Abstract
Transdermal drug delivery systems (TDDS) are non-invasive, self-contained dosage forms designed to deliver therapeutic agents through the skin and into the systemic circulation. TDDS offer a valuable alternative to oral and injectable routes, providing improved patient compliance, reduced side effects, and sustained drug release. These systems utilize various technologies, including patches, gels, and sprays, to facilitate drug permeation across the skin barrier. Key benefits of TDDS include: Enhanced bioavailability Reduced first-pass metabolism Prolonged drug action Minimized systemic side effects Improved patient adherence. TDDS have been successfully employed for managing conditions such as pain management (e.g., fentanyl, lidocaine), hypertension (e.g., clonidine), and hormonal imbalances (e.g., estrogen, testosterone). Ongoing research focuses on optimizing formulation design, enhancing skin permeability, and expanding the range of deliverable drugs.
Keywords
Drug Penetration, Matrix, Reservoir, Polymer, Pressure Sensitive Adhesive, Pro-Liposomes.
Introduction
Scopolamine was the medication used in the first transdermal drug delivery (TDD) system, Transderm -Scope, which was created in 1980 to treat motion sickness. A membrane-moderated system powers the transdermal device. In this technology, a microporous polypropylene sheet serves as the membrane. The medicine is dissolved in a mixture of mineral oil and poly-isobutylene to form the drug reservoir. This research release is sustained for a duration of three days.[3] By applying a drug formulation to intact and healthy skin, the transdermal drug delivery system, commonly referred to as patches, provides a painless method of systemically distributing therapeutically active drugs.[1,2] They have the ability to deliver innovative, genetically engineered medications to the site of action without changing the desired outcome.[4] The greatest organ for medication delivery is the skin, which serves as the body’s first line of defense. However, because of its primary function, the skin is not as effective for this purpose.[5]
Advantages of transdermal drug delivery systems:
1) Transdermal delivery is quickly rising to the top of the list of accepted drug delivery techniques because of the ongoing innovations in the field and its capacity to transfer the medication to the site of action without causing any disruption to the skin.[4,6]
2) Prevents GIT-induced enzymatic disruption and first-pass metabolism.
3) Self-administration is an option with transdermal medications.
4) Topical patches have a continual medication release in the bloodstream.
5) A less unpleasant way to administer medication.
6) Transdermal patches have less side effects than oral techniques.
7) Prevents medication incompatibility with the gastrointestinal tract.[1]
Disadvantages of transdermal drug delivery systems:
1)A few patients cease using the system after developing contact dermatitis at the application site from one or more system components.
2)No ionic medication use.
3)Could result in allergic responses.
4) Drug molecules must have a molecular weight of fewer than 500 daltons.
5) Only certain strong medicines are appropriate for transdermal treatment. 6)Ionic medications are not administered via transdermal treatment.[1,2]
Anatomy and Physiology of Skin :-
-Human skin consists of three distinct layers
1)Epidermis
2)Dermis
3) Hypodermis
The surface area of the human body’s epidermis is around 2 square meters. Because it gets about one-third of the body’s total blood volume, it is essential to our general health. Stated differently, our skin operates as a big canvas that interacts with a substantial percentage of our blood supply to facilitate vital tasks and preserve the overall health of our body.[7]]
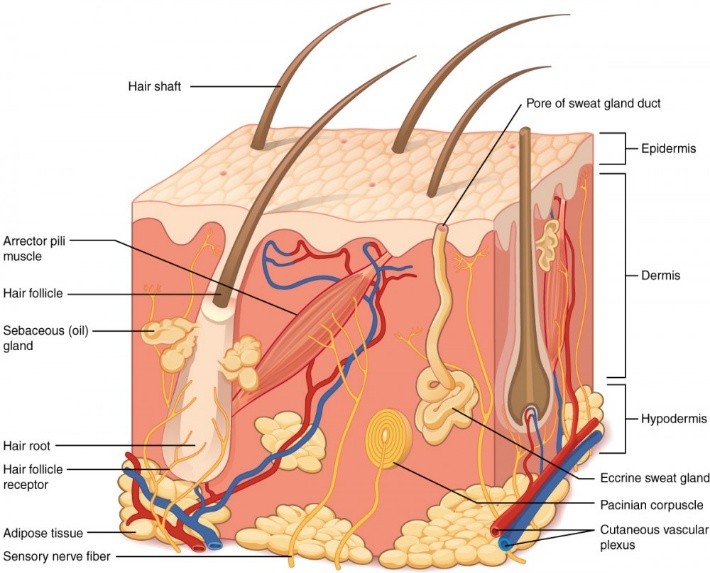
Figure 1: Skin Anatomy and Physiology.
Epidermis:
The outermost layer of the skin is called the epidermis. It doesn’t have any blood vessels and offers a watertight barrier. Keratinocytes, which generate the protein keratin and contribute to the skin’s protective and tough qualities, are among the cells that make up the epidermis. Melanocytes, which determine skin tone, and Langerhans cells, which are essential elements of the immune system, are found in the epidermis.
There are multiple sublayers in the epidermis, including: -
1)Stratum corneum
2)Stratum granulosum
3)Stratum spinosum
4) Stratum basale
On our palms and the soles of our feet, it is roughly 0.8 mm thick, although its thickness varies according on the area of our body.[13,8]
They comprise over 95% of the epidermis’s cells. Merkel cells, Langerhans cells, and melanocytes are among the cell types found in the epidermis. [14,9]
Dermis:
The dermis is a layer that is between 3 and 5 mm thick and is made up of a connective tissue matrix that includes nerve tissue, blood vessels, and lymph vessels. The blood supply to the skin is essential for controlling body temperature. It also gives the skin nourishment and oxygen, which helps to get rid of waste and pollutants. Most molecules that penetrate the skin barrier find a place to sink thanks to the capillaries, which extend up to 0.2 mm into the skin’s surface. As a result, blood supply maintains a permeant’s dermal concentration at a very low level. The concentration gradient that results across the epidermis is what is necessary for transdermal penetration.[15,10]
Hypodermis:
The innermost layer of the skin is called the hypodermis or subcutaneous tissue, which is made up of connective tissue and adipocytes, or fat cells.
This layer protects the body’s organs and bones from impact and works as an insulator, assisting in the regulation of body temperature. These three layers must be crossed by medications administered topically, such as creams or patches, in order for them to enter our bloodstream. Certain medications require even more penetration—that is, to enter our bodies’ bloodstream. However, in order for most skin treatments to be successful, they only need to pass through the stratum corneum, the skin’s outermost layer, and remain within the skin layers. [18,11]
LIMITATIONS ON TDDS SELECTION: Not all medications can be taken using this method; the medication needs to have certain desired pharmacological and/or chemical qualities. [11,24]
-Unsuitable for medications requiring elevated plasma levels. Unsuitable for medications that cause contact dermatitis and skin irritation. Not appropriate for large molecular weight medications.
-Unsuitable for medications that are metabolized while being absorbed through the skin.
-Since the skin acts as an extremely effective barrier against drug penetration, many medications cannot be administered by the transdermal route. It is only possible to provide a tiny dose.
THE FOLLOWING CONDITIONS FOR THE USE OF TRANSDERMAL PATCHES: Transdermal patches are utilized when a patient requests an alternate drug delivery mechanism, has unbearable side effects (such as constipation), and is unable to take oral medication due to dysphagia. Where effective administration could potentially improve pain control. Patients with cognitive impairment or those unable to self-medicate with their analgesia for various reasons may find this helpful.[25]
SETTLEMENTS WHERE TRANSDERMAL PATCHES ARE NOT APPLIED: When: (1) as well treating acute pain is necessary, transdermal patches should not be used.
(2) When a quick dose titration is necessary.
(3) When the dosage needed is 30 mg or less per 24-hour period [ 26].
Therapeutic Applications of TDDS:- [27]
Hisetal, a medication used to treat multiple sclerosis, can be produced in TDDS with oleic acid acting as a permeability enhancer to ensure proper drug delivery
TDDS can be used to synthesize non-steroidal anti-inflammatory medications (NSAIDs) like celecoxib and diclofenac sodium in order to prevent the stomach lesions that come with oral dose. Medications used for long-term dose in chronic illnesses, such as captopril, verapamil, terbutaline sulphate, pinacidil, and propranolol, can be manufactured as TDDS to achieve prolonged steady state plasma concentration. These medications have a short biological half-life and significant first pass metabolism
The development of an efficient controlled release transdermal system may result from the employment of a gel formulation with a betahistine lipid dispersion system
Hydrophilic polymers, such as polyvinylpyrrolidone, can speed up drug release, whereas hydrophobic polymers, such as ethyl cellulose, can slow down drug delivery.
Types of Transdermal Drug Delivery Systems
Single-layer drug- in–adhesive:
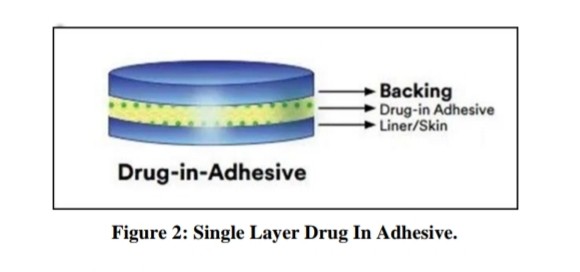
The adhesive layer in this type of system also contains the drug. In this system of patches, the adhesive layer not only serves to adhere the number of layers together, along with the whole system, to the skin but is also responsible for the release of the drug. The adhesive layer is bounded by a temporary liner and a backing Membrane.[28]
Multi-layer drug in adhesive
This type of drug in adhesive works similarly to a single-layer system in that the medication is released from both sticky layers.
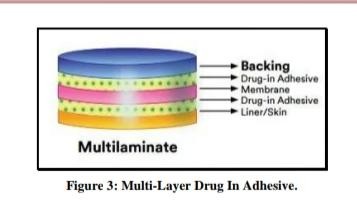
However, it differs in that a further layer of drug-in-adhesive is added, usually divided by a membrane.
This patch also offers long-lasting backing in addition to a short-medication.[28]
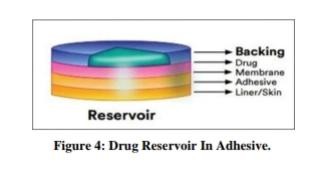
Drug reservoir-in-adhesive:
There is a separate drug layer in this transdermal device. The drug layer is a liquid compartment that is separated from the backing layer by a drug solution or suspension. The rate of release in this reservoir system is zero order.[28]
Drug matrix-in-adhesive:
This matrix system comprises a semisolid matrix drug layer that holds a drug suspension or solution.
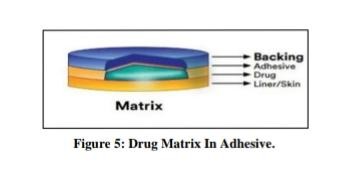
This patch’s medication layer, which partially covers the adhesive layer, surrounds it.[28]
Evaluation or characterization of TDDS:
Physical appearance
Visual examination was performed on each of the created patches to evaluate various aspects like color, clarity, opacity, transparency, flexibility, and smoothness.[11]
Thickness of patch
Various spots on each patch were measured using digital micrometers, traveling microscopes, micrometer screw gauges, or vernier calipers to find the thickness of the formed patches. On each patch, the measurements were obtained at several locations. We made sure that the thickness of the formed patches was accurately assessed by computing the average thickness and standard deviation.[12]
Weight Uniformity: Prior to weighing, the patches are dried at 60°C. One centimeter squares are cut from three patches and weighed separately to determine weight homogeneity. In order to make sure that the individual weights do not considerably differ from the average weight, the weight variation is computed. The weight of the patch is determined by averaging the three individual sections’ weights.[14]
Folding endurance: Folding endurance is measured by folding a patch or film strip repeatedly at one spot until it breaks or can be folded up to 300 times. The folding endurance of a patch is determined by how many folds it can withstand without breaking. This statistic shows how flexible the patch is.[15]
Drug Content :-A predetermined portion of the patch needs to dissolve in a predetermined volume of a suitable solvent. After that, the solution must be filtered via a filter medium before the drug content is determined using the appropriate technology (UV or HPLC). Every value is the average across three samples. [16]
Percentage Moisture Uptake:-To maintain 84% RH, the weighted films must be stored in desiccators with saturated potassium chloride solution for 24 hours at room temperature. The films must be reweighed after 24 hours in order to calculate the percentage of moisture uptake using the formula below [17].
[Final weight-Initial weight/initial weight] × 100 = Percentage moisture uptake.
Research on Water Vapor Transmission Rate (WVTR): Transmission cells were equal-diameter glass vials. These transmission cells underwent extensive washing before being dried for a while at 100 ° C in an oven. The cells were filled with approximately 1g of anhydrous calcium chloride, and the corresponding polymer film was fixed over the brim. Precise weight measurements were taken, and the cells were stored in a closed desiccator with a saturated potassium chloride solution to preserve an 84 percent relative humidity. After being stored, the cells were removed and weighed. The following formula was used to determine the amount of water vapor transmitted[18,1]
Formula: Water Vapor Transmission Rate is equal to Time X Area – Final Weight – Initial Weight. The measurement is given in grams of moisture gained/hour/square centimeter.
Rolling Ball Tack Test: This assessment measures the length of time a stainless steel ball travels along an adhesive facing upward. A rolling ball that moves forward suggests that the sticky membrane is becoming less tacky.[18,2]
Peel Adhesion Properties: The force needed to separate the adhesive film from the substrates is referred to as the peel adhesion. This test measures the force needed to pull a single-coated tape. The coat needs to be applied at 180°C to a substrate.[19]
Peel-Tack (Quick Stick) Test:- By removing the tape, or adhesive layer, from the substrate, or stainless steel plate, at a pace of 12 inches per minute, the peel force necessary to break the bond between the adhesive and the substrate can be measured. [23,20]
Skin Irritation Test:-Sensitization and skin penetration tests are conducted on healthy rabbits. The dorsal surface of the rabbits’ skin is where the specially made patches are applied with caution. The hair on the rabbits’ skin is removed before the patch is attached. The skin is carefully monitored and inspected for any possible indications of irritation or negative reactions after a 24-hour period. [16,21]
In vitro release study [58,22]: A USP dissolving equipment is used at 37°C and 50 rpm in the in vitro release test. Using an adhesive, a transdermal film is attached to a glass slide and then immersed in a dissolution medium that contains 900 milliliters of phosphate buffer at a pH of 7.4. Over the course of 24 hours, 5 ml samples are taken out hourly, and the dissolution media is supplied with an equivalent volume of buffer. Following spectrophotometric analysis of these samples, the cumulative drug release is computed using the data gathered.
Stability studies [23]:- The International Council for Harmonization's (ICH) criteria are followed in conducting the stability tests. For a duration of six months, the transdermal patches that have been produced are kept at a temperature of 40°C ± 0.5°C and a relative humidity of 75% ± 5%. Samples are taken out at predetermined times—0, 30, 60, 90, and 180 days—and properly tested to ascertain the amount of drug present. Through this testing, the transdermal patches' quality and stability are guaranteed for a prolonged length of time under predetermined storage settings
REFERENCES
- Soni Ankita et al., “Article Reviewing Transdermal Drug Delivery System”, Journal of Drug Delivery and Therapeutics, 2022; 176-180.
- N. Chaithanya et al., “A Review Article of Transdermal Drug Delivery System (TDDS)”, International Journal of Research in Engineering, Science and Management, 2019; 2:111-116.
- Chowdary K.P.R and Naidu R.A.S, Transdermal Drug Delivery, A Review of Current Status, Indian Drugs, 1995, 32(9), 414-422
- Hafeez A, Jain U, Singh J, Maurya A, Rana L. Recent advances in transdermal drug delivery system (TDDS): an overview. J Sci Innov Res. 2013;2(3):695–709. [Google Scholar]
- Ramadon D, McCrudden MTC, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug DelivTransl Res. 2022;12(4):758–91. Doi: 10.1007/s13346-021-00909-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Aundumbar Digambar Mali, Ritesh Bathe and Manoj kumar Patil, “An Updated Review of Transdermal Drug Delivery Systems”, International Journal of Advances in Scientific Research, 2015; 1(06): 244-254.
- Benson HA, Watkinson AC. Topical and Transdermal Drug Delivery: Principles and Practice. Hoboken, NJ, USA: Wiley; 2012.
- Suh H, Shin J, Kim Y. Microneedle Patches for Vaccine Delivery. Clin Exp Vaccine Res. 2014;3(1):42–9.
- Nikhil Sharma et al., “A Review:Transdermal Drug Delivery Systems: A Tool For Novel Drug Delivery Systems”, Int. J. Drug Dev. & Res., July-Sep., 2011; 3(3): 70-84.
- Kumar D. Sharma N. Rana AC, Agarwal G, Bhat ZA. A review: transdermal drug delivery system: tools for novel drug delivery system. Int J Drug Dev Res. 2011;3(3):70-84
- Singh J, Tripathi KT, Sakia TR. Effect of penetration enhancers on the Invitro transport of ephedrine through rate skin and human epidermis From matrix based Transdermal formulations. Drug Dev Ind Pharm. 1993;19:1623–8.
- Rhaghuram Reddy K., Muttalik S., and Reddy S., “Once Daily Sustained-Release Matrix Tablets of Nicorandil: Formulation and Invitro Evaluation”, AAPS Pharm. Sci. Tech., 2003; 4: 4.
- Das A, Ghosh S, Dey B, Das S. A Novel Technique for Treating the Type-II Diabetes by Transdermal Patches Prepared by Using MultiplePolymer Complexes. Int J Pharm Res Dev. 2010;2:195–204.(45)Ubaidulla U, Reddy MV, Rukmani K, Ahmad FJ, Khar RK.Transdermal Therapeutic System of Carvediol: Effect of Hydrophilic And Hydrophobic Matrix On in-vitro and in- vivo Characteristics. AAPS Pharm Sci Tech. 2007;8(1):E13–20.
- Shinde AJ, Garala KC, More HN. Development and Characterization Of Transdermal Therapeutics System of Tramadol Hydrochloride.AAPS Pharm Sci Technol. 2008;2(4):265–9.
- Prabhu Prabhakara, Marina Koland, Preparation and Evaluation Of Transdermal Patches of Papaverine Hydrochloride ,International Journal of Research Pharmaceutical Sciences, 2010,1(3), 259-266
- Koteshwar K.B, Udupa N and Vasantha Kumar, Design and Evaluation of Captopril Transdermal Preparations, Indian Drugs, 15 (29), 680-685.
- Sankar V, Velrajan G, Palaniappan R and Rajasekar S, Design And Evaluation of Nifedipine Transdermal Patches, Indian Journal of Pharmaceutical, Sciences, 2003, 65(5), 510-515.
- Shingade et al., “Review on Recent Trends in Transdermal Drug Delivery Systems”, Journal of Drug Delivery and Therapeutics, 2012; V-2(1): 66–75.
- Chetan Ghulaxe et al., “Review on Transdermal Drug Delivery System”, World Journal of Pharmaceutical and Medical Research, 2017; V3-(8): 63–71.
- Chetan Ghulaxe et al., “Review on Transdermal Drug Delivery System”, World Journal Of Pharmaceutical and Medical Research, 2017; V3-(8): 63–71.
- D.Prabhakar, J. Sreekanth, and K.N. Jayaveera, “Transdermal Drug Delivery Patches: A Review”, Journal of Drug Delivery and Therapeutics, 2013; 3(4): 213-221.
- Sood A, Panchagnula R. Role of Dissolution Studies in Controlled Release Drug Delivery System. STP Pharma Sci. 1999;9:157-68.
- Sozio P, Cerasa LS, Marinelli L., Stefano AD. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2012:8:361-8. Doi:10.2147/NDT.S16089.
- Jain N. K, Controlled and Novel Drug Delivery, 1997, 100-115.
- Kamal Saroha, Bhavna Yadav, Benika Sharma, Transdermal Patch, A Discrete Dosage Form, International Journal of Current Pharma Research, 2011,3(3), 98-108.
- Shah S, Transdermal Drug Delivery Technology Revisited, Recent Advances, Pharmainfo Net, 2008, 6(5),
- zhang L. Mas & Appicalan of quality by dealga in the curveal drag developmzal As. J. Pharm Scl. 2017:12:1-8 001 blipa dal.org/10.1016 sjp: 2016.07. 006
- Kharat Rekha Sudam and Bathe Ritesh Suresh, “A Comprehensive Review on: Transdermal Drug Delivery Systems”, International Journal of Biomedical and Advance Research, 2016; 7(4): 147-159.


 Padale Ankita* 1
Padale Ankita* 1
 Ajit Tuwar 2
Ajit Tuwar 2
 Dr. Megha Salve 3
Dr. Megha Salve 3





 10.5281/zenodo.14211648
10.5281/zenodo.14211648