Abstract
Benzothiophene derivatives have possessed diverse pharmacological applications due to their structural versality and their potential therapeutic effects. They exhibit various biological activities such as antidiabetic, anticancer, anti-inflammatory , anti-oxidant ,anti-tubercular ,antimicrobial and anticonvulsant activity. Beginning with an introduction to the structural diversity and synthetic strategies for benzothiophene derivatives, the review highlights their broad spectrum of biological effects. Key sections delve into the multifaceted roles of benzothiophene compounds in medicinal chemistry, including their anti-inflammatory, antimicrobial, anticancer, and neuroprotective activities. Furthermore, their interactions with specific biological targets such as enzymes, receptors and ion channels are discussed. This review is based on various pharmacological effects of Benzothiophene derivatives.
Keywords
Docking , Benzothiophene , Anti-Diabetic, Anti-Cancer, Anti-Tubercular
Introduction
Docking
Molecular docking is an important tool in computer-assisted drug design and structural molecular biology. The aim of ligand—protein docking is to predict the predominant binding modes of a particular ligand with a protein of known three-dimensional structure. The docking methods find high-dimensional spaces and use a scoring function that properly ranks candidate dockings. Docking is normally used to perform virtual screening on large libraries of compounds, rank the results, and gives structural hypotheses on how the ligands inhibit the target. The setting up of the input structures is important for docking . This article discusses the background and theory of molecular docking software, and includes the usage of some of the most-cited docking software.[1,2,3]
Benzothiophene
Benzothiophene is a bicyclic system containing heteroatom S , where a benzene ring is fused with a thiophene ring at 4,5-positions . The various activities are anti-diabetic , anti-cancer , anti-bacterial , anti-tubercular , anti-inflammatory , anti-oxidant , anti-microbial , anti-viral activities.[4]
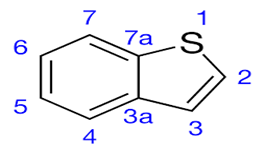
Fig no:1 Benzothiophene
Table no:1 Physical properties of 1-Benzothiophene

Some of the Benzothiophene derivatives which are available in the market , include Ipragliflozin , Raloxifene , Zileuton , Setaconazole , Benocyclidine.
Chemistry of benzothiophene
Benzothiophene is an aromatic organic compound consisting of S as heteroatom in the first position of thiophene which is fused with benzene ring at 4,5-positions. It is a stable compound due to the resonance stabilization given by its aromatic ring system and delocalization of ? electron cloud.1-Benzothiophene shows geometric isomerism due to restricted rotation around C-C bond and their reactivity is influenced by its ring fusion , aromaticity and presence of S atom.[5]
Synthesis of benzothiophene
Benzothiophene derivatives where synthesised using coupling and cyclization reactions. Benzothiophene can be prepared by intramolecular cyclization of various aryl sulfides in the presence of different catalyst .[6]
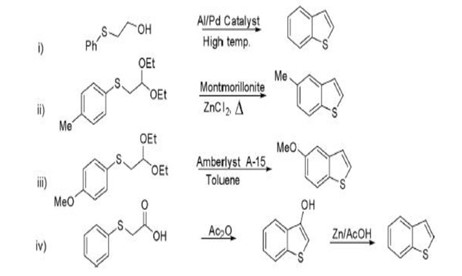
Fig no: 2 Synthesis of benzothiophene
PHARMACOLOGICAL ACTIVITIES
Anti-Diabetic activity
Type-2 Diabetes Mellitus a metabolic disorder which is characterized by hyperglycemia due to impaired insulin secretion or insulin resistance. Agents which inhibit Sodium glucose co-transporters (SGLTs) , are a good option in the management of T2DM as they have distinct mechanism of action to reduce blood glucose levels. Renal glucose absorption is mediated by SGLT1 and SGLT2.Selective SGLT2 inhibitors (Ipragliflozin) have shown enhanced urinary glucose excretion and reduce hyperglycemia in patients with T2DM.[7,8]

Fig no:3 Ipragliflozin
Anti-Bacterial activity
Bacterial infections, which are mainly caused by Gram-positive and Gram-negative organisms, which are proliferations of harmful strains of bacteria on or inside the body. A Cup plate method with the help of Hi-media agar medium was used to study about antibacterial activity of synthesized derivatives such as (1) 2-[5-(4-Chlorophenyl)-1, 3, 4-oxadiazol-2-yl]-1-benzothiophen-3-amine (3a) ,( 2) 2-[5-(2,4-Dinitrophenyl)-1,3,4-oxadiazol-2-yl]-1-benzothiophen-3-amine (3b) , (3) 2-[5-(4-Aminophenyl)-1,3,4-oxadiazol-2-yl]-1-benzothiophen-3-amine (3c) , (4) 2-[5-(2-Chlorophenyl)-1,3,4-oxadiazol-2-yl]-1-benzothiophen-3-amine (3d) , (5) 1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(3-nitrophenyl)-1,3,4-oxadiazol-3(2H)-yl]ethanone (5a) , (6)1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(4-chlorophenyl)-1,3,4-oxadiazol-3(2H)-yl]ethanone (5b) , (7) 1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(3-hydroxyphenyl)-1,3,4-oxadiazol-3(2H)-yl] ethanone (5c) , (8) 1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(4-methoxyphenyl)-2-methyl-1,3,4-oxadiazol-3(2H)-yl]ethanone.(7b) , (9) 1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(4-hydroxyphenyl)-2-methyl-1,3,4-oxadiazol-3(2H)-yl]ethanone (7c) , (10) 1-[5-(3-Amino-1-benzothiophen-2-yl)-2-(3-aminophenyl)-2-methyl-1,3,4-oxadiazol-3(2H)-yl]ethanone (7d) .
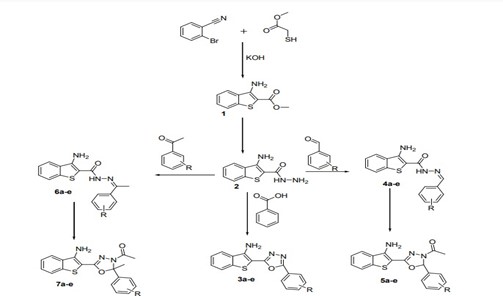
Fig no: 4 Synthesis of benzothiophene derivatives having Anti-Bacterial activity
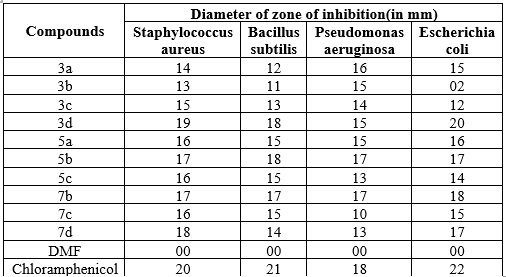
The activity was studied against two gram positive bacteria Staphylococcus aureus – ATCC 25923 and Bacillus subtilis -ATCC 6633 and gram negative bacteria Pseudomonas aeruginosa – ATCC 10145 and Esherichia coli-ATCC 35218.[9,10]
Table no:2 The derivatives which were tested showed antibacterial activity when compared to standard drugs against all microorganisms are listed in the table 1.2.
Anti-Cancer activity
Benign tumors, characterized by their self-limiting nature within a specific group of cells and their lack of invasion or metastasis, stand in stark contrast to the vast majority of cancers, which arise from genetic abnormalities induced by various carcinogens like tobacco smoke, radiation, chemicals, and infectious agents.A study in the International Journal of Advances in Pharmaceutical Sciences has identified Benzo[b]thienyl hydroxamic acids as a potent anticancer agent. The researchers undertook a quantitative structure-activity relationship (QSAR) analysis on a series of Benzo[b]thiophene based compounds to elucidate their pharmacological properties. The investigation unveiled that steric and electrostatic interactions, along with electro-topological parameters, estate numbers, and alignment-independent descriptors, play pivotal roles in dictating the anticancer efficacy. Moreover, the QSAR model showcased a correlation between anticancer activity and specific parameters such as Polar Surface Area, Saa CHE-Index, and SaasCE-Index. This study aims to introduce amide type of ring system as anticancer agents, demonstrating the potential of benzothiophene derivatives in drug discovery and design.[11,12,13]
Anti-Tubercular activity
Tuberculosis is an infectious disease caused by the Mycobacterium tuberculosis affecting mainly the lungs . It mainly spreads through the air when an infected person sneeze or cough .Small droplets containing Tuberculosis bacteria may be inhaled by normal people which leads to infection. A study showed that 3-substituted benzothiophene -1,1-dioxide class of compounds act as effective inhibitors of Mycobacterium tuberculosis under aerobic conditions. The oxidation of 3-bromothionaphthalene with hydrogen peroxide produced 3-bromo-benzo thiophene-1,1-dioxide.Benzo[b]thiophene moiety is a drug which is known to possess potential medicinal value in FDA approved drugs like raloxifene, sertaconazole, zileuton, and benocyclidine. Therefore, in continuation of the program on the discovery of antitubercular compounds, various 1,3-diketones, flavones, pyrazoles, and carboxamides were synthesized from benzo[b]thiophene carboxylic acid and demonstrated inhibitory activity against MTB H37Ra and M. bovis BCG. The cytotoxicity of compounds has been also tested.[14,15,16]
Anti- Convulsant activity
Epilepsy is characterized by the periodic and unpredictable occurrence of seizures and is the most frequent neurological disorder affecting approximately about 50 million people throughout the world .Almost 90% of these people belong to developing countries. Two sets of spiro[[1]benzothiophene-3,2'-[1,3]thiazolidine]-2,4'-diones, denoted as (5a-e) and (6a-e), were synthesized, characterized, and subjected to assessment for their anticonvulsant properties. Following these findings, our attention has been focused on a group of spiro benzothiophene thiazolidinone derivatives (5a-e) and (6a-e). ). All the title compounds comprised four pharmacophoric elements that are necessary for good anticonvulsant activity.[17,18,19]
Fig no:5 Synthesis of benzothiophene derivatives having Anti-Convulsant activity
Anti-Inflammatory activity
Inhibiting leukotriene synthesis has granted significant interest in pharmaceutical research for its potential in combating inflammatory and vascular diseases. This study focuses on bioactive 2-substituted benzo[b]thiophene derivatives, akin to Zileuton analogues, synthesized from readily available 5-nitrobenzo[b]thiophene-2-carboxylic acid. The newly synthesized compounds were meticulously evaluated for their biological activities and structure-activity relationships (SAR), juxtaposed with the classic NSAID, piroxicam. Compounds such as phenylthiosemicarbazide and 4-(5-nitrobenzo[b]thiophene-2-yl)semicarbazide demonstrated heightened potency as anti-inflammatory and anti-nociceptive agents. By concurrently inhibiting 5-LOX/COX, the compounds exhibit augmented anti-inflammatory effects with reduced side effects, highlighting the significance of designing novel dual inhibitors for treating inflammation. The design rationale of these target compounds involves integrating benzothiophene or its bioisostere benzofuran, housing various anti-inflammatory pharmacophore heterocycles, through distinct atom spacers.[20]
CONCLUSION
Benzothiophene and its derivatives exhibit a wide range of bioactive effects, making them promising candidates for various therapeutic applications. Their structural versatility allows for the development of compounds with diverse pharmacological properties, including anti-inflammatory, anticancer, antibacterial, antitubercular, antidiabetic, anticonvulsant. The significant bioactivity of benzothiophene underscores its importance in medicinal chemistry and its potential to contribute to future advancements in drug development.
ACKNOWLEDGEMENT
We want to offer this endeavour to GOD ALMIGHTY for all the blessings showered on us during the course of this project. We take the privilege to acknowledge to all those who helped in the completion of the review. At first, our express deep sense of gratitude indebtedness to The Department of Pharmaceutical Chemistry of Mar Dioscorus College of Pharmacy , for helping in the completion of our review .We are extremely grateful to our Principal, for her guidance and valuable suggestions which made to complete our work. We are deeply obliged to Dr . Sreeja S , our guide as well as mentor, for her guidance , immense knowledge insightful comments , constant support and encouragement which completing our work within time schedule .We express our sincere gratitude to Mr .Vani V , our co- guide for sharing her expertise by giving constructive comments and suggestions upon reviewing our study.
REFERENCE
- Garrette M. Morris and Marguerita Lim -Wilby , Molecular Docking , Molecular Modelling of Proteins , 11 Mar 2012 ; 443.
- Jakhar, Ritu, Dangi, Mehak, Khichi, Alka, Chhillar, Anil , Relevance of Molecular Docking Studies in Drug Design ,Current Bioinformatics, 4 Nov 2020 ;15 ,270-278(9).
- L. Scotti, F.J.B. Mendonça Júnior, H. Ishiki, F.F. Ribeiro, Rajeev K Singla, J.M. Barbosa Filho, M.S. Da Silva1 and M.T. Scotti, Docking Studies for Multi-Target Drugs ,Current Drug Targets,10 July 2015;18, 592-604(13).
- Rangappa S. Keri, Karam Chand, Srinivas Budagumpi, Sasidhar Balappa Somappa, Siddappa A.Patil, Bhari Malllanna Nagaraj , An overview of benzo[b]thiophene-based medicinal chemistry, Oriental Journal of Chemistry, 29 September 2017; 138,1002-1033.
- Mio Matsumura , Atsuya Muranaka, Rina Kurihara , Misae Kanai , Kengo Yoshida , Naoki Kakusawa ,DaisukeHashizum, Masanobu Uchiyama ,Shuji Yasuike ,General synthesis, structure, and optical properties of benzothiophene-fused benzoheteroles containing Group 15 and 16 elements,Tetrahedron,8 December 2016;72(49),8085-8090.
- Arun M. Isloor , Balakrishna Kalluraya , K. Sridhar Pai ,Synthesis, characterization and biological activities of some new benzo[b]thiophene derivatives, European Journal of Medicinal Chemistry,February 2010;45(2),825-830.
- Huayi Shao ,Dong Li ,Yi Yang ,Hui-Fang Guo ,Zong-Ying Liu ,Shu-Yi Si ,Ze Yang Zhuo-Rong Li , The effect of substituted thiophene and benzothiophene derivates on PPAR? expression and glucose metabolism, Journal of Enzyme Inhibition and MedicinalChemistry,11 March 2010 ; 25(2),283-289.
- Qingmei Liu , Lei Ma , Fangyuan Chen , Shuyun Zhang , Zexin Huang, Xiufen Zheng, Zikai Chen, Junwei Ye, Ning Hou, Wei Yi, Zhi Zhou, Raloxifene-driven benzothiophene derivatives: Discovery, structural refinement, and biological evaluation as potent PPAR? modulators based on drug repurposing, European Journal of Medicinal Chemistry,5 April 2024;269.
- Gadada Nagangowda , Patchanita Thamyongkit and Amorn Petsom , Synthesis and Anti-microbial activities of Benzothiophene Derivatives, Journal of Chilean Chemical Society, Mar 2021 ; 57,1043-1047.
- Isabel C. F. R. Ferreira,a,* Ricardo C. Calhelha,a Let?´cia M. Estevinhoa and Maria-Joa˜o R. P. Queirozb, Screening of antimicrobial activity of diarylamines in the 2,3,5-trimethylbenzo[b]thiophene series: a structure–activity evaluation study, Bioorganic & Medicinal Chemistry Letters,17 September 2004;14,5831-5833.
- Satish K. Sarankar , Kalpna Tomar , Jitendra Bajaj , Parul Mehta , A.K Pathak and Mukul Tailang , QSAR Study of Novel Benzothiophene Derivatives as Potent Anticancer Agent ,International Journal of Advances in Pharmaceutical Science ,2010;309-318.
- Mishra, Raghav, Kumar, Nitin, Isha, Sachan, Neetu, A Review on anticancer activities of thiophene and its analogues, Mini Reviews in Medicinal Chemistry ;20(19); 1944-1965(22) .
- Narsimha Reddy Penthala, Vijayakumar N. Sonar, Jamie Horn, Markos Leggas, Jai Shankar K.B Yadlapalli and Peter A. Crooks, Synthesis and evaluation of a series of benzothiophene acrylonitrile analogs as anticancer agents, MedChemComm.,2013;(7).
- N Susantha Chandrasekera , Mai A. Bailey , Megan Files, Torey Alling, Stephanie and Tanya Parish , Synthesis and anti-tubercular activity of 3-substituted benzo [b] thiophene-1,1-dioxides , European Journal of Medicinal Chemistry ,7 0ct 2014;138
- Tarfah Al-Warhi, Nermeen M. Rashad, Hadia Almahli, Marwa M. Abdel-Aziz, Zainab M. Elsayed, Mai I. Shahin, Wagdy M. Eldehna, Design and synthesis of benzo[b]thiophene-based hybrids as novel antitubercular agents against MDR/XDR Mycobacterium tuberculosis, Arch Pharm,9 November 2023;357(2), 2300529
- Gopal Krishna Rao1 and Rajasekaran Subramaniam2, Synthesis, Antitubercular and Antibacterial Activities of Some Quinazolinone Analogs Substituted with Benzothiophene, Chemical Sciences Journal,2015;6(2).
- Ashraf F. Zaher, Nadia A. Khalil and Eman M. Ahmed , synthesis and anticonvulsant activity of new 3'-aryl7-bromo-spiro[ [1]benzothiophene-3,2'-[1,3] thiazolidine]-2,4'-dione derivatives, Oriental Journal of Chemistry, 2010;26(4),1241-1248.
- Deep, Aakash; Narasimhan, Balasubramanian; Aggarwal, Swati; Kaushik, Dhirender; K. Sharma, Arun Thiophene Scaffold as Prospective Central Nervous System Agent: A Review, Central Nervous System Agents in Medicinal Chemistry,2016; 16(2),158-164.
- K. A. El-Sharkawy1 , N. N. E. El-Sayed2,3 and M. Y. Zaki, Uses of 2-Amino-5,6-dihydro-4H-cyclopenta [b] thiophene-3-carbonitrile in the Synthesis of Heterocyclic Compounds with Anticonvulsant, Behavioural and CNS Antidepressant Activities, International Research Journal of Pure & Applied Chemistry,2012;2(1),91-104.
- Chandramani Pathak1 Rajiv Ranjan Singh1 Saurabh Yadav1,2 Neha Kapoor1 Varshiesh Raina1 Sarika Gupta1 Avadhesha Surolia, Evaluation of Benzothiophene Carboxamides as Analgesics and Anti-inflammatory Agents , International Union of Biochemistry and Molecular Biology,26 March 2014;66(3),201-211.


 Sreeja S. * 1
Sreeja S. * 1






 10.5281/zenodo.11550514
10.5281/zenodo.11550514