Abstract
Cancer has recently been identified as the leading cause of mortality worldwide. Several conventional treatments and cytotoxic immunotherapies have been developed and made available to the market. Considering the complex behaviour of tumors and the involvement of numerous genetic and cellular factors involved in tumorigenesis and metastasis, there is a need to develop a promising immunotherapy that targets tumors’ at both the cellular and genetic levels. Chimeric antigen receptor (CAR) T cell therapy has emerged as a novel therapeutic T cell engineering practice, in which T cells derived from patient blood are engineered in vitro to express artificial receptors targeted to a specific tumors antigen. These directly identify the tumor antigen without the involvement of the major histocompatibility complex. The use of this therapy in the last few years has been successful, with a reduction in remission rates of up to 80% for hematologic cancer, particularly for acute lymphoblastic leukemia (ALL) and Non-Hodgkin- lymphomas, such as large B cell lymphoma.
Keywords
Car – T cells, T cell remedy, therapy.
Introduction
Chimeric antigen receptors (CARs) are unique receptors that are designed to target a specific tumors antigen to functionally reprogram T lymphocytes. As T lymphocytes are genetically engineered to express these artificial receptors to target cancer cells, the type of therapy may be termed immunotherapy, gene therapy or cancer therapy. (1). However, cancer cells have the potential to subvert the immune system to their advantage, resulting in inadequate antitumor immunity, and tumors survival and progression. (2) CAR T cell treatment has achieved success in treating hematopoietic malignancies; however, its effectiveness against solid tumors remains to be determined. In the following sections, the rapid progression in implementing the adoptive transfer of T cells and their mechanism of tumor cell eradication are discussed. (3) The absence of efficacy was credited to lack of specific trafficking of the T cells to excrescence and short continuity of the transferred T cells. First generation buses deliver the primary activation signal to the T cells, but the actuated T cells are susceptible to energy, or activation convinced cell death AICD) in the absence of exogenous stimulation and fail to persist in vivo. Further, T cells were expanded ex vivo for over to 56 days with incompletely inadequate stimulation, a lengthy process known presently to reduce the figures of less- discerned cells that maintain proliferative capacity and produce a nonstop source of effector get after consanguineous transfer. (4)
T Cell remedy in cancer:
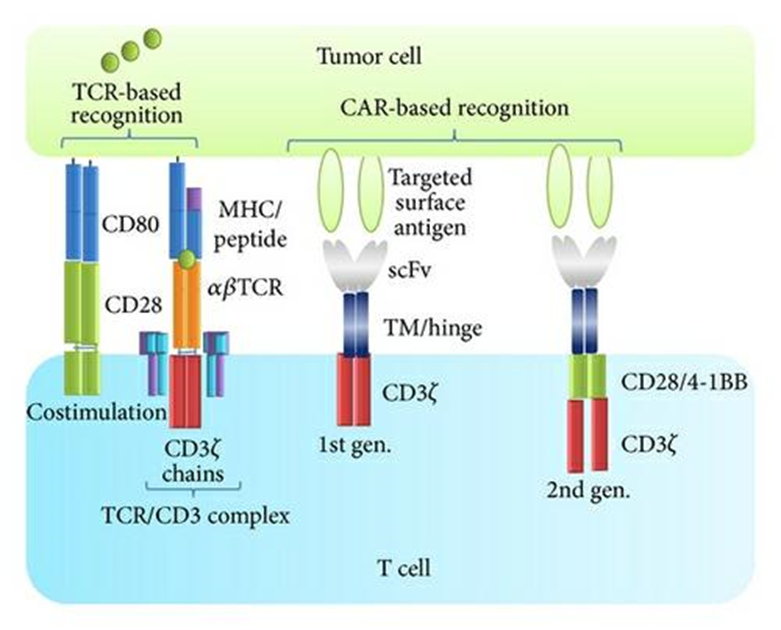
The efficacy of consanguineous T cell remedy (ATC) in mortal cancers was first demonstrated by the induction of molecular absolution after patron lymphocyte infusion (DLC). in myeloid malice returning following bone gist transplantation. (5,6) farther studies demonstrated that expanded excrescence insinuating lymphocytes (TLC). could induce complete, long- lasting retrogression of large vascularized metastatic tubercles. (7,8) ATC using Epstein Barr contagion-(EBV-) specific T cells showed clinical benefit in colorful EBV- associated malice, including Hodgkin’s complaint, Burkitt’s carcinoma, and nasopharyngeal melanoma Auto is an arising immunotherapy for several malice. (9) This remedial approach is an experimental form of gene remedy that redirects T lymphocytes to annihilate cancerous cells. The original step in this remedy is leukapheresis or the insulation of a case’s supplemental blood. Apheresis is extensively used to insulate blood from cases and separate it into its factors, which are also genetically altered before- injecting them into the case’s body. presently, apheresis is used by blood banks to collect platelecasnd other blood factors for the treatment of several conditions, including hematologic and renal diseases. Thus, it’s regarded as a safe practice for healthy Individualities and case. (10)
Ideology of T cell engineering and Car T cell Design:
The oneness of Fantastic receptors exists in their capacity to fuse or resolve separate vital functions, similar recognition, co-simulative and activation, in different chains of a receptors patch by imitating the complexity of the native T cell receptors structure .(11)T cells don`t generally bear stimulation for activation and to initiate proliferation, but in the process of establishing Auto T cells, the activation and proliferation of T cells bear the presence of costimulatory motes, which also help in Auto T cells , the cell cytokine product. The strategy involves constructing A finagled fantastic receptors for T cells grounded on the integration of scFv fractions in the hinge area that separates scFv from the cell membrane. The exposure of scFv on the cell face, in addition to other small functional motes, enhances induction of the cytolysis function of the finagled T cell. Together, this collaboration of a ‘living medicine’ in the vulnerable system fights against cancer. (12)
Advanced features of CAR T cell Therapy:
Although cancer cells have multiple lineages and diversity, they retain common target antigens, similar as CD19, CD20, CD22 and multitudinous others that allow Auto T cells to fete excrescence cells irrespective of Cell lineage. Thus, recent advances in this fashion include more precise target antigens expressed by
Excrescence cells. (13,14)
Bispecific CARs:
bispecific receptor is one that contains two distinct antigen recognition disciplines attached And placed with two distinct intracellular signaling disciplines that are expressed as two different buses on a Single cell face. At present, bispecific Auto CD19/ CD20 has been introduced as a new synthetic patch that Can fete and bind to further than one targeted excrescence antigen on the cancer cell face. Thus, it can produce A synergistic waterfall of effector motes when it encounters two excrescence antigens. (15) Targeting T cells with
Bispecific buses was shown to exclude transplanted pediatric ALL in a study, whereas T cells targeted by CD20 CAR did not control the complaint. (16)
Tandem CARs (Tan -CARs):
Conventional buses can not meet advanced prospects under certain Circumstances, similar as the down regulation or revision of targeted antigens that can do in cancerous cells. (17) These conditions latterly lead to antigenic loss or escape variations lately, a trivalent Auto T cell was designed By a group of scientists by contemporaneously co -targeting multiple antigens, similar as HER2, IL- 13 Receptor ?2 and Ephron A2, to overcome integration insecurity with a propensity to target nearly 100 of
Excrescence cells. (18)
Inhibitory CARs (I – CARs):
Reported that certain new immunoinhibitory receptors are involved in T cell activation and The attenuation or termination of T cell responses, similar as programmed death- 1(PD- 1), programmed Death- ligand 1(PD- L1) and cytotoxic T- lymphocyte- associated antigen 4( CTLA- 4). These pathways are Regarded as cancer immunotherapy improvements several Auto constructions contained scFv of murine
Origin. (19)
Physiological CARs:
This is associated with the threat of an vulnerable response to the modified cells, and the performing
Anaphylaxis of Auto T cell transfer can not be avoided. These disadvantages limit the continuity of the a physiological Auto has been developed, also known as invested cells. Auto has been developed, alas a Receptor- ligand Auto, which can fete and bind to excrescence antigens, similar as HER3 and HER4. (20)
Natural Killer (NK -CARs):
Are a type of cytotoxic T cell that are essential for natural impunity. The function of NK cells is analogous to That of cytotoxic T cells in the adaptive vulnerable response of invertebrates. Immune cells generally identify MHC complexes expressed on contagious cell shells to evoke cytokine product, therefore generating an Vulnerable response, climaxing in the apoptosis or death of contagious cells. (21)
CRISPR CARs:
CRISPR is a gene modifying tgen, That uses a companion gRNA to modify a DNA sequence. As this technology is an integration-free gene Objectification system, it offers a reliable and competent gene knock- in process. Following the significant Progress associated with CRISPR technology, it has the implicit to crop as a promising immunotherapy (91). The CRISPR system can be directly applied to mammalian cells via transfection using a plasmid that contains Both nuclease and sgRNA. Cas9 encodes a large patch with a multifunctional DNA endonuclease and is Known to excise dsDNA from 3- bp upstream of the PhotoScape conterminous motif (PAM). (22)
Mode Of Delivery:
Gene remedy involves the delivery of DNA into cells, and this can be fulfilled using a few styles epitomized below. The most traditional system utilizes recombinant contagions (also known as viral vectors), natural nanoparticles Andon-viral styles grounded on the direct delivery of naked DNA.
1)Viral vector: Viral vectors, similar as ?- retrovirus, lentivirus and adenovirus vectors are generally used in gene remedy. Retrovirus transduction is one of the generally used delivery styles in gene remedy. The system involves a rear transcriptase that promotes the stable integration of artificial genes into the host genome. (23)
2)Non-viral: vector the medium of transposon transfection is different from that of viral transfection. Delivery via transposons is an on-viral process that uses transposon DNA and a transposase enzyme for stable gene transfer. Two important vectors, videlicet piggyBac (PB) and sleeping beauty (SB), are constantly used.(24)
A) Electroporation: has evolved as a useful fashion for modifying genes of different cell types.
The target cells are exposed to electric fields to temporarily disrupt their cell membranes. This allows the charged motes to enter the cells. Forecourt- surge and palpitation- grounded systems are new electroporation bias. (25)
B) Nanoparticles: The most critical step in Auto T cell remedy is T cell activation, for which cells need to be incubated with the viral vector including an Auto gene. Still, stable insertional mutagenesis can be dangerous as its possible effects on humans remain to be completely illustrated. (26)
Factors affecting Efficacy of Car T Cell therapy:
1)Numerous known and multitudinous yet unidentified factors are likely to contribute to the variability observed in clinical responses across trials and between individual cases. Even though differences in clinical protocols avert direct comparisons, clinical data inclusively point at T cell expansion and continuity after consanguineous transfer as crucial critical factors for achieving an effective concurrence. (27)
2) The in vivo fate of the T cells is told by several factors astronomically related to the Auto design, the composition of the invested T cells, the excrescence type and medium, and philanthropist preconditioning authority. Savoldi and associates elegantly demonstrated the significance of introducing costimulatory disciplines into alternate generation buses by treating six carcinoma cases with an admixture of first and alternate generation Auto T cells, furnishing substantiation for the enhanced continuity of T cells expressing the ultimate Auto configuration. (28)
3)Single- target remedy may elect for and lead to escape of the variants and targeting multiple antigens on excrescences would increase the chances of remedial effectiveness. Combination of buses with different particularity, or the use of bispecific tandem buses, which join two antigen- recognition halves, may help relapses due to escape of variants but bear farther studies. (29)
CONCLUSION AND FUTURE PERSPRECTIVE:
The consanguineous transfer of gene modified T cells is a fleetly evolving innovative treatment for cancer. Auto diverted T cells are renewable medicines with the capacity to gain in the case after infusion and further to persist and give sustained functional impunity. The efficacy has been demonstrated in a range of hematological cancers including ALL, CLL, DLBCL, FL, and multiple myeloma. (30) Also, the Auto T cell technology must be capitalized at an respectable cost. Airman- scale processes for Auto T cell generation were firstly developed in academic centers for early phase I clinical exploration, taking small figures of T cell products. These processes are grounded in homemade and open- running way in safety closets and are not suited for marketable manufacturing of thousands of remedial T cell bonuses demanded for an unborn approved remedy. Industrialized T cell processing can only be achieved with significant investments in robotizing and the entablement of a completely unrestricted process T cell selection, and expanse ion ways, as well as the development of safe and more effective viral and nonviral vectors, will further enhance the integration of T cell gene curatives. (30) Eventually, to overcome the constraints associated with complicated logistics and manufacturing of the personalized T cell remedy in the autologous setting, significant sweats are under way to develop universal and off- the- shelf, allogeneic T cell medicines. While out- the- shelf T cells might allow more effective manufacturing and reduce lead time for the administration of the T cell medicine, there are some enterprises regarding their use, including their eventuality to drive GVHD and their limited life span after transfer. (30)
REFERENCES
- Restifo NP, Dudley ME and Rosenberg SA: Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol. 12:269–281. 2012. View Article : Google Scholar : PubMed/NNCB
- Perales MA, Kebriaei P, Kean LS and Sadelain M: Building a safer and faster CAR: Seatbelts, airbags, and CRISPR. Biol Blood Marrow Transplant. 24:27–31. 2018. View Article : Google Scholar : PubMed/NCB
- Miliotou AN and Papadopoulou LC: CAR T-cell therapy: A new era in cancer immunotherapy. Curr Pharm Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
- Kolb H. J., Mittermüller J., Clemm C., Holler E., Ledderose G., Brehm G., Heim M., and Wilmanns W., Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients, Blood. (1990) 76, no. 12, 2462–2465, 2-s2.0-0025678601.
- Deol A. and Lum L. G., Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited, Cancer Treatment Reviews. (2010) 36, no. 7, 528–538, https://doi.org/10.1016/j.ctrv.2010.03.004, 2-s2.0-77957934068.
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A., Simpson C., Carter C., Bock S., Schwartzentruber D., Wei J. P., and White D. E., Use of tumor-infiltrating lymphocyts and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report, The New England Journal of Medicine. (1988) 319, no. 25, 1676–1680, https://doi.org/10.1056/nejm198812223192527, 2-s2.0-0024166189.
- Hinrichs C. S. and Rosenberg S. A., Exploiting the curative potential of adoptive T-cell therapy for cancer, Immunological Reviews. (2014) 257, no. 1, 56–71, https://doi.org/10.1111/imr.12132, 2-s2.0-84890147150
- Bollard C. M., Aguilar L., Straathof K. C., Gahn B., Huls M. H., Rousseau A., Sixbey J., Gresik M. V., Carrum G., Hudson M., Dilloo D., Gee A., Brenner M. K., Rooney C. M., and Heslop H. E., Cytotoxic T lymphocyte therapy for epstein-barr virus+ Hodgkin?s disease, Journal of Experimental Medicine. (2004) 200, no. 12, 1623–1633, https://doi.org/10.1084/jem.20040890, 2-s2.0-19944426160.
- Heslop H. E., Slobod K. S., Pule M. A., Hale G. A., Rousseau A., Smith C. A., Bollard C. M., Liu H., Wu M.-F., Rochester R. J., Amrolia P. J., Hurwitz J. L., Brenner M. K., and Rooney C. M., Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients, Blood. (2010) 115, no. 5, 925–935, https://doi.org/10.1182/blood-2009-08-239186, 2-s2.0-77649221824.
- Abate-Daga D and Davila ML: CAR models: Next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 3:160142016. View Article : Google Scholar : PubMed/NCBI
- Yang QY, Yang JD and Wang YS: Current strategies to improve the safety of chimeric antigen receptor (CAR) modified T cells. Immunol Lett. 190:201–205. 2017. View Article : Google Scholar : PubMed/NNCBI
- Mirzaei HR, Mirzaei H, Namdar A, Rahmati M, Till BG and Hadjati J: Predictive and therapeutic biomarkers in chimeric antigen receptor T-cell therapy: A clinical perspective. J Cell Physiol. 234:5827–5841. 2019. View Article : Google Scholar : PubMed/NCBI
- Paietta E: Immunobiology of acute leukemia. In: Neoplastic diseases of the bloodSpringer; Cham: pp. 237–279. 2018
- Zah E, Lin MY, Jensen MC, Silva-Benedict A and Chen YY: Abstract IA12: Combating antigen escape with CD19/CD20 bispecific CAR-T cell therapy. Cancer Immunol Res 5 (3 Suppl). IA122017.
- Zhang W, Liu Y, Wang Y, Wang C, Yang QM, Zhu HL and Han WD: Long-term safety and efficacy of CART-20 cells in patients with refractory or relapsed B-cell non-Hodgkin lymphoma: 5-years follow-up results of the phase I and IIa trials. Signal Transduct Target Ther. 2:170542017. View Article : Google Scholar : PubMed/NCBI
- Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al: Tandem CAR T cells targeting HER2 and IL13R?2 mitigate tumor antigen escape. J Clin Invest. 126:3036–3052. 2016. View Article : Google Scholar : PubMed/NCBI
- Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al: HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncol. 3:1094–1101. 2017. View Article : Google Scholar : PubMed/NCBI
- Ott PA, Hodi FS and Robert C: CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI
- Shi H, Sun M, Liu L and Wang Z: Chimeric antigen receptor for adoptive immunotherapy of cancer: Latest research and future prospects. Mol Cancer. 13:2192014. View Article : Google Scholar : PubMed/NCBI
- Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L and Koehl U: Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 6:212015. View Article : Google Scholar : PubMed/NCBI
- Hsu PD, Lander ES and Zhang F: Development and Applications of CRISPR-Cas9 for genome engineering. Cell. 157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI
- Giacca M and Zacchigna S: Virus-mediated gene delivery for human gene therapy. J Control Release. 161:377–388. 2012. View Article : Google Scholar : PubMed/NCBI
- Munñoz-López M and García-Perez JL: DNA transposons: Nature and applications in genomics. Curr Genomics. 11:115–128. 2010. View Article : Google Scholar : PubMed/NCBI
- Yarmush ML, Golberg A, Serša G, Kotnik T and Miklav?i? D: Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu Rev Biomed Eng. 16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
- Ramamoorth M and Narvekar A: Non viral vectors in gene therapy-an overview. J Clin Diagn Res. 9:GE01–GE06. 2015.PubMed/NCBI
- Maus M. V., Grupp S. A., Porter D. L., and June C. H., Antibody-modified T cells: CARs take the front seat for hematologic malignancies, Blood. (2014) 123, no. 17, 2625–2635, https://doi.org/10.1182/blood-2013-11-492231, 2-s2.0-84899631361.
- Savoldo B., Ramos C. A., Liu E., Mims M. P., Keating M. J., Carrum G., Kamble R. T., Bollard C. M., Gee A. P., Mei Z., Liu H., Grilley B., Rooney C. M., Heslop H. E., Brenner M. K., and Dotti G., CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients, Journal of Clinical Investigation. (2011) 121, no. 5, 1822–1826, https://doi.org/10.1172/JCI46110, 2-s2.0-79955517235.
- Grada Z., Hegde M., Byrd T., Shaffer D. R., Ghazi A., Brawley V. S., Corder A., Schönfeld K., Koch J., Dotti G., Heslop H. E., Gottschalk S., Wels W. S., Baker M. L., and Ahmed N., TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy, Molecular Therapy—Nucleic Acids. (2013) 2, article e105, https://doi.org/10.1038/mtna.2013.32, 2-s2.0-84880259439.
- Gill S. and June C. H., Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies, Immunological Reviews. (2015) 263, no. 1, 68–89, https://doi.org/10.1111/imr.12243, 2-s2.0-84924750926.
- Kaiser A. D., Assenmacher M., Schröder B., Meyer M., Orentas R., Bethke U., and Dropulic B., Towards a commercial process for the manufacture of genetically modified T cells for therapy, Cancer Gene Therapy. (2015) 22, no. 2, 72–78, https://doi.org/10.1038/cgt.2014.78, 2-s2.0-84924801689.
- Morgan R. A. and Kakarla S., Genetic modification of T cells, Cancer Journal. (2014) 20, no. 2, 145–150, https://doi.org/10.1097/ppo.0000000000000033, 2-s2.0-84897494749.


 Rutuja Jotiram sawant*
Rutuja Jotiram sawant*

 10.5281/zenodo.14018247
10.5281/zenodo.14018247