Diabetes often leads to the significant complication of impaired wound healing, primarily due to reduced insulin production or sensitivity. A key factor in this process is Nrf2, which is crucial for regulating phase 2 detoxifying enzymes and antioxidant gene expression, both of which are essential in wound formation. Additionally, glucose levels influence cytokine levels, and it is suggested that type 2 diabetes mellitus may be an innate immune system disorder that perpetuates a cytokine-driven acute phase response. This condition is also linked to delayed recovery following acute vascular events, such as delayed wound re-epithelialization. Oxidative stress plays a role in the dysfunction of bone marrow-derived angiogenic cells (BMAC) in diabetic patients, with reactive oxygen species (ROS) levels often measured using flow cytometry. Heme oxygenase-1 (HO-1) has demonstrated anti-inflammatory and antioxidant effects, which contribute to granulation tissue maturation in diabetic wounds. Thrombospondin-2 (TSP-2), a protein involved in the response to injury, has been linked to delayed wound healing in diabetes. Additionally, various plants and herbs with wound healing properties have been identified in Africa and other developing regions.
Diabetes, wound healing, phytopharmaceuticals, Nrf2, TSP-2, HO-1.
Diabetes is a diverse set of metabolic disorders marked by elevated blood glucose levels. A major complication of diabetes is the impaired healing of wounds.[1] Foot ulcers are seen in about 4% to 10% of individuals with diabetes, frequently leading to infections. These infections can cause significant health issues, including high rates of lower limb amputation and considerable economic costs.[2] The global rate of diabetes is continuously increasing, affecting both genders and becoming more common with age. Prevalence rates are about 0.19% for individuals under 20 years, 8.6% for those over 20 years, and as high as 62.5% for people over 65 years.[1] Diabetes often leads to prolonged wound healing due to disruptions in insulin production or sensitivity. Insulin is essential for enabling cells to use glucose from the blood for energy. When insulin is not functioning properly, it becomes difficult to regulate blood glucose levels, resulting in elevated sugar levels that can damage white blood cells. These cells are crucial for the immune system, and when they are impaired, the body’s ability to fight infections is reduced, adversely affecting wound healing. Additionally, poor circulation is commonly observed in individuals with uncontrolled diabetes, further hindering the healing process. Poor circulation in people with diabetes slows blood flow, which impedes the delivery of nutrients to wounds and results in delayed healing or even non-healing injuries. Diabetes also causes neuropathy, where nerve damage reduces sensation in affected areas. Elevated blood glucose levels contribute to this nerve damage, making it difficult to feel foot injuries, especially during trauma. Additionally, diabetes can affect wound healing by decreasing the production of growth factors and healing hormones, impairing the formation of new blood vessels, weakening the skin barrier, and reducing collagen production.[3] In Type 1 diabetes mellitus, the primary problem is a complete lack of insulin production due to the destruction of beta cells. A rare form of Type 1 diabetes, known as idiopathic Type 1 diabetes, occurs without a known cause. Immune-mediated diabetes is an autoimmune disorder in which the immune system attacks and destroys the pancreatic cells responsible for insulin production. In Type 2 diabetes mellitus, the condition often involves insulin resistance accompanied by a relative deficiency of insulin, or it may involve a defect in insulin secretion, with or without the presence of insulin resistance.[4]
PATHOPHYSIOLOGY OF WOUND HEALING IN DIABETIC PATIENTS:
Non-healing wounds are those that do not close within a typical timeframe, with ulcers that persist for more than 12 weeks being categorized as chronic, especially in individuals with diabetes. These chronic wounds often originate from acute injuries but encounter various disruptions in the healing process, which impedes normal repair and results in prolonged open wounds.
The Wound Healing Process:
The process of wound healing is intricate and involves several key components:
Growth Factors:
These are essential proteins that stimulate cell growth, proliferation, and tissue repair. They help orchestrate various stages of healing, including cell migration and tissue regeneration.
Cytokines:
These are small proteins that act as signaling molecules to regulate immune responses and inflammation. They play a critical role in coordinating the inflammatory phase and transition to the repair phase of wound healing.
Proteases:
These enzymes are responsible for breaking down proteins in the extracellular matrix, which is crucial for remodeling and tissue repair. They help in removing damaged tissue and facilitating the formation of new tissue.
Cellular Components:
Various cell types, such as fibroblasts, endothelial cells, and immune cells, are involved in the healing process. Fibroblasts contribute to collagen production and tissue formation, while endothelial cells are important for new blood vessel formation.
Extracellular Matrix:
This network of proteins and other molecules provides structural support to tissues and influences cellular activities during the repair process. It is essential for maintaining the integrity and function of the healed tissue.[5]
RESPONSIBLE FACTORS FOR WOUND FORMATION IN DIABETES:
Nuclear Factor Erythroid 2–Related Factor 2 (Nrf2) is a key transcription factor responsible for regulating the expression of phase II detoxifying enzymes and antioxidant genes. It is a member of the CNC (Cap 'n' Collar) family of basic-leucine zipper (b-Zip) transcription factors, which also includes p45 NF-E2, Nrf1, and Nrf3. Nrf2 functions by forming heterodimers with small Maf proteins, which enhances its ability to bind to specific DNA sequences and regulate gene expression.
CNC Transcription Factors Overview:
p45 NF-E2:
This factor was initially recognized for its role in binding to the NF-E2 binding motif. It was the first member of the CNC family to be identified and characterized in terms of its gene regulation capabilities.
Nrf1, Nrf2, Nrf3:
These proteins share a conserved motif located in the N-terminal region of their b-Zip domains, a feature that classifies them within the CNC family. This conserved motif is crucial for their function in regulating genes with Antioxidant Response Elements (AREs), which are involved in cellular defense mechanisms.
Nrf2 and Its Biological Role:
Importance:
Nrf2 is especially significant due to its role as a master regulator of antioxidant and detoxification responses. It is expressed in various organs that are central to metabolism and detoxification, such as the liver and kidneys. Additionally, Nrf2 is present in tissues that are frequently exposed to environmental stressors, including the skin, lungs, and gastrointestinal tract.
Mechanism of Action:
Nrf2 regulates the expression of numerous phase II detoxifying and antioxidant enzymes by binding to AREs found in the promoter regions of these genes. This binding is a critical aspect of the cellular response to oxidative stress and exposure to harmful substances.
Research Findings:
Studies using Nrf2-deficient mice have highlighted the vital role of Nrf2 in maintaining cellular redox balance and facilitating detoxification. These studies demonstrate that Nrf2 is essential for protecting cells from oxidative damage, thereby supporting overall metabolic health and resilience against environmental stressors.[6]
INFLAMMATORY CYTOKINES:
Inflammatory Cytokines and Type 2 Diabetes Risk:
Interleukin-6 (IL-6):
This cytokine is involved in the body's acute inflammatory response and is known to affect glucose metabolism. Elevated IL-6 levels are associated with insulin resistance and an increased likelihood of developing Type 2 diabetes (T2DM).
Tumor Necrosis Factor-alpha (TNF-alpha):
TNF-alpha is a critical inflammatory cytokine that disrupts insulin signaling, leading to insulin resistance. High levels of TNF-alpha are often seen in individuals with T2DM.
Interleukin-18 (IL-18):
IL-18 is associated with inflammation and immune system activation. Recent research suggests it may play a role in cardiovascular issues by contributing to the instability of arterial plaques. Increased IL-18 levels are observed in inflammatory and metabolic conditions.
2. Effect of Acute Hyperglycemia on Cytokines:
Research Objective:
The study explores how short-term increases in blood glucose levels impact the concentration of cytokines such as TNF-alpha, IL-6, and IL-18 in people with normal glucose tolerance or impaired glucose tolerance (IGT).
Hyperglycemia Effects:
Rapid spikes in blood glucose might trigger systemic inflammation, leading to elevated levels of these cytokines. This inflammation could be a contributing factor to insulin resistance and the development of T2DM.
3. Intermittent vs. Continuous Hyperglycemia:
Episodic Hyperglycemia:
The study also examines whether intermittent spikes in glucose levels are more detrimental to cells and induce a stronger inflammatory response compared to sustained high glucose levels.
Cellular Impact:
Intermittent glucose spikes may cause greater cellular damage due to the frequent inflammatory responses they generate, potentially worsening insulin resistance and inflammation.
4. Antioxidants and Cytokine Responses:
Glutathione:
This antioxidant is evaluated for its potential to reduce the inflammatory response triggered by acute hyperglycemia.
Oxidative Stress:
Elevated glucose levels can lead to increased oxidative stress, which may stimulate the release of inflammatory cytokines like TNF-alpha. The study investigates whether glutathione can counteract this oxidative stress and reduce inflammation, potentially offering a new approach for managing T2DM and related conditions.
Potential Implications:
Insights into Inflammation and T2DM:
The study could enhance understanding of how different glucose patterns influence inflammation and insulin resistance, leading to better-targeted treatments.
Management Strategies:
If intermittent hyperglycemia proves more harmful, strategies to control glucose spikes may become crucial in diabetes management. Antioxidants like glutathione might also provide new avenues for reducing inflammation and improving metabolic health.
Cardiovascular Risk:
Understanding IL-18's role in glucose metabolism and inflammation could have implications for preventing cardiovascular diseases in diabetic individuals.[7]
DELAYED RE-EPITHELIALIZATION:
Diabetes is associated with adverse outcomes following acute vascular occlusive events, primarily due to the impaired formation of new blood vessels in response to ischemia. This process is heavily reliant on vascular endothelial growth factor (VEGF), which is crucial for promoting angiogenesis under low oxygen conditions (hypoxia). The aim of this study was to examine whether diabetes negatively affects the ability of cells to upregulate VEGF in response to hypoxia.
Study Findings and Mechanisms:
1. Diminished VEGF Response in Diabetes:
Fibroblasts derived from Type 2 diabetic patients, as well as normal fibroblasts exposed to chronic high glucose conditions, showed a reduced capacity to increase VEGF levels in response to hypoxic conditions. This suggests that diabetes impairs the normal regulatory mechanism of VEGF production in response to low oxygen.
2. Decreased HIF-1 Activity:
In individuals with diabetes, there is also a diminished ability to enhance VEGF production when confronted with tissue hypoxia. This impairment is linked to reduced activation of hypoxia-inducible factor-1 (HIF-1), a critical transcription factor that drives VEGF expression under low oxygen conditions.
3. Impaired HIF-1 and p300 Interaction:
The study identified that the reduced activity of HIF-1 in diabetic conditions is associated with a decreased ability of HIF-1 to bind to its coactivator p300. This impaired interaction is caused by the modification of p300 by methylglyoxal, a reactive dicarbonyl compound that accumulates in diabetes.
4. Restoration of VEGF Production:
Treatment with deferoxamine, which inhibits the modification of p300 by methylglyoxal, successfully restored the interaction between HIF-1 and p300. This restoration improved HIF-1 activity, leading to enhanced VEGF production, better neovascularization, and improved wound healing.
Implications and Significance:
Impact on Vascular Health in Diabetes:
The findings underscore how diabetes can disrupt the body’s ability to respond effectively to ischemia by impairing VEGF production. This impairment affects the ability to form new blood vessels, which is critical for recovery from vascular events and wound healing.
Potential Therapeutic Interventions:
The study suggests that targeting the modification of p300 by methylglyoxal could be a promising strategy to restore normal VEGF production and improve vascular repair mechanisms in diabetic patients. Deferoxamine and similar compounds might offer new avenues for enhancing angiogenesis and wound healing in the context of diabetes.[8]
OVER GENERATION OF REACTIVE OXYGEN SPECIES:
In individuals with diabetes, reactive oxygen species (ROS) levels are significantly elevated across various tissues. This increase arises from several factors that both enhance ROS production and weaken the body's antioxidant defenses. Consequently, wounds in diabetic patients often exhibit high concentrations of ROS, such as superoxide (O??) and hydrogen peroxide (H?O?). Chronic hyperglycemia is a primary factor driving the accumulation of ROS in these wounds.
Mechanisms Leading to Elevated ROS in Diabetes:
1. Excessive Mitochondrial Superoxide Production:
High blood glucose levels in diabetes lead to an overload of the mitochondrial electron transport chain, resulting in excessive production of superoxide. This byproduct of mitochondrial respiration significantly contributes to the overall increase in ROS levels within tissues.
2. Accumulation of Advanced Glycation End Products (AGEs):
Elevated glucose levels accelerate the formation of AGEs through glycation, where glucose binds to proteins, lipids, and nucleic acids. AGEs accumulate in tissues and interact with cellular structures, promoting oxidative stress and further ROS generation, exacerbating oxidative damage in diabetic wounds.
3. Reduced Antioxidant Capacity:
Diabetes often leads to decreased levels and activity of key antioxidant enzymes and molecules, such as superoxide dismutase (SOD), catalase, and glutathione. These antioxidants are essential for neutralizing ROS and protecting cells from oxidative damage. In diabetic individuals, the reduced efficacy of these antioxidants impairs the body’s ability to manage ROS levels effectively.
4. Enhanced Activity of ROS-Generating Enzymes:
In diabetic conditions, enzymes like NADPH oxidase become overactive. NADPH oxidase normally produces ROS, but in diabetes, its activity is increased, contributing to higher ROS levels in tissues and wounds.
Implications for Wound Healing:
The disruption of redox balance where ROS production outstrips the capacity of antioxidant defenses has several consequences for wound healing:
Delayed Healing:
Elevated ROS levels can damage critical cellular components, including proteins, lipids, and DNA. This damage impairs the functions necessary for effective wound repair, resulting in slower or stalled healing processes.
Heightened Inflammation:
Increased ROS levels can trigger and sustain inflammatory responses by activating inflammatory pathways and promoting the release of cytokines. Persistent inflammation further complicates and delays the healing process, contributing to chronic wound conditions.
Cellular Dysfunction:
Oxidative stress from high ROS levels can impair the function of various cells involved in wound healing, such as fibroblasts, endothelial cells, and immune cells. This dysfunction disrupts the normal repair mechanisms and contributes to the chronicity of wounds in diabetic patients.[9]
DIABETIC INFLAMMATION MEDIATED BY HEME OXYGENASE(HO-1):
Heme oxygenase-1 (HO-1) is a key enzyme in regulating inflammation that results from elevated blood glucose levels. It plays a role in reducing the inflammatory response by modulating the levels of cytokines such as IL-1?, IL-6, and MCP-1, which are typically upregulated in hyperglycemic conditions.
HO-1 and Inflammation Management:
Cytokine Modulation:
HO-1 has been observed to lower the levels of proinflammatory cytokines like IL-1?, IL-6, and MCP-1, which are often elevated in response to high blood sugar. This reduction in cytokine levels suggests that HO-1 may help mitigate the inflammatory effects of hyperglycemia.
Indirect Mechanisms:
Although HO-1 is associated with decreased levels of these inflammatory cytokines, direct evidence showing that HO-1 directly regulates their expression is lacking. Instead, HO-1 is thought to influence cytokine levels through various indirect mechanisms. These may include its effects on oxidative stress and cellular signaling pathways that affect inflammatory responses.
Mechanisms Through Which HO-1 Affects Inflammation:
1. Reduction of Oxidative Stress:
HO-1 has antioxidant properties that help reduce oxidative stress, a major contributor to inflammation. By lowering oxidative stress, HO-1 can modulate cellular processes that lead to the production of inflammatory cytokines. This reduction in oxidative stress can help maintain a balanced inflammatory response.
2. Impact on Cellular Signaling Pathways:
HO-1 may influence key cellular signaling pathways involved in inflammation. For instance, it can affect the activity of transcription factors such as NF-?B, which play a central role in regulating inflammatory gene expression. By modulating these pathways, HO-1 indirectly affects the production of cytokines.
3. Effects on Immune Cell Function:
HO-1 might also impact the function and activation of immune cells. This can have downstream effects on cytokine production, as immune cells are primary sources of these inflammatory signals. HO-1’s influence on immune cell behavior can thus contribute to the overall regulation of inflammation.[10]
PROMOTION OF DELAYED WOUND HEALING BY HO-1:
Heme oxygenase-1 (HO-1) provides significant anti-inflammatory and antioxidant benefits, which aid in the maturation of granulation tissue in diabetic wounds. Additionally, sulfide production linked with HO-1 may influence the healing process in diabetic wounds.[11] Thrombospondin-2 (TSP2), a matricellular protein crucial for injury response, is associated with delayed wound healing in diabetes. Research indicated that TSP2 levels are elevated in diabetic mice and in skin samples from diabetic patients. To investigate the role of TSP2 in impaired wound healing, scientists developed a diabetic model lacking TSP2. Although these TSP2-deficient mice displayed similar levels of obesity and hyperglycemia as control diabetic mice, they showed markedly improved wound healing. This improvement was characterized by faster re-epithelialization, increased connective tissue formation, enhanced fibroblast migration, and better vessel development. Elevated TSP2 expression in fibroblasts, a key cellular source of TSP2 in wounds, was observed in response to hyperglycemia. Mechanistically, high glucose levels activate the hexosamine pathway and nuclear factor-?B signaling, leading to increased TSP2 expression. These results suggest that the increased expression of TSP2 induced by hyperglycemia contributes to the impaired healing process seen in diabetes.[11]
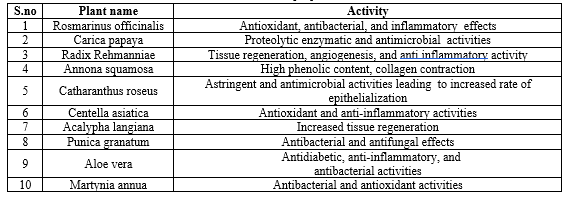
MAJOR ROLE OF MEDICINAL PLANTS IN WOUND HEALING IN DIABETES PATIENTS:
Plants and herbs are valuable for wound healing, particularly in Africa and other developing regions. The use of these medicinal plants in wound care encompasses medical treatment, surgical procedures, and creating conditions that facilitate natural healing. Compared to conventional therapies, medicinal plants are often less toxic and have fewer side effects, which has led to increased interest in their use for both diabetic and non-diabetic wound healing. Challenges in healing diabetic wounds are a significant global health concern and are sometimes linked to undefined causes. In resource-limited settings, one effective approach to treatment is the use of medicinal plants. Their application can offer beneficial outcomes in areas with limited access to traditional medical resources. Some examples of medicinal plants and their healing properties are listed below.[12]


 Bethanabelli Naveen Kumar* 1
Bethanabelli Naveen Kumar* 1
 Anjana Male 2
Anjana Male 2
 10.5281/zenodo.13764943
10.5281/zenodo.13764943