dexamethasone, Dtx, DOX,
Erlotinib, etoposide, exemestane, flutamide, fulvestrant, gefitinib, ifosfamide, imatinib, irinotecan, letrozole, medroxyprogesterone, acetate, mitoxantrone, paclitaxel, tamoxifen, targretrin, teniposide, topotecan, vincristine, vinblastine.
|
4. STRATEGIES FOR CONTROLLED DRUG DELIVERY
4.1. TRIGGERED RELEASE
Noninvasive stimuli-responsive controlled drug conveyance is appealing since such frameworks allow remote, repeatable, and solid exchanging on or off drug discharge based on requirement. A complete noninvasive remote-controlled drug conveyance system comprises drug, an external/internal impetus, stimulus-sensitive materials, and stimulus-responsive carriers. The extrinsic stimulus can be a magnetic field, light, ultrasound, or radio-frequency. The internal stimulation can be pH, temperature, or cellular proteins. With the offer assistance of these response-triggered conveyance system, not only can accomplish targeted conveyance of restorative agents, but can control the length and degree of drug discharge moreover into tumor cell. A schematic has been given to clarify the diverse sorts of triggered release systems (Fig. 3).
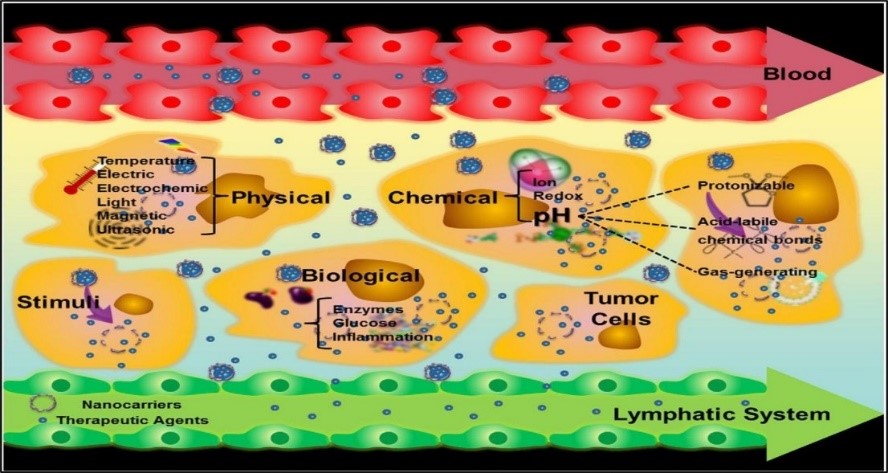
Fig. 3. Nanocarriers improve the permeability and retention of drugs in tumors. The figure depicts various stimuli that can be used to trigger drug-release from appropriately responsive materials in tumor cells [140].
4.2 THERMO-RESPONSIVE RELEASE
Temperature-responsive or thermos-responsive polymers manifest a sharp and irregular change of their physical properties with temperature [142]. These polymers can be functionalized with groups that bind to particular biomolecules. The polymer-biomolecules-conjugate can be accelerated from solution by a little alter in temperature. Various examples of thermos-responsive conveyance systems have been debated. Gnaim et al. synthesized and characterized a novel ?-cyclodextrin (?-CD) based carrier for atomic encapsulation of cancer chemotherapeutic agent DOX. The ?-CD subsidiary, with a ?- naphthyl alanine residue joined in its foremost surface, revealed potent binding with DOX. The embodiment effectiveness was surveyed beneath different temperatures and pH. The carrier-DOX incorporation complex is exceedingly steady under a wide range of acidic conditions (pH 1.0 –7.0). Although, the encapsulated drug is gradually discharged under hyperthermic conditions (up to 50°C). Cell culture ponders demonstrated that the complexation of DOX with the carrier inhibited cellular uptake subsequently significantly lowering toxicity. Thermos-triggered DOX release was approved and rise in cellular uptake was watched [143]. Magnetic-based core shell particles (MBCSPs) were evolved to target skin cancer cells whereas conveying chemotherapeutic drugs in a controlled design. MBCSPs comprise a thermos-responsive shell of poly (N-isopropyl acrylamide-acrylamide-allylamine) and a core of poly (lactic-co-glycolic acid) (PLGA) implanted in magnetite nanoparticles. To target melanoma tumor cells, MBCSPs were fused with Gly–Arg–Gly–Asp–Ser (GRGDS) peptides that specifically bind to the a5b3 receptors of melanoma cells. MBCSPs comprise interesting multifunctional controlled drug conveyance properties. Extraordinarily, these particles can provide dual drug discharge mechanisms (a sustained discharge through degradation of PLGA core and a controlled release in reaction to changes in temperature through thermos-responsive polymer shell), as well as dual targeting mechanisms (attractive localization and receptor-mediated targeting). Results from in vitro ponders illustrated that GRGDS-conjugated MBCSPs were less than 300nm in diameter. No cytotoxicity was watched in human dermal fibroblasts. These particles sustained the release of curcumin. A temperature-dependent release of doxorubicin from the shell of MBCSPs was watched. The particles moreover created a dull contrast signal in magnetic resonance imaging. At last, the particles amassed at the tumor location in a B16F10 melanoma orthotopic mouse demonstrate, particularly in the presence of a magnet [144]. To advance the viability of gemcitabine (Gem) in the treatment of advanced pancreatic cancer a multi-functional nanoplatform allowing both in vivo warming and drug delivery was flourished by Kim et al. [145]. The analysts utilized a chemo-hypothermia approach to achieve high intra-tumoral Gem concentrations at the same time instigating hyperthermia for improved tumor cell death as well as development hindrance. MRI visible hydroxypropyl cellulose (HPC) united permeable magnetic drug carrier may allow in vivo realization of bioequivalence. The magnetic drug carriers delivered strong T2 weighted image contrast and allowed proficient heating with low magnetic field force. The thermos-mechanical reaction of HPC activated Gem discharge was confirmed by in vitro drug release ponders. In vitro ponders affirmed that, pancreatic cancer cell development was significantly restrained (~82% reduction) with chemo hyperthermia compared to chemotherapy or hyperthermia alone. Chemo hyperthermia with intra-tumoral infusions of GEM-magnetic carriers (followed by heating) resulted in critical rise in apoptotic cells compared to tumors treated with GEM-magnetic carrier infusions. Chemo hyperthermia with GEM-magnetic carrier offers the potential to essentially progress the restorative efficacy of gemcitabine in the treatment of pancreatic cancer [145]
4.3 ENZYME RESPONSIVE RELEASE
Enzymes are key components of the bio-nanotechnology tool kit that gives extraordinary bio-recognition capabilities and exceptional catalytic properties. Combined with distinctive physical properties of nanomaterials, the emerging enzyme-responsive nanoparticles can be designed to perform proficiently with specificity for the triggering stimulus (Fig.4). This powerful concept has been effectively applied in the manufacture of drug conveyance systems where the tissue of enthusiasm is targeted through release of cargo triggered by the biocatalytic action of an enzyme [141] [146]. When the enzymatic action correlated to a specific tissue is intimated at higher concentrations at the target location, the nanomaterial can be modified to provide drugs by means of enzymatic transformation of the carrier [147]. Excitingly, the detection of enzyme action can be an amazingly valuable apparatus in diagnostics, up and down regulation of enzyme expressions that may be related with numerous disorders. Moreover, the extraordinary efficiency of enzymes in catalyzing a chemical response can be tackled to intensify the signal produced by the acknowledgment of a certain analyte, i.e. immunoreaction in enzymelinked immunoassays. In a few cases, the nanoparticles are prepared with a material that is sensitive to enzymatic change stemming from acknowledgment of chemical structure by a biocatalyst and/or change of an enzymatic response by the product. Polymeric nanoparticles consolidating natural motifs are cleaved through such enzymatic excretion. Under these conditions, it is possible to program the nanomaterials to discharge cargo (e.g. anticancer agent) by activating the degradation of the polymeric shell [148]. Nazli et al. developed magnetic iron oxide nanoparticles (MIONPs) coated with matrix-metalloproteinase (MMP)-sensitive PEG-hydrogel. High concentrations of proteolytic enzymes such as MMP are discharged by tumor tissues. These proteins can degrade the basement membrane and characteristic extracellular lattice opening up more space for tumor growth. MMP-2 and MMP-9 are exceedingly expressed in some threatening tissues such as breast, colon and brain tumors [149]. Raised concentrations of MMP in tumor tissues can lead to proteolytic degradation of peptide linkages conjugated to drugs and/or conveyance systems. Multifunctional MIONP was outlined to serve as contrast agent for MRI as well as carry chemotherapeutic doxorubicin to target overexpressed receptors on cancer cell layer. Nanoparticles comprise proteolytically degradable PEG hydrogel coatings with integrin-binding RGDS domains. In existence of MMP, such nanoparticles may corrupt due to cleavage of the MMP sensitive domains causing anticancer drug discharge. Ponders demonstrated that such nanoparticles may enter cancer cells eleven times more efficiently than blank nanoparticles. Confocal laser scanning microscopy assist showed that the targeted nanoparticles discharged doxorubicin into the cores of HeLa cells within 2 hrs. This procedure may become a promising and profoundly proficient alternative to existing strategies of cancer treatment [149].
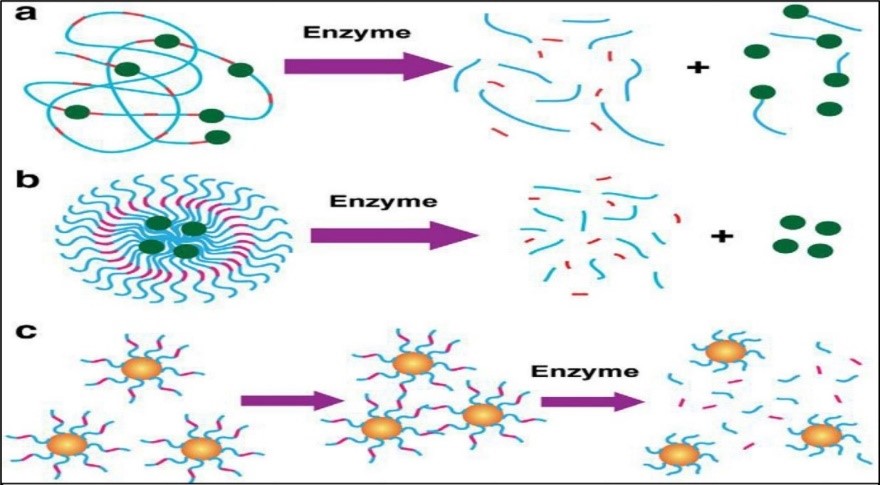
Fig. 4. Enzyme-responsive nanomaterials for drug delivery and diagnostics. a) polymer-based nanoparticles covalently modified with drugs through an enzyme cleavable linker with enzyme triggered drug delivery in malignant tissue; b) polymer stabilized liposomes loaded with drugs, where programmed degradation triggered by an enzyme; c) Inorganic nanoparticles used for diagnostics where the activity of the target hydrolase the assembly or disassembly of the nanoparticles. [141]
4.4 pH RESPONSIVE RELEASES
Among the diverse sorts of stimuli, pH sensitive system has been the most broadly employed nano-systems in cancer treatment [140]. It is well known that pH changes essentially in distinctive tissues or organs, such as stomach and liver, and in illness states, such as ischemia, infection, swelling, and tumorigenesis. Due to high rate of glycolysis in cancer cells, oxygen consuming and anaerobic conditions and lower pH in tumors can be exploited to target chemotherapeutic to these cells. Tumors have been illustrated to exhibit acidic pH values extending from 5.7 to 7.8, whereas the pH of ordinary tissue is 7.4 [150][151]. Various approaches have been developed to design pH-responsive drug discharge. One of the most commonly utilized approach is to instigate an “ionizable” chemical group such as amines, phosphoric and carboxylic acids among others, into polymeric structures. These groups, with distinctive chemical structures and pKa values, can acknowledge or give protons and experience pH-dependent changes in physical or chemical properties such as swelling ratio and/or solubility, resulting in drug discharge [152]. Another approach is to establish acid-labile chemical bonds either by covalently conjugating drug atoms directly to the surface of existing nanocarrier or to build a modern nanocarrier. These acid-labile linkages are stable at neutral pH but effortlessly degraded or hydrolyzed under acidic environment. The acid-labile linkers most commonly utilized are acetal, Ortho ester, hydrazine, imine, and cis-aconyl bonds. A novel approach to prepare pH-responsive conveyance systems is to incorporate carbon dioxide-generating antecedents that produce CO2 in an acidic environment, leading to disintegration of the carrier and release of active molecules [153]. For viable conveyance of anticancer drugs, pH-sensitive nano systems may encapsulate and stabilize the anticancer agent at physiological pH but quickly release the cargo at tumor pH.
5. Cancer Immunotherapy
Cancer immunotherapy is a revolutionary treatment aiming to activate the immune system to induce disease stabilization [154]. In contrast to the other conveyance approaches, immunotherapy basically points to avoid the metastatic spread of the illness. Cancer immunotherapy can utilize specificity of the resistant system for the treatment of melanoma. Although the immune system is efficient of recognizing and killing cancer cells but the tumor hinders with immune reactions [155]. Amid tumor development, cancer cells gain the ability to overcome the surrounding healthy tissue, subsequently spreading the infection. Owing to genetic instability, cancer cells ordinarily express anomalous proteins. Tumor-associated antigens (TAAs), deliver none or restricted expression on non-cancer cells. Such TAAs expose modern, potentially immunogenic epitopes, which can be recognized by the host immune framework. Hence, tumor cells must be determined at early stages by resistant system causing annihilation of these anomalous cells [156]. As of now, immune check point inhibitors have been utilized to treat cancer. The immune system check points incorporate PD-1 (programmed cell death protein 1) and CLTA-4 (Cytotoxic T-lymphocyte-associated antigen 4) which can secure ordinary cells. Targeting of these check focuses may be a modern instrument for the treatment of cancer [157]. Approaches to cancer immunotherapy incorporate, active and passive immunotherapy. Cytokines control both the cells of the natural safe framework and the adaptive safe framework. Cytokines apply the impacts upon binding to their particular receptors on the target cells. US Food and Drug Organization (FDA) affirmed IL-2 and IFN-?2b for treatment of a variety of cancers. IL-2 is demonstrated in the treatment of renal cell carcinoma, leukemia and lymphoma. IFN-?2b has been affirmed for the treatment of Kaposi’s sarcoma and different types of leukemia. Active immunotherapy incorporates cancer vaccines in which tumor antigen(s) are co-administered with an adjuvant to raise a particular T cell or B cell reaction. Passive immunotherapy incorporates monoclonal antibodies that piece immune checkpoints such as CTLA-4 and PD-1 [156], [154]
6. Magnetic nanoparticles (MNP)
Widder and colleagues were the foremost to utilize attractive microsphere to coordinate anticancer agents to tumor tissue with the help of external magnetic field [158]. Magnetism-assisted therapy has experienced a noteworthy advancement to become a modern therapeutic (combination of therapeutics and diagnostics) approach. Magnetic nanoparticles (MNP) are flexible systems advertising a diversity of alteration for restorative and diagnostic applications. Owing to super paramagnetic property, MNP have demonstrated to be fabulous magnetic resonance imaging (MRI) contrast agents. These agents are biocompatible and well endured. As a result, a number of MNP’s are in clinical trials or endorsed as contrast agent for MRI to identify a variety of cancers. Iron oxide NP may deliver reactive oxygen species in vivo (Fenton reaction) leading to DNA vandalism and toxicity [159]. In any case, this can be effectively dodged by coating MNP’s with polymers, surfactant, inorganic metals or oxides. Some of the commonly utilized materials for preparation of MNP’s incorporate magnetite (Fe3O4), maghemite (?-Fe2O3), iron-based metal oxides (CoFe2O4, NiFe2O4, MnFe2O4), iron amalgams (FePt and FeAu), rare earth metal alloys and transition metals. Cobalt, nickel and chromium are less favored as biomedical agents, since these metals are profoundly toxic and require impenetrable coating. Iron oxide and iron amalgam-based materials are generally more secure to use.
6.1. Therapeutic applications of MNP’s
MNP are ordinarily 10 to 100 nm in estimate with exceptionally narrow size dispersion, which permit them to exploit improved penetration and retention impact (passive targeting). This molecule size range helps in evasion of both renal clearance and phagocytosis of circulating particles. Chemotherapeutic agents can be consolidated in the polymeric film of MNP or chemically conjugated to polymer chains through appropriate linker. MNP can moreover be coordinated to the tumor site by utilizing an outside magnetic field to provide a localized conveyance of anticancer agents thereby restricting off-target toxicity. In a clinical trial, MNP’s were utilized to provide DOX hydrochloride to hepatocellular carcinoma assisted by outside magnetic field. Molecule localization was observed by MRI. In a comparative ponder Wilson et al. treated inoperable hepatocellular carcinoma with MNP targeted DOX hydrochloride. Utilizing an outside magnet DOX hydrochloride coated MNP’s were coordinated to tumor. The division of tumor volume (ranged from 0.64 to 0.91) was treated with negligible off target effect [160]. Surface coating moreover gives numerous advantages such as avoidance of agglomeration of sub-nano particles. It also limits nonspecific interactions, progresses pharmacokinetics and allows ligand intervened active targeting. Coating moreover empowers development of multifunctional nanocarriers facilitating conveyance of a numerous class of restorative agents simultaneously. A schematic representation of multifunctional nanoparticles is appeared in Fig.5. MNP can be actively targeted to cancer cells through receptor-ligand interceded particular interactions. Much cancer cell particular surface biomarkers have been targeted for this purpose. Ligand-targeted MNP have been utilized for determination as well as theranostics application for MR imaging. Area of tumor injuries as small as ~2–3 mm in diameter can be recognized following cellular uptake [161]. Chlorotoxin-conjugated MNP was utilized to target glioma cells. Active targeting brought about in higher aggregation in cancer cells with significantly improved differentiate MR imaging [162]. Targeting ligands conjugated to MNP was exploited for theranostics purposes extending from little molecule nutrient transporters such folate [163–165] to macromolecules like Trastuzumab [166-168], single-chain antiepidermal development factor antibody [169], anti-epidermal development factor receptor antibody [170], anti-vascular endothelial development factor antibody (VEGF) [171, 172] and aptamers [173], [174]. A number of small atomic weight peptide have moreover been explored including arginylglycylaspartic acid (RGD) [165, 175], bombesin [176, 177], and luteinizing hormone releasing hormone [178, 179]; profoundly cationic peptides like CPP [180], myristoylated poly-arginine peptide (MPAP) [181, 182] and HIV-TAT [183]. Multifunctional MNP have moreover been created where nanocomposites are surface modified with fluorescent tests empowering both fluorescence and MR imaging for improved in vivo tracking. Lin et al. developed methotrexate loaded folate receptor targeted double probe MNP where cyanin color (Cy5.5) was utilized as fluorescent test [184]. Such theranostic nanocomposite can be conveyed to treat tumors and observed in real time through MRI or fluorescence imaging.Hyperthermia is another application of superparamagnetic iron oxide nanoparticles ( SPIONs) [185]. MNP can raise local temperature to ~ 41–46 °C under substituting magnetic field which has been appeared to annihilate cancer cells [186]. Introduction to moderate temperature 41–46 °C leads to actuation of heap of intra and extracellular degradation mechanisms such as protein denaturation, protein folding, conglomeration and DNA cross-linking. Temperatures over 46 °C (up to 56 °C) causes coordinate tissue corruption, coagulation or carbonization leading to cell death. MNP in the 14–16 nm range have been allegedly most effective in producing hyperthermia [187]. However, this strategy requires exact special controls to dodge damage to typical cells. Hyperthermia has too been utilized as a mechanism to trigger chemotherapeutic release inside tumor microenvironment, possibly minimizing off-target impact due to premature drug discharge. Hyperthermia-mediated medicate release is accomplished through either bond cleavage, where drug is chemically conjugated to MNP via thermolabile bond or improved penetrability where chemotherapeutic are encapsulated in polymer film coated MNP. Thermoresponsive poly (N-isopropyl acrylamide) ( PNiPAM) microgel coated MNP containing anticancer agents were encapsulated inside microgel. Release is accomplished through improved permeation mechanism [188]. Essentially, PNiPAM based hydrogel was enclosed with SPIONs. Demonstrate agents (vitamin B12 and methylene blue) showed drug discharge in the presence of wavering magnetic field through enhanced penetration mechanism [189]. Derfus et al. have detailed multifunctional MNP for inaccessible controlled delivery of fluorescein-labeled DNA through oscillating magnetic field [190]

Fig.5. Schematic representation of core shell type magnetic nanoparticles. The external polymer coating entrapping anticancer agent can be decorated with imaging agent for enhanced imaging and/or targeting ligand for active targeting via a suitable linker.
7. Different Non-invasive Strategies for Cancer Treatment
7.1 Ultrasound-Mediated Treatment
Ultrasound as a conventional diagnostic tool has been specified in a non-invasive therapy and anti-cancer drug delivery. The prospective mechanism of ultrasound for drug delivery required three plans involving the thermal result effect, cavitation, and radiation forces. Ultrasound has been employee to make easier intracellular delivery of a particular drug as well as to increase the overall regulation of the cytotoxic result from the careers such as micro bubbles and Nano bubbles. [191-196] Ultrasound as an essential of drug delivery complex has the ability to be coupled with different drug bearer for the cure of cancer. [197-199]
7.1.1. Thermal Effects- Thermal Effects-Localized tissue warming depends on the assimilation of vitality, intensity, and recurrence of the ultrasound and the rate of thermal dissemination and change diffusion and change. Even a direct temperature increment may significantly increase porousness of blood capillaries and cause cell layer fluidization [200-201]. Thermal impact of ultrasound has been utilized with temperature touchy lies on which is the most commonly investigated ultrasound responsive drug-delivery vehicle. In combination with localize hyperthermia beneath ultrasound, thermosensitive liposomes made strides the delivery of different anticancer drugs to tumor [202-204]. Liposomes experience a gel to fluid stage move in the phospholipid film and ended up more penetrable. These prepare permits quick medicate release in the target region within non-threatening hyperthermia over(39-42°C) [201,202,205,206] an expansion in local drug conveyance causes consequential hindrance of tumor development [207].
7.1.2. Cavitation - Acoustic cavitation causes wavering, and collapse of little stabilized gas bubbles beneath an ultrasonic field in a liquid medium. It is considered to be the most important of all non-thermal ultrasound instruments. This component has the potential to produce cavitation in organic tissues, particularly for upgrading sedate conveyance. Cavitation can be impressively moved forward by combining gas-filled microbubbles [193]. There are two distinct sorts of acoustic cavitation action such as non-inertial (or steady) cavitation and inertial (or transitory) cavitation [208]. The non-inertial cavitation bubbles may steadily oscillate and hold on for a numerous acoustic cycle. Non-inertial cavitation of systemically injected microbubbles actuates rotating invagination and distention of vascular dividers. In turn, it causes harm to the endothelial lining and briefly raises blood vessel permeability for improved extravasation and can make strides conveyance to entire tissue [209-211] In ultrasound, inertial (temporal) cavitation can to change the penetrability of individual cells for progressed conveyance of qualities and drugs. Inertial cavitation bubbles develop and grow two to three times of their thunderous measure, and at long last collapse. Inertial cavitation is for the most part considered as the essential instrument for basically changing intaglio cells including irreversible harm and non-destructive layer penetrability [212, 213]. Inertial cavitation of microbubbles causes microjets and stun waves which make gaps in blood vessels and cell films causing higher penetrability of drugs and the carriers. The process of ultrasound-induced creation of pores in cell layers is known as sonoporation [214–217]. Acoustic cavitation approach includes the way of ultrasound for sending drugs from the carrier. In any case, the correct instrument by which the intuitive between the bubbles and the carriers make these impacts is still hazy. Ultrasound-induced cavitation has been consolidated for opening liposomal films and ultrasound-responsive steady liposomes illustrated delayed circulation time and compelling tumor focusing on [218 –221]. Drug-loaded microbubbles are appealing and ultrasound-responsive medicate carriers may be very useful for sedate focusing on to intravascular targets [222–228]. Focused on and ultrasound-triggered liposomes co-modified with single stranded DNA aptamers can target platelet-derived development calculate receptors (PDGFRs) communicated in breast cancer cells. Poly (NIPMAM-co-NIPAM) as the thermosensitive polymer can sensitize these liposomes at high temperature [229]. Perfect ultrasound-mediated tumor-targeted medicate carrier requires great drug soundness in circulation, long medicate maintenance until actuated, little measure permitting extravasation through inadequate tumor vasculature and high ultrasound responsiveness [230]. However, right now utilized differentiate operators as tumor-targeted sedate carriers have numerous inalienable difficulties. The commercially accessible microbubbles fizzled to viably extravagate into tumor tissue for successful sedate focusing on due to their exceptionally brief circulation time and relatively expansive measure (2–10 microns). After discharge from microbubbles into circulation, a majority of sedate division may circulate inside the systemic circulation and inevitably reach off-target locales. As a result, as it were a little division comes to the tumor location.
7.1.3 Radiation Force - Radiation has been characterized as a unidirectional force that is generated with an exchange of energy from the ultrasound wave to the medium under relatively high amplitudes of ultrasound exposures. Radiation powers are relative to the rate of vitality being connected and the retention coefficient of the medium where as it is inversely corresponding to the speed of the ultrasound wave in the medium. [231]. Acoustic streaming can decrease heating through the handle of expanding the mass transport of nanoparticles for progressed transdermal conveyance [232, 233]. Acoustic streaming and radiation constrain can to encourage nanoparticle exchange over blood capillaries, consequently enhancing extravasation of medicate carriers and/or macromolecular drugs [234–239]. Additionally, ultrasound radiation drive may help in modulating ligand presentation onto the surface of nanoparticles. The ligand in the bead of essential nanoparticle can be uncovered to the cell receptor with ultrasound [240]. Ultrasound impact on medicate carriers and natural tissues is to upgrade perfusion, extravasation of drugs and/or carriers, and sedate dissemination through tumor tissue. In general impact is medicated entrance through different organic boundaries. Enhanced intracellular take-up of nanoparticles, qualities, and drugs broadly make strides therapeutic viability of these operators [234, 241–247]. However, ultrasound treatment has also been related with an actuated resistant reaction to tumors [248–250].
CONCLUSION
Specific conveyance with low systemic toxicity. An ebbing tumor illustrates a dynamic environment with changes in its angiogenic status, cell mass, and extracellular lattice composition, among other variables. With recent progressions and advance in drug conveyance approaches, preventive intercessions are being recognized. Modern chemo preventive agents maybe conveyed through novel cell targeting approaches. Center of such novel approaches is based on cancer treatment with inventive strategies. Subsequently, these promising technologies offer modern opportunities for cancer avoidance and treatment with minimal/lower toxicity to typical cells which can be realized in the clinic in the very near future. In this survey, we examined different novel approaches such as targeting, triggered release strategies, gene delivery, approach and analogue/chemical conjugation to treat cancer cells. This comprehensive survey paper has examined many later approaches such as intracellular medicate targeting, enzyme responsive release, tumor microenvironment, efflux protein, cancer stem cells treatment, cancer immunotherapy, magnetic drug targeting and ultrasound-mediated drug treatment to convey cancer therapeutics. Such approaches have been explored to overcome the restrictions of ordinary treatment. This article covers several perspectives of multidrug resistance (MDR), metabolizing enzymes and consequently response to chemotherapy
REFERENCES
- Ali, Imran, Mohammad Nadeem Lone, Zeid A. Al-Othman, Abdulrahman Al-Warthan, and Mohd Marsin Sanagi. "Heterocyclic scaffolds: centrality in anticancer drug development." Current drug targets 16, no. 7 (2015): 711-734.
- Siegel, Rebecca L., Kimberly D. Miller, and Ahmedin Jemal. "Cancer statistics, 2018." CA: a cancer journal for clinicians 68, no. 1 (2018): 7-30.
- Miller, Kimberly D., Leticia Nogueira, Theresa Devasia, Angela B. Mariotto, K. Robin Yabroff, Ahmedin Jemal, Joan Kramer, and Rebecca L. Siegel. "Cancer treatment and survivorship statistics, 2022." CA: a cancer journal for clinicians 72, no. 5 (2022): 409-436.
- Boyle, P. & Bernard, L. World Cancer Report 2008. (IARC Press, Lyon, 2008).
- Soppimath, Kumaresh S., Tejraj M. Aminabhavi, Anandrao R. Kulkarni, and Walter E. Rudzinski. "Biodegradable polymeric nanoparticles as drug delivery devices." Journal of controlled release 70, no. 1-2 (2001): 1-20.
- Su, Jing, Feng Chen, Vincent L. Cryns, and Phillip B. Messersmith. "Catechol polymers for pH-responsive, targeted drug delivery to cancer cells." Journal of the American Chemical Society 133, no. 31 (2011): 11850-11853.
- Kumar, Sunil, Shikha Singh, Sudipta Senapati, Akhand Pratap Singh, Biswajit Ray, and Pralay Maiti. "Controlled drug release through regulated biodegradation of poly (lactic acid) using inorganic salts." International journal of biological macromolecules 104 (2017): 487-497.
- Shim, Min Suk, and Young Jik Kwon. "Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications." Advanced drug delivery reviews 64, no. 11 (2012): 1046-1059.
- Alvisi G, Poon IK, Jans DA. Tumor-specific nuclear targeting: promises for anti-cancer therapy? Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2006; 9:40–50.
- Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Current medicinal chemistry. 2008; 15:2648–2655. [PubMed: 18991627]
- Sui M, Liu W, Shen Y. Nuclear drug delivery for cancer chemotherapy. Journal of controlled release: official journal of the Controlled Release Society. 2011; 155:227–236. [PubMed: 21846484]
- Wagstaff KM, Fan JY, De Jesus MA, Tremethick DJ, Jans DA. Efficient gene delivery using reconstituted chromatin enhanced for nuclear targeting. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008; 22:2232–2242. [PubMed: 18356302]
- Nakamura Y, Martin C, Krapcho K, White R. Isolation and mapping of a polymorphic DNA sequence (pCMM8.1) on chromosome 1p [D1S63]. Nucleic acids research. 1988; 16:9370. [PubMed: 2902597]
- Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS letters. 2003; 538:85–88. [PubMed: 12633858]
- Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochemical and biophysical research communications. 2007; 353:26–32. [PubMed: 17184736]
- Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008; 9:435–443. [PubMed: 18189360]
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Molecular cell. 2004; 16:319–330. [PubMed: 15525506]
- Ryan KJ, Wente SR. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Current opinion in cell biology. 2000; 12:361–371. [PubMed: 10801463
- Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. The EMBO journal. 2001; 20:1320–1330. [PubMed: 11250898]
- Mohr D, Frey S, Fischer T, Guttler T, Gorlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. The EMBO journal. 2009; 28:2541–2553. [PubMed: 19680228]
- Wei W, Ma GH, Hu G, Yu D, McLeish T, Su ZG, Shen ZY. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. Journal of the American Chemical Society. 2008; 130:15808–15810. [PubMed:18980322]
- Tagliazucchi M, Peleg O, Kroger M, Rabin Y, Szleifer I. Effect of charge, hydrophobicity, and sequence of nucleoporins on the translocation of model particles through the nuclear pore complex. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110:3363–3368. [PubMed: 23404701]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999; 285:1569–1572. [PubMed: 10477521]
- Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, Altmann A, Haberkorn U, Mier W. The pharmacokinetics of cell-penetrating peptides. Molecular pharmaceutics. 2010; 7:2224–2231. [PubMed: 20845937]
- Hoang B, Ekdawi SN, Reilly RM, Allen C. Active targeting of block copolymer micelles with trastuzumab Fab fragments and nuclear localization signal leads to increased tumor uptake and nuclear localization in HER2-overexpressing xenografts. Molecular pharmaceutics. 2013; 10:4229–4241. [PubMed: 24066900]
- Yuan W, Kuai R, Ran R, Fu L, Yang Y, Qin Y, Liu Y, Tang J, Fu H, Zhang Q, Yuan M, Zhang Z, Gao F, He Q. Increased delivery of doxorubicin into tumor cells using extracellularly activated TAT functionalized liposomes: in vitro and in vivo study. Journal of biomedical nanotechnology. 2014; 10:1563–1573. [PubMed: 25016656]
- Zhu JY, Lei Q, Yang B, Jia HZ, Qiu WX, Wang X, Zeng X, Zhuo RX, Feng J, Zhang XZ. Efficient nuclear drug translocation and improved drug efficacy mediated by acidity-responsive boronate-linked dextran/cholesterol nanoassembly. Biomaterials. 2015; 52:281–290. [PubMed: 25818434]
- Swift LP, Rephaeli A, Nudelman A, Phillips DR, Cutts SM. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer research. 2006; 66:4863–4871. [PubMed: 16651442]
- Wang C, Wu C, Zhou X, Han T, Xin X, Wu J, Zhang J, Guo S. Enhancing cell nucleus accumulation and DNA cleavage activity of anti-cancer drug via graphene quantum dots. Scientific reports. 2013; 3:2852. [PubMed: 24092333]
- Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. Journal of controlled release: official journal of the Controlled Release Society. 2008; 129:228–236. [PubMed: 18539355]
- Shen Y, Zhou Z, Sui M, Tang J, Xu P, Van Kirk EA, Murdoch WJ, Fan M, Radosz M. Chargereversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010; 5:1205–1217. [PubMed: 21039198]
- Misra R, Sahoo SK. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2010; 39:152–163. [PubMed: 19961929]
- Aronov O, Horowitz AT, Gabizon A, Fuertes MA, Perez JM, Gibson D. Nuclear localization signal-targeted poly (ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues. Bioconjugate chemistry. 2004; 15:814–823. [PubMed: 15264869]
- Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angewandte Chemie. 2007; 46:4999–5002. [PubMed: 17526044]
- Cao S, Cripps A, Wei MQ. New strategies for cancer gene therapy: progress and opportunities. Clinical and experimental pharmacology & physiology. 2010; 37:108–114. [PubMed: 19671071]
- Alexandrova R. Experimental strategies in gene therapy of cancer. Journal of B.U.ON.: official journal of the Balkan Union of Oncology. 2009; 14(Suppl 1): S23–S32. [PubMed: 19785067]
- Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biology direct. 2006; 1:29. [PubMed: 16984643]
- Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. International journal of pharmaceutics. 2014; 459:70–83. [PubMed: 24286924]
- Luo J, Luo Y, Sun J, Zhou Y, Zhang Y, Yang X. Adeno-associated virus-mediated cancer gene therapy: current status. Cancer letters. 2015; 356:347–356. [PubMed: 25444906]
- Chiu TL, Peng CW, Wang MJ. Enhanced anti-glioblastoma activity of microglia by AAV2- mediated IL-12 through TRAIL and phagocytosis in vitro. Oncology reports. 2011; 25:1373–1380. [PubMed: 21399879]
- Han T, Abdel-Motal UM, Chang DK, Sui J, Muvaffak A, Campbell J, Zhu Q, Kupper TS, Marasco WA. Human anti-CCR4 minibody gene transfer for the treatment of cutaneous T-cell lymphoma. PloS one. 2012; 7: e44455. [PubMed: 22973452]
- Pan J, Zhang Q, Zhou J, Ma D, Xiao X, Wang DW. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Molecular cancer therapeutics. 2009; 8:2754–2761. [PubMed: 19723890]
- He SS, Shi HS, Yin T, Li YX, Luo ST, Wu QJ, Lu L, Wei YQ, Yang L. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in mice. Oncology reports. 2012; 27:1142–1148. [PubMed: 22218393]
- Di L, Zhu Y, Jia J, Yu J, Song G, Zhang J, Che L, Yang H, Han Y, Ma B, Zhang C, Yuan Y, You M, Wan F, Wang X, Zhou X, Ren J. Clinical safety of induced CTL infusion through recombinant adeno-associated virus transfected dendritic cell vaccination in Chinese cancer patients. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012; 14:675–681.
- Xie Y, Hicks MJ, Kaminsky SM, Moore MA, Crystal RG, Rafii A. AAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancer. Gynecologic oncology. 2014; 135:325–332. [PubMed: 25108232]
- Alam S, Bowser BS, Israr M, Conway MJ, Meyers C. Adeno-associated virus type 2 infection of nude mouse human breast cancer xenograft induces necrotic death and inhibits tumor growth. Cancer biology & therapy. 2014; 15:1013–1028. [PubMed: 24834917]
- Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Molecular therapy: the journal of the American Society of Gene Therapy. 2012; 20:1831–1832. [PubMed: 23023051]
- Ibraheem D, Elaissari A, Fessi H. Gene therapy and DNA delivery systems. International journal of pharmaceutics. 2014; 459:70–83. [PubMed: 24286924]
- Castro MG, Candolfi M, Wilson TJ, Calinescu A, Paran C, Kamran N, Koschmann C, MorenoAyala MA, Assi H, Lowenstein PR. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert opinion on biological therapy. 2014; 14:1241–1257. [PubMed: 24773178]
- Fukazawa T, Matsuoka J, Yamatsuji T, Maeda Y, Durbin ML, Naomoto Y. Adenovirus-mediated cancer gene therapy and virotherapy (Review). International journal of molecular medicine. 2010; 25:3–10. [PubMed: 19956895]
- Yao X, Yoshioka Y, Morishige T, Eto Y, Watanabe H, Okada Y, Mizuguchi H, Mukai Y, Okada N, Nakagawa S. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene therapy. 2009; 16:1395–1404. [PubMed: 19641532]
- Yao X, Yoshioka Y, Morishige T, Eto Y, Narimatsu S, Kawai Y, Mizuguchi H, Gao JQ, Mukai Y, Okada N, Nakagawa S. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2011; 19:1619–1625. [PubMed: 21673661]
- Breckpot K, Aerts JL, Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene therapy. 2007; 14:847–862. [PubMed: 17361214]
- Wang W, Zhu H, Zhang H, Zhang L, Ding Q, Jiang H. Targeting PPM1D by lentivirus-mediated RNA interference inhibits the tumorigenicity of bladder cancer cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.]. 2014; 47:1044–1049.
- Zhang ZD, Li Y, Fan Q, Zhao B, Tan B, Zhao XF. Annexin A2 is implicated in multi-drugresistance in gastric cancer through p38MAPK and AKT pathway. Neoplasma. 2014; 61:627–637. [PubMed: 25150310]
- Zhang C, Chen Y, Wang M, Chen X, Li Y, Song E, Liu X, Kim S, Peng H. PPM1D silencing by RNA interference inhibits the proliferation of lung cancer cells. World journal of surgical oncology. 2014; 12:258. [PubMed: 25123458]
- Yin H, Yan Z, Liang Y, Liu B, Su Q. Knockdown of protein phosphatase magnesium-dependent 1 (PPM1D) through lentivirus-mediated RNA silencing inhibits colorectal carcinoma cell proliferation. Technology in cancer research & treatment. 2013; 12:537–543. [PubMed: 23745790]
- Passer BJ, Cheema T, Wu S, Wu CL, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer gene therapy. 2013; 20:17–24. [PubMed: 23138870]
- Zeng WG, Li JJ, Hu P, Lei L, Wang JN, Liu RB. An oncolytic herpes simplex virus vector, G47Delta, synergizes with paclitaxel in the treatment of breast cancer. Oncology reports. 2013; 29:2355–2361. [PubMed: 23525624]
- Goshima F, Esaki S, Luo C, Kamakura M, Kimura H, Nishiyama Y. Oncolytic viral therapy with a combination of HF10, a herpes simplex virus type 1 variant and granulocyte-macrophage colonystimulating factor for murine ovarian cancer, international journal of cancer. Journal international du cancer. 2014; 134:2865–2877. [PubMed: 24265099]
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature reviews. Genetics. 2014; 15:541–555.
- Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Accounts of chemical research. 2012; 45:971–979. [PubMed: 21870813]
- Bonamassa B, Hai L, Liu D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharmaceutical research. 2011; 28:694–701. [PubMed: 21191634]
- Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene therapy. 2007; 14:99–107. [PubMed: 17167496]
- Yazawa H, Murakami T, Li HM, Back T, Kurosaka K, Suzuki Y, Shorts L, Akiyama Y, Maruyama K, Parsoneault E, Wiltrout RH, Watanabe M. Hydrodynamics-based gene delivery of naked DNA encoding fetal liver kinase-1 gene effectively suppresses the growth of pre-existing tumors. Cancer gene therapy. 2006; 13:993–1001. [PubMed: 16763608]
- Fioretti D, Iurescia S, Rinaldi M. Recent advances in design of immunogenic and effective naked DNA vaccines against cancer. Recent patents on anti-cancer drug discovery. 2014; 9:66–82. [PubMed: 23444943]
- Pharmaceuticals, A. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. Phase 2 Study of ALN-RSV01 in Lung Transplant Patients Infected with Respiratory Syncytial Virus (RSV). [cited 2015 July 13]
- Pharmaceuticals, Q. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. I5NP for Prophylaxis of Delayed Graft Function in Kidney Transplantation. [cited 2015 July 13]
- Mazda O, Kishida T. Molecular therapeutics of cancer by means of electroporation-based transfer of siRNAs and EBV-based expression vectors. Frontiers in bioscience. 2009; 1:316–331.
- Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annual review of biomedical engineering. 2014; 16:295–320
- Heller R, Heller LC. Gene electrotransfer clinical trials. Advances in genetics. 2015; 89:235–262. [PubMed: 25620013]
- Bosnjak M, Prosen L, Dolinsek T, Blagus T, Markelc B, Cemazar M, Bouquet C, Sersa G. Biological properties of melanoma and endothelial cells after plasmid AMEP gene electrotransfer depend on integrin quantity on cells. The Journal of membrane biology. 2013; 246:803–819. [PubMed: 23649038]
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008; 26:5896–5903. [PubMed: 19029422]
- Cha E, Daud A. Plasmid IL-12 electroporation in melanoma. Human vaccines & immunotherapeutics. 2012; 8:1734–1738. [PubMed: 23151447]
- Sun Y, Peng S, Qiu J, Miao J, Yang B, Jeang J, Hung CF, Wu TC. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene therapy. 2015; 22:528–535. [PubMed: 25786869]
- Zhang Y, Li H, Sun J, Gao J, Liu W, Li B, Guo Y, Chen J. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. International journal of pharmaceutics. 2010; 390:198–207. [PubMed: 20116418]
- Qin F, Zhou Y, Shi J, Zhang Y. A DNA transporter based on mesoporous silica nanospheres mediated with polycation poly (allylamine hydrochloride) coating on mesopore surface. Journal of biomedical materials research. Part A. 2009; 90:333–338. [PubMed: 18508328]
- Qureshi HY, Ahmad R, Zafarullah M. High-efficiency transfection of nucleic acids by the modified calcium phosphate precipitation method in chondrocytes. Analytical biochemistry. 2008; 382:138–140. [PubMed: 18703012]
- Lu W, Zhang G, Zhang R, Flores LG 2nd, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer research. 2010; 70:3177–3188. [PubMed: 20388791]
- Saccardo P, Villaverde A, Gonzalez-Montalban N. Peptide-mediated DNA condensation for non-Sviral gene therapy. Biotechnology advances. 2009; 27:432–438. [PubMed: 19341789]
- Costa PM, Cardoso AL, Mendonca LS, Serani A, Custodia C, Conceicao M, Simoes S, Moreira JN, Pereira de Almeida L, Pedroso de Lima MC. Tumor-targeted Chlorotoxin-coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Molecular therapy. Nucleic acids. 2013; 2: e100. [PubMed: 23778499]
- Costa PM, Cardoso AL, Custodia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. Journal of controlled release: official journal of the Controlled Release Society. 2015; 207:31–39. [PubMed: 25861727]
- Ekin A, Karatas OF, Culha M, Ozen M. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. The journal of gene medicine. 2014; 16:331–335. [PubMed: 25331590]
- Kim HJ, Takemoto H, Yi Y, Zheng M, Maeda Y, Chaya H, Hayashi K, Mi P, Pittella F, Christie RJ, Toh K, Matsumoto Y, Nishiyama N, Miyata K, Kataoka K. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS nano. 2014; 8:8979–8991. [PubMed: 25133608]
- Mitra M, Kandalam M, Rangasamy J, Shankar B, Maheswari UK, Swaminathan S, Krishnakumar S. Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells. Molecular vision. 2013; 19:1029–1038. [PubMed: 23687439]
- Fang S, Wu L, Li M, Yi H, Gao G, Sheng Z, Gong P, Ma Y, Cai L. ZEB1 knockdown mediated using polypeptide cationic micelles inhibits metastasis and effects sensitization to a chemotherapeutic drug for cancer therapy. Nanoscale. 2014; 6:10084–10094. [PubMed: 25032749]
- Ikegami S, Yamakami K, Ono T, Sato M, Suzuki S, Yoshimura I, Asano T, Hayakawa M, Tadakuma T. Targeting gene therapy for prostate cancer cells by liposomes complexed with anti-prostate-specific membrane antigen monoclonal antibody. Human gene therapy. 2006; 17:997– 1005. [PubMed: 17032155]
- Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013; 34:3489–3502. [PubMed: 23410679]
- Chen Y, Wang X, Liu T, Zhang DS, Wang Y, Gu H, Di W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. International journal of nanomedicine. 2015; 10:2579–2594. [PubMed: 25848273]
- Chen Y, Gu H, Zhang DS, Li F, Liu T, Xia W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials. 2014; 35:10058–10069. [PubMed: 25277774]
- Yang H, Li Y, Li T, Xu M, Chen Y, Wu C, Dang X, Liu Y. Multifunctional core/shell nanoparticles cross-linked polyetherimide-folic acid as efficient Notch-1 siRNA carrier for targeted killing of breast cancer. Scientific reports. 2014; 4:7072. [PubMed: 25400232]
- Ki MH, Kim JE, Lee YN, Noh SM, An SW, Cho HJ, Kim DD. Chitosan-based hybrid nanocomplex for siRNA delivery and its application for cancer therapy. Pharmaceutical research. 2014; 31:3323–3334. [PubMed: 24858398]
- Li TS, Yawata T, Honke K. Efficient siRNA delivery and tumor accumulation mediated by ionically cross-linked folic acid-poly (ethylene glycol)-chitosan oligosaccharide lactate nanoparticles: for the potential targeted ovarian cancer gene therapy. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2014; 52:48–61. [PubMed: 24178005]
- Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta pharmacologica Sinica. 2013; 34:732–740. [PubMed: 23685952]
- Kunnimalaiyaan S, Trevino J, Tsai S, Gamblin TC, Kunnimalaiyaan M. Xanthohumol-Mediated Suppression of Notch1 Signaling Is Associated with Antitumor Activity in Human Pancreatic Cancer Cells. Molecular cancer therapeutics. 2015; 14:1395–1403. [PubMed: 25887885]
- Ihle MA, Trautmann M, Kuenstlinger H, Huss S, Heydt C, Fassunke J, Wardelmann E, Bauer S, Schildhaus HU, Buettner R, Merkelbach-Bruse S. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Molecular oncology. 2015
- Ho-Yen CM, Jones JL, Kermorgant S. The clinical and functional significance of c-Met in breast cancer: a review. Breast cancer research: BCR. 2015; 17:52. [PubMed: 25887320]
- Brinkhof B, van Tol HT, Groot Koerkamp MJ, Riemers FM, SG IJ, Mashayekhi K, Haagsman HP, Roelen BA. A mRNA landscape of bovine embryos after standard and MAPK-inhibited culture conditions: a comparative analysis. BMC genomics. 2015; 16:277. [PubMed: 25888366]
- Zoni E, van der Pluijm G, Gray PC, Kruithof-de Julio M. Epithelial Plasticity in Cancer: Unmasking a MicroRNA Network for TGF-beta-, Notch-, and Wnt-Mediated EMT. Journal of oncology. 2015; 2015:198967. [PubMed: 25883651]
- Noort AR, van Zoest KP, Weijers EM, Koolwijk P, Maracle CX, Novack DV, Siemerink MJ, Schlingemann RO, Tak PP, Tas SW. NF-kappaB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. The Journal of pathology. 2014; 234:375–385. [PubMed: 25043127]


 Darshan Waghmare*
Darshan Waghmare*
 Mohd. Saqib Raza
Mohd. Saqib Raza
 Mohini Shivam Kale
Mohini Shivam Kale
 Nilesh Omprakash Chachda
Nilesh Omprakash Chachda





 10.5281/zenodo.14304062
10.5281/zenodo.14304062